Abstract
Twelve laboratories representing 4 countries participated in an interlaboratory study conducted to determine all-trans-β-carotene and total β-carotene in dietary supplements and raw materials. Thirteen samples were sent as blind duplicates to the collaborators. Results obtained from 11 laboratories are reported. For products composed as softgels and tablets that were analyzed for total β-carotene, the reproducibility relative standard deviation (RSDR) ranged from 3.35 to 23.09% and the HorRat values ranged from 1.06 to 3.72. For these products analyzed for trans β-carotene, the reproducibility relative standard deviation (RSDR) ranged from 4.28 to 22.76% and the HorRat values ranged from 0.92 to 3.37. The RSDr and HorRat values in the analysis of a beadlet raw material were substantial and it is believed that the variability within the material itself introduced significant variation in subsampling. The method uses high pressure liquid chromatography (LC) in the reversed-phase mode with visible light absorbance for detection and quantitation. If high levels of α-carotenes are present, a second LC system is used for additional separation and quantitation of the carotene species. It is recommended that the method be adopted as an AOAC Official Method.
Carotenes and,inparticular,β-carotene, have long been recognized for their efficacy in providing vitamin A activity from vegetable sources in the human food supply. In addition, it is accepted that carotenoids in general and carotenes in particular provide significant antioxidant activity to the human food and animal feed supply, and thus may be responsible for some of the significant correlations between increased intake of vegetables providing significant carotenoid content and improved health status. The cis and trans isomers of β-carotene exhibit different biological activities; therefore, their separation and quantitation is important to accurately define the health benefits of the supplement or ingredient.
A single laboratory validation study (SLV; Schierle, J., Pietsch, B., Ceresa, A., & Fizet, C. (2004) J. AOAC Int. 87, 1070–1082) was previously conducted to optimize a high pressure liquid chromatography (LC) method for the determination of total and all-trans-β-carotene in a variety of dietary supplements, including multivitamin tablets, softgels, capsules, and raw material beadlets. Extraction variants were developed for the different types of supplements tested based upon the supplement type and level of β-carotene. The procedure consisted of enzymatic digestion, extraction with ethanol—dichloromethane, followed by dilution or concentration as appropriate. Quantitative LC analysis of the final test solution uses either a reversed-phase C18 column or, in products containing high levels of α-carotene relative to the β-carotene content, a reversed-phase C30 column. The systems were shown to be linear in the range of 0.1–50 μg β-carotene/mL. The predominant mono-cis-β-carotene isomers were well separated from all-trans-β-carotene as well as from α-carotene, lycopene, lutein, and zeaxanthin. Duplicate determinations performed on 8 different test materials by 2 analysts on 5 different days resulted in relative standard deviation (RSD) values of 1.2–4.4%. Recoveries determined for supplements and beadlet raw material spiked with β-carotene levels of 10 μg to 100 mg/test portion and 0.2–40%, respectively, ranged from 97.5 to 102.1%. On the basis of the accuracy, precision, and recovery results for this SLV study, it was recommended that the method be collaboratively studied to validate it as an AOAC Official Method for the determination of β-carotene in dietary supplements.
β-Carotene Standard Purity
During review of the SLV and the data available with regard to the purity of commercially available β-carotene standards, it was apparent that a satisfactory procedure for accurately determining the purity of a particular standard or lot being used by the assaying laboratory is necessary. The purity of the β-carotene standard is determined by a 2-step process wherein the spectrophotometric and chromatographic purities are independently determined and the overall purity of the standard is calculated as the product of the 2 measured purities.
The first step in determining the overall purity is the determination of the spectrophotometric purity. The absorbance at 457 nm of a solution containing a known amount of the standard is taken and the spectrophotometric purity is calculated by using Beer’s Law. The accuracy of the spectrophotometric purity is compromised if the solution contains other components that also absorb at the same wavelength, therefore, the presence of interfering components is determined by chromatographic analysis.
The second step is the determination of the chromatographic purity. The standard is analyzed chromatographically and the chromatographic purity is calculated as the ratio of the all-trans-β-carotene peak relative to all peaks in the chromatogram, because the total area of all the peaks represents the total spectrophotometric absorbance. The true purity of the β-carotene standard can then be calculated as the product of the spectroscopic and chromatographic purities. Each laboratory used its individually determined purities in its calculations.
In this approach, the LC accurately compensates for the presence of impurities that absorb at the spectrophotometric wavelength, while the spectrophotometric determination accurately quantifies only those materials present that absorb at the wavelength used. The 2-step process is incorporated into the method as collaboratively studied.
Collaborative Study
Protocol
Twelve laboratories representing commercial, industrial, and governmental laboratories in 4 countries agreed to participate in the collaborative study, which was conducted using a blind duplicate design. Each laboratory was requested to first assay 3 practice samples to ensure that laboratory personnel understood the entire procedure before proceeding with the collaborative study. All laboratories were asked to provide data with regard to determining the purity of the standard used and to provide chromatograms with results to aid Study Directors, should troubleshooting be necessary.
Laboratories providing inadequate data for the practice samples were provided with assistance in troubleshooting their procedures before repeating the practice samples. Laboratories providing satisfactory data on the practice samples received a shipment of the collaborative study samples. This test set contained blind duplicates, and each laboratory analyzed each test in singlet and reported only the singlet test results.
Test samples to be assayed were commercially available products either purchased at local retail stores or supplied by Joseph Schierle (DSM Nutritional Products, Ltd., Basel, Switzerland). Tablets were ground and homogenized in a high speed, hand-held food processor by the Study Directors and placed in nitrogen overcapped glass jars before submission to the collaborating laboratories. Beadletted raw materials and solutions were mixed thoroughly by manually stirring in the original container before subsampling, and the receiving laboratories were directed to thoroughly mix the test sample they received before assay. For capsule products, the entire quantity of capsules obtained was shaken together to mix the capsules; then capsules were randomly selected and separated out into portions to be sent to the collaborating laboratories. Gelcaps and soft gelcaps were handled similarly.
As the collaborative study proceeded, it became apparent to the Study Directors that the commercial products chosen as “high α-carotene” products requiring additional LC analysis using the C30 reversed-phase columns had undergone a change in formulation and no longer contained >5% α-carotenes. To ensure that the C30 reversed-phase portion of the method was adequately studied, 2 additional products of high relative α-carotene content were obtained, split into blind duplicates, and the 4 resulting test samples were sent to each laboratory.
Variability of Gelcap Weights
Because the methodology for supplements requires the selection of discrete numbers of capsules for the assay, a certain amount of variability in results will be due to differences in the contents of the capsules or gelcaps. To separate this variability factor from the methodology variability factors, the Study Directors randomly selected 10 items from the thoroughly mixed master lot of each relevant formulation type. Variability was determined as follows.
For the analysis of the β-carotene gelcaps, the analytical method prescribes that the weight of the entire gelcap be used to determine test portion size. These commercially available gelcaps are sold to contain consistent levels of oil content and β-carotene concentration; however, the weights of the outer shells vary according to production parameters. This variation introduces a source of variability outside the scope of the analytical method and, as such, a study was undertaken to determine the variability of the shell and content weights for the gelcaps. The variation in the shell weights and contents weights was then accounted for so that the gelcap to gelcap variability could be segregated from the analytical variability determined by this study.
To determine the shell weight and content weight variability of the gelcaps, 10 representative gelcaps of each type of gelcap were randomly chosen from the test samples sent to the collaborating laboratories. Each gelcap was weighed separately and then carefully sliced open with a razor to ensure against any loss of capsule material. The contents were removed by flushing the shell-halves with isopropyl alcohol or methanol until all contents were visibly removed. The empty shells were then dried at room temperature overnight and reweighed. Table 1 lists the average weights and variations of the gelcaps, their contents, and their shells.
Table 1.
Liquid capsule weight variation study
| Sample ID | Capsule description | Avg. capsule wt, g |
Capsule wt (SD)a |
Avg. shell wt, g |
Empty shell wt (SD) |
Avg. contents wt, g | Contents (SD) |
Contents wt (RSD), %b |
|---|---|---|---|---|---|---|---|---|
| 2 | β-Carotene in soy oil | 0.3339 | 0.00299 | 0.1124 | 0.00362 | 0.2214 | 0.00273 | 0.54 |
| 3 | β-Carotene in fish oil | 1.1225 | 0.01315 | 0.3970 | 0.01146 | 0.7255 | 0.01169 | 1.23 |
| 6 | β-Carotene in oil | 0.2554 | 0.00406 | 0.0860 | 0.00879 | 0.1693 | 0.00395 | 2.33 |
| 7 | β-Carotene in oil with vitamin E present | 0.2353 | 0.01614 | 0.0784 | 0.01576 | 0.1569 | 0.00416 | 1.61 |
| 10 | β-Carotene with lycopene present | 0.7586 | 0.01825 | 0.2628 | 0.01743 | 0.4958 | 0.00268 | 2.65 |
| Set 2, No. 1 | β-Carotene in glycerin | 0.5173 | 0.00750 | 0.1932 | 0.00853 | 0.3241 | 0.00493 | 1.52 |
| Set 2, No. 2 | β-Carotene in glycerin | 0.5613 | 0.01909 | 0.2349 | 0.01871 | 0.3264 | 0.00162 | 0.50 |
SD = Standard deviation.
RSD = Relative standard deviation.
During statistical evaluation of the data, the Study Directors determined that by reporting the results in units of mg β-carotene/gelcap as opposed to mg β-carotene/g, the effect of the variation in the gelcap shell weights was removed. The only remaining nonanalytical sources of variation were the weights and the homogeneity of the gelcap contents. Because each set of test samples was obtained from a single manufacturer’s lot, the gelcap contents were taken as homogeneous and of consistent β-carotene concentration. Therefore, the only remaining variable (in addition to analytical variability) was the gelcap content weight. As can be seen in Table 1, this relative variability ranged from 0.5 to 2.65%. This variation in weight from gelcap content to gelcap content was then taken into account to separate the gelcap content variability from the analytical variability of the method.
Use of HPLC System A vs HPLC System B
The method extracts carotene isomers efficiently. However, to accurately quantify β-carotene levels in products that contain elevated levels of α-carotene, a separate LC analysis was used to better resolve the α-carotene and β-carotene isomers. All test samples were initially analyzed using LC System A. If the tentative α-carotene content exceeded 5% of the total carotenes (i.e., the sum of tentative α- and β-carotenes), the test extract was then reanalyzed using LC system B.
AOAC Official Method 2005.07 β-Carotene in Supplements and Raw Materials
Reversed-Phase High Pressure Liquid Chromatographic Method First Action 2005
(Applicable to the determination of all-trans- and total β-carotene in raw materials and supplements from 0 to 200 mg/g except for beadlet materials and tablet materials made from beadlets.)
See Tables 2005.07A-D for the results of the interlaboratory study supporting acceptance of the method.
Table 2005.07A.
Interlaboratory study resutls for the determination of total β-carotene
| Sample No. |
Sample matrix | Unit | Mean | sr | sR | RSDr,% | RSDR,% | r | R | HorRat value |
|---|---|---|---|---|---|---|---|---|---|---|
| 101, 114 | Softgel with β-carotene and lycopene present | mg/capsule | 0.527 | 0.032 | 0.052 | 6.17 | 9.88 | 0.091 | 0.146 | See Table 2005.07C |
| 102, 120 | Capsule, β-carotene from carrot oil | mg/g | 0.505 | 0.053 | 0.072 | 10.52 | 14.29 | 0.148 | 0.202 | 2.28 |
| 103, 118 | Multivitamin tablet | mg/g | 0.535 | 0.122 | 0.124 | 22.88 | 23.09 | 0.343 | 0.346 | 3.72 |
| 104, 112 | Softgel, β-carotene in soy oil | mg/capsule | 15.68 | 1.05 | 1.19 | 6.70 | 7.60 | 2.94 | 3.33 | See Table 2005.07C |
| 105, 109 | Softgel, β-carotene in unknown oil | mg/capsule | 6.65 | 0.061 | 0.801 | 9.09 | 12.04 | 1.69 | 2.24 | See Table 2005.07C |
| 106, 110 | Placebo tablet | mg/g | 0 | NAa | NA | NA | NA | NA | NA | NA |
| 107, 119 | Beadlets used as raw material | mg/g | 196 | 11 | 24 | 5.39 | 12.3 | 29.57 | 67.43 | 4.81 |
| 108, 116 | Multivitamin/multimineral tablet | mg/g | 0.516 | 0.019 | 0.034 | 3.68 | 6.61 | 0.053 | 0.095 | 1.06 |
| 111, 121 | Softgel, β-carotene in fish oil | mg/capsule | 0.056 | 0.0041 | 0.0076 | 7.23 | 13.56 | 0.011 | 0.021 | See Table 2005.07C |
| 113, 117 | Hard tablet | mg/g | 49 | 3 | 3.6 | 6.22 | 7.31 | 8.53 | 10.03 | 2.32 |
| 115, 122 | Softgel with vitamins A and E present | mg/capsule | 15.19 | 1.10 | 1.1 | 7.25 | 7.25 | 3.09 | 3.09 | See Table 2005.07C |
| 2–1, 2–4 | Ultracarotenoid softgel | mg/capsule | 16.92 | 0.44 | 0.87 | 2.60 | 4.87 | 1.23 | 2.31 | See Table 2005.07C |
| 2–2, 2–3 | Carotenoid softgel | mg/capsule | 3.55 | 0.029 | 0.32 | 0.82 | 9.10 | 0.081 | 0.90 | See Table 2005.07C |
NA = Not applicable.
Table 2005.07D.
Interlaboratory study results for the determination of all-trans-β-carotene
| Sample No. | Original RSDR, %a | RSDR, % of capsule contentsb |
Net RSDSR, % |
Mean capsule wt, g |
C (g β-carotene/ g capsule) |
Expected RSDR |
Adjusted HorRat values |
|---|---|---|---|---|---|---|---|
| 101, 114 | 15.35 | 0.54 | 15.04 | 0.7586 | 0.000475 | 6.33 | 2.38 |
| 104, 112 | 9.20 | 1.23 | 8.49 | 0.3339 | 0.044325 | 3.20 | 2.66 |
| 105, 109 | 9.19 | 2.33 | 7.84 | 0.2554 | 0.024393 | 3.50 | 2.24 |
| 111, 121 | 14.08 | 1.61 | 13.15 | 1.1225 | 0.0000292 | 9.50 | 1.37 |
| 115, 122 | 5.81 | 2.65 | 4.28 | 0.2353 | 0.060901 | 3.05 | 1.40 |
| 2–1, 2–4 | 5.60 | 1.52 | 4.72 | 0.5173 | 0.029963 | 3.39 | 1.39 |
| 2–2, 2–3 | 8.84 | 0.50 | 8.55 | 0.5613 | 0.004596 | 4.50 | 1.90 |
From Table 2005.07A.
From Table 2.
A. Principle
Water dispersible formulations such as powders, emulsions, tablets, and capsules are digested with protease and extracted with dichloromethane and alcohol. Oily suspensions are dissolved directly in dichloromethane and alcohol. The extract is chromatographed on a C18 isocratic high-performance liquid chromatography (LC) system that separates the predominant geometrical isomers of β-carotene from each other and from other carotenoids such as all-trans-α-carotene, lycopene, cryptoxanthin, lutein, and zeaxanthin. In the case of products with relatively high α-carotene content, where the cis-isomers of α-carotene are known to interfere with the quantitation of β-carotene, the extract is further chromatographed with a more selective C30 reversed-phase LC system in the gradient mode that minimizes this interference.
B. Reagents
β-Carotene, type II.—Sigma-Aldrich (St. Louis, MO; USA; CAS 7235-40-7). Purity >95%, grade: type II.
Alkaline protease R.—Bio-Cat, Inc. (Troy, VA, USA; www.bio-cat.com).
n-Hexane.—CAS 110-54-3, purity ≥99%, peroxide free (GC).
Cyclohexane.—CAS 110-82-7, purity 99.5% (GC).
Tetrahydrofuran.—CAS 109-99-9, purity ≥99.5%, peroxide free (GC).
Ethanol.—CAS 64-17-5, purity ≥99.5% (GC).
Dichloromethane.—CAS 75-09-2, purity ≥99.5% (GC).
Protease.—CAS 9014-01-1, bacterial alkaline protease enzyme preparation in water, Synonym: subtilisin, IUB 3.4.21.62.
Methanol.—CAS 67-56-1, purity 99.8% (GC).
Butylated hydroxytoluene (BHT).—CAS 128-37-0, purity≥99%.
N-ethyldiisopropylamine.—CAS 7087-68-5, purity ≥98% (GC).
2-Propanol.—CAS 67-63-0, purity ≥98% (GC).
Ammonium acetate.—CAS 631-61-8, purity ≥98% (NT).
Acetonitrile.—CAS 75-05-08, purity ≥99.9% (GC).
Methyl-tert-butyl ether (MTBE).—CAS 1634-04-4, purity ≥99.5% (GC).
Water.—Distilled or demineralized.
LC control solution.—(1) Preparation.—Dissolve about 3 mg β-carotene reference substance and 1 g BHT in 50 mL tetrahydrofuran. Add 200 mL ethanol and reflux for 2 h in a water bath at 80°C. Cool, dilute to 500 mL with ethanol, and transfer the solution to a dispenser bottle. Mix well, leave overnight at room temperature, and dispense solution into a large number of LC vials. Carefully seal the vials immediately after filling with Teflon/silicone septa, and store at ca 5°C in the dark. (2) Use of the LC control solution.—Measure the initial β-carotene content of the control solution when the LC system is calibrated. Inject in parallel with the standard solution, at least six 20 μL aliquots into the LC system. Calculate themean β-carotene content of the control solution from the resulting chromatograms using the newly determined response factor. Subsequently, inject the control solution together with each series of test extracts. The response factor is regarded as constant as long as the measured β-carotene content of the control solution corresponds to the initial value within ±2%. As long as the response factor remains within these limits, the original response factor can be used for calculations. But the LC system must be recalibrated if the measured β-carotene content exceeds the tolerance.
Mobile phase for LC System A.—In a 1 L volumetric flask, dissolve 50 mg BHT in 20 mL 2-propanol and add 0.2 mL N-ethyldiisopropylamine, 25 mL 0.2% ammonium acetate solution, (m), 455 mL acetonitrile, and ca 450 mL methanol. Mixture cools and contracts. Warm to room temperature and dilute to volume with methanol.
Mobile phase for LC System B.—(1) Solvent1.—In a 1 L volumetric flask, dissolve 200 mg vitamin C in 500 mL methanol (sonicate if necessary), add 100 mL MTBE, and dilute to volume with methanol. Discard after 2 days. (2) Solvent 2.—MTBE.
C. Apparatus
Analytical balance.—Readability ±0.0005 g.
Spectrophotometer.—Dual beam, wavelength range of 190–900 nm, 1.5 nm fixed spectral bandwidth, wavelength accuracy of 0.07 nm (at 541.92 nm), wavelength reproducibility of 0.01 nm.
Ultrasonic water bath.—150 W, 35 kHz, 4 L.
Rotary evaporator.—5–240 rpm, 20°–100°C connected to a vacuum pump, absolute minimum pressure of 10 mbar.
Homogenizer.—750 W, 12 mm diameter dispersing aggregate.
Syringe.—Disposable, 2mL.
Filter.—Disposable, 0.45 μm pore size, 25 mm diameter, for organic solvents.
Liquid chromatograph.—Consisting of online degasser, pump, autosampler unit, column thermostat, UV-Vis detector, integrator. (1) Operating conditions for System A.—Column, Suplex pKb-100, Supelco, 5 μm, 250 × 4.6mm; column temperature, 30°C; autosampler temperature, 15°C; mobile phase, see B(r); flow rate, 0.6 mL/min; pressure, ca 33 bar; injection volume, 20 μL; detection wavelength, 448 nm; run time, ca 30 min. (2) Operating conditions for System B.—Column, YMC-Pack C30, 5 μm, 250 × 4.6 mm; column temperature, 30°C; autosampler temperature, 15°C; pressure, 40–80 bar; injection volume, 20 μL; detector wavelength, 445 nm; run time, ca 65 min; mobile phase, step gradient using solvent 1 and solvent 2 [see B(s)]: 0–57 min: 100% solvent 1 (0.9 mL/min); 57–60 min: 100% solvent 2 (2.0 mL/min); 60–65 min: 100% solvent 1 (0.9 mL/min).
D. Determination of Purity of Standard
Determine the purity of the β-carotene standard by a 2-step process where the spectrophotometric and chromatographic purities are independently determined and the overall purity of the standard is reported as the product of the 2 measured purities.
- Spectrophotometric purity (SP).—Weigh ca 6 mg all-trans-β-carotene reference standard, recorded to the nearest 0.01 mg and dissolve in 100 mL tetrahydrofuran (identify as Stock Solution A). Pipet 5 mL of this solution into a 100 mL volumetric flask and dilute to volume with cyclohexane to give a standard measuring solution of 3 μg/mL β-carotene in cyclohexane—tetrahydrofuran (95 + 5). Measure the absorption (ABS) of the standard measuring solution against cyclohexane at the absorbance maximum (457 nm).Calculate the apparent absorbance constant, Eapp{1%, 1 cm}, of the reference standard:
where A is the absorbance at the maximum, 2 is the dilution factor in L, W is the mass of reference standard in mg, 10 000 is the factor to convert from % to mg/L, and B is the cell path length in cm.Calculate the SP of the all-trans-β-carotene reference standard:
where 2505 is the reported E{1%, 1 cm} value for pure all-trans-β-carotene in cyclohexane (Schwieter, U., & Isler, O. (1967) The Vitamins, Vol. 1, W.H. Sebrell Jr & R.S. Harris (Eds), Academic Press, New York, NY, p. 73). -
Chromatographic purity(CP).—Pipet exactly 5.0 mL Stock Solution A into 100 mL volumetric flask. Add 5.0 mL tetrahydrofuran and dilute to volume with ethanol. This is the standard working solution ca 3 μg/mL β-carotene in ethanol—tetrahydrofuran (9 + 1). Immediately analyze six 20 μL aliquots using LC System A as described below. Determine the CP of the all-trans-β-carotene relative to all compounds in the chromatogram. (Ignore the solvent front or any peak present in a solvent blank.)
CP = (Area of all-trans-β-carotene)/(sum of the areas of all relevant peaks)
Calculate the CP as the average of the individual CP values of all 6 injections.
- Reference standard purity (P).—Calculate the purity (P) of the reference standard:
where 100 is the conversion of decimal to percent.
E. Calibration of LC Systems
- Calculate the concentration (C) of all-trans-β-carotene in D(c), the standard working solution.
where W is the weight of the test portion in mg, P is the purity in %, and 200 is the factor for the dilution of 2000 mL, the conversion of mg to μg, and the conversion of percent to decimal for the purity (i.e., 2000/(1000 × 100). - Calculate the response factor (RF) for all-trans-β-carotene:
where Areasws is the average peak area of all-trans-β-carotene in the 6 injections of D(c), the working standard solution. Inject at least six 20 μL aliquots into LC System B. Determine the instrument’s response factor as in (b) above.
F. Extraction
Prior to analysis, store test samples at 4°C in the dark. Grind hard tablets to homogeneity. Analyze capsules and softgels containing formulations as such.
Extract the test portion according to the physical form of the material, the expected content of β-carotene, and the weight of the dosage form.
Oily solutions or suspensions.—Weigh to the nearest 0.1 mg a test portion containing ca 20 mg β-carotene. Add 250 mg BHT and transfer into a 250 mL volumetric flask with 120 mL dichloromethane. Add 100 mL ethanol and shake until completely dissolved or suspended. Let stand in the dark until room temperature is reached (about 2 h). Add dichloromethane to volume and shake again vigorously. Pipet a 5 mL aliquot into a 50 mL volumetric flask and dilute to volume with dichloromethane—ethanol (1 + 1). Continue to chromatographic analysis, G.
Powders, beadlets, or emulsions.—Weigh to the nearest 0.1 mg a test portion containing ca 10 mg β-carotene and place into a 250 mL volumetric flask. Add 250 mg BHT, 0.5 mL alkaline Protease R, and 15 mL water. Tilt the flask gently to wet the entire contents. Place the flasks in an ultrasonic bath at ca 50°C for 30 min, and swirl at 10 min intervals. Add 100 mL ethanol to the warm suspension and shake vigorously. Add 135 mL dichloromethane and shake again. Let the mixture stand in the dark until it reaches room temperature (ca 2 h), dilute to volume with dichloromethane, shake vigorously, and allow solids to settle in the dark. Pipet a 5 mL aliquot into a 50 mL volumetric flask and dilute to volume with dichloromethane—ethanol (1 + 1). Filter cloudy solutions through a 0.45 μm membrane filter and proceed to chromatographic analysis, G.
-
Tablets and capsules.—Randomly select a test portion equivalent to 3 tablets or capsules (but a mass ≤5 g). If concentration by weight result is desired, weigh to the nearest 0.1 mg. Grind tablets to a fine powder and place into 250 mL volumetric flask. For powder-containing capsules, empty the shell, and place shell and contents into 250 mL volumetric flask. Place capsules containing liquid formulations directly into 250 mL volumetric flask.
Add 250 mg BHT, 0.5 mL alkaline Protease R, and 15 mL water, and tilt the flask gently to wet the entire contents. Place flask into an ultrasonic bath at ca 50°C for 30 min, and swirl at 10 min intervals. Add 100 mL ethanol to the warm suspension and shake vigorously. Add 120 mL dichloromethane and shake vigorously again. Disperse any clumps in the homogenizer, and rinse the homogenizer probe with 15 mL dichloromethane into the volumetric flask. Allow the extract to stand in the dark until room temperature is reached (ca 2 h), dilute to volume with dichloromethane, shake vigorously, and allow solids to settle.
Depending upon the estimated β-carotene content of the test portion, continue with steps 1, 2, or 3:
(1) β-Carotene content of test portions below 0.1 mg.— Evaporate a representative aliquot, e.g., 50 mL, of the supernatant to dryness under reduced pressure at 40°C, using a rotary evaporator and a 250 mL round-bottom flask. Dissolve the dry residue in a volume of ethanol—dichloromethane (1 + 1) so that the extract is concentrated by a factor of 10, e.g., residue from 50 mL dissolved in 5 mL. Filter cloudy solutions through a 0.45 μm membrane filter and proceed to chromatographic analysis, G.
(2) β-Carotene content of the test portion between 0.1 and 10 mg.—Filter cloudy solutions through a 0.45 μm membrane and proceed to chromatographic analysis, G.
(3) β-Carotene content of the test portion above 10 mg.— Dilute an aliquot of the supernatant with ethanol—dichloromethane (1 + 1) so that the β-carotene content of the final solution is 1–10 μg/mL. Filter cloudy solutions through a 0.45 μm membrane and proceed to chromatographic analysis, G.
G. Chromatography
First an a lyze test extract using LC System A. If the tentative α-carotene content exceeds 5% of the total (sum of tentative α and β) carotenes, then the extract must be analyzed by using LC System B.
-
(1) LC System A conditions.—Column, Suplex pKb-100, Supelco, 5 μm, 250 × 4.6 mm; column temperature, 30°C; autosampler temperature, 15°C; mobile phase, see B(r); flow rate, 0.6 mL/min; pressure, ca 33 bar; injection volume, 20 μL; detection, 448 nm; run time, ca 30 min.
Retention times.—all-trans-β-carotene: ca 20–25 min. Approximate retention times relative to all-trans-β-carotene: all-trans-lutein, 0.30; all-trans-zeaxanthin, 0.32; all-trans-β-cryptoxanthin, 0.58; all-trans-lycopene, 0.67; nonidentified cis-lycopene, 0.69, 0.75, 0.79; 9′-cis-α-carotene, 0.91; all-trans-α-carotene, 0.93; 9-cis-α-carotene, 0.98; 13-cis and 13′-cis-α-carotene, 1.03; nonidentified cis-α-carotene, 1.08; all-trans-β-carotene, 1.00; 9-cis-β-carotene, 1.07; 13-cis-β-carotene, 1.17; 15-cis-β-carotene,1.21.
Other minor cis-isomers of β-carotene elute in the range of the trans- and mono-cis-isomers of β-carotene (e.g., with relative retention times of 1.02 for 13,15-di-cis-β-carotene, 1.11 for 9,9′-di-cis-β-carotene, and 1.12 for 9,15-di-cis-β-carotene).
Representative chromatograms for the heat isomerized beta-carotene control sample and a dietary supplement are shown in Figure 2005.07A.
-
(2) LC System B conditions.—Column, YMC-Pack C30, 5 μm, 250 × 4.6 mm; column temperature, 30°C; autosampler temperature, 15°C; mobile phase, step gradient using solvent 1 and solvent 2, see B(s): 0—ca 57 min [adjust the start point of the flush to the retention time of 9-cis-β-carotene (see Retention times listed below)]: 100% solvent 1; ca 57–60 min [adjust the start point of the flush to the retention time of 9-cis-β-carotene (see Retention times listed below)]: 100% solvent 2 (flush); ca 60–65 min: 100% solvent 1; flow rate, 0—ca 57 min [adjust the start point of the flush to the retention time of 9-cis-β-carotene(see Retention times listed below)]: 0.9 mL/min; ca 57–60 min [adjust the start point of the flush to the retention time of 9-cis-β-carotene (see Retention times listed below)]: 2.0 mL/min; ca 60–65 min: 0.9 mL/min; pressure, 40–80 bar; injection volume, 20 μL; detector wavelength 445 nm; run time, ca 65 min.
Retention times.—all-trans-β-carotene: ca 40–55 min. Approximate retention times relative to all-trans-β-carotene: all-trans-lutein, 0.22; all-trans-zeaxanthin, 0.26; all-trans-β-cryptoxanthin, 0.51; 13-cis-α-carotene, 0.48; nonidentified cis-β-carotene, 0.50 (this cis-isomer ofβ-carotene is only taken into account if total β-carotene exceeds total α-carotene by far, e.g., by factor 10); nonidentified cis-α-carotene, 0.50; 13′-cis-α-carotene, 0.53; 15-cis-β-carotene, 0.56; 13,15-di-cis-β-carotene, 0.58; 13-cis-β-carotene, 0.61; 9,15-di-cis-β-carotene, 0.64; nonidentified cis-β-carotene, 0.73; all-trans-α-carotene, 0.76; 9-cis-α-carotene, 0.79; 9,9′-di-cis-β-carotene, 0.91; 9′-cis-α-carotene, 0.95; all-trans-β-carotene, 1.00; 9-cis-β-carotene, 1.12; lycopene isomers, >1.20.
Representative chromatograms for the heat isomerized β-carotene control sample and a dietary supplement are shown in Figure 2005.07B.
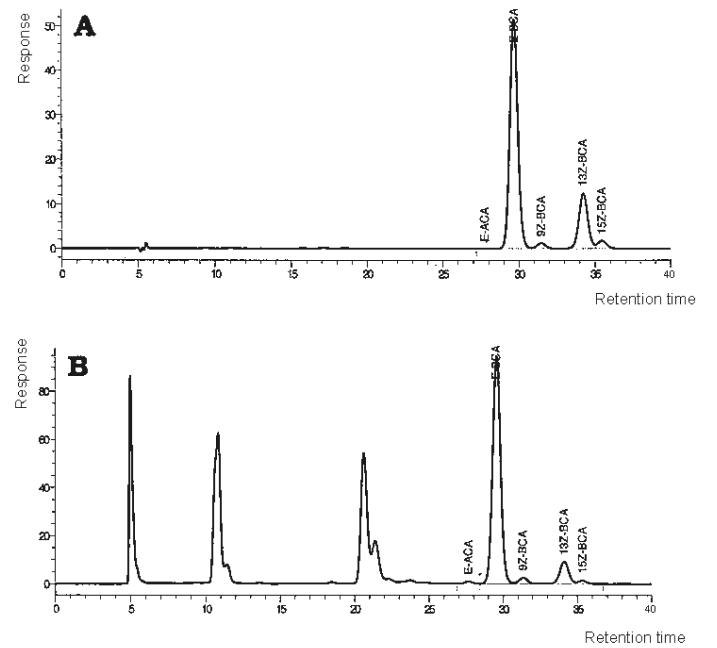
Figure 2005.07A.
Chromatograms using LC System A. (A) Control sample; (B) multivitamin tablet sample 108.
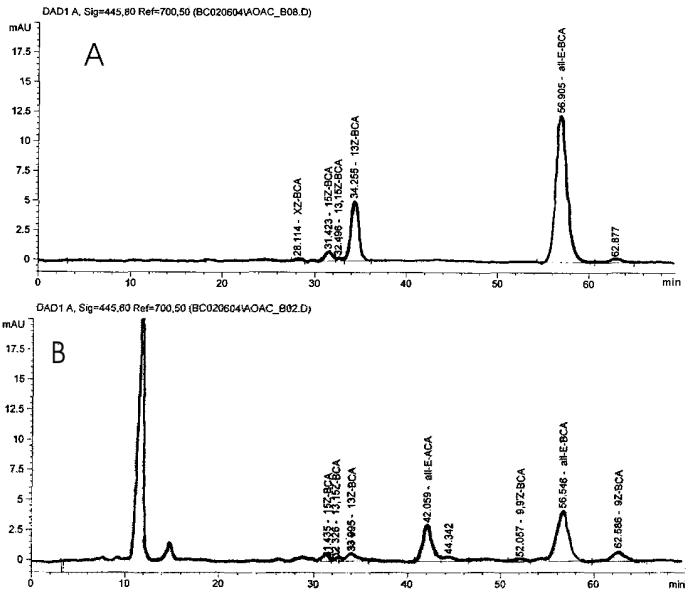
Figure 2005.07B.
Chromatograms using LC System B. (A) Control sample; (B) softgel sample set 2, No. 2.
H. Calculations
The contents of all-trans- and total β-carotene in the test samples are determined as follows
where Ctot = total β-carotene content (mg/g or mg/dose); Ctrans = all-trans β-carotene content (mg/g or mg/dose); Atrans = peak area of all-trans-β-carotene (area units = AU); A9cis = peak area of 9-cis-β-carotene (AU); A13cis = peak area of 13-cis-β-carotene (AU); A15cis = peak area of 15-cis-β-carotene (AU); AXcis = sum of peak area of other cis-isomers of β-carotene (AU); 1.2 and 1.4 are relative response factors (correction factors to compensate for lower specific absorption of 13-cis- and 15-cis-β-carotene compared to all-trans-β-carotene at this wavelength); M = test portion amount (g); N = number of tablets or capsules used as amount of test portion; RFtrans = response factor of all-trans-β-carotene (AU*L/mg); V = theoretical volume in which the test portion is dissolved (L) = [V1 × (V3/V2)] where V1 = volume of the flask used for extraction with dichloromethane—ethanol (L; 0.25); V2 = volume aliquot which is diluted or evaporated (L); V3 = volume to which aliquot V2 is diluted or in which the residue after evaporation of aliquot V2 is dissolved (L).
When LC System A is used, calculate a tentative total α-carotene using the same equation as for total β-carotene, except that RFα = RFβ × 1.053. If the tentative α-carotene content of the product exceeds 5% of the total (sum of tentative α and β) carotenes, immediately analyze the extract using HPLC System B. Calculate the total and all-trans-β-carotene as above using this system.
Reference: J. AOAC Int. 88, 1279 (2005).
Results and Discussion
Eleven of the 12 laboratories that agreed to participate successfully completed the purity assay of their standards, established instrument linearity, analyzed the practice samples, and were able to submit complete data packages. One laboratory was unable to successfully analyze the practice samples and was excluded from the study. The remaining laboratories did not report any difficulties in conducting the method and the study.
Purity of Standards
Carotenes, by nature, are susceptible to deterioration by various factors. For this reason, it is virtually impossible to ensure that a high purity all-trans-β-carotene standard will be of that high purity at the time of injection unto the LC system. Therefore, it is necessary to ascertain the purity of the all-trans-β-carotene at the time of injection for calibration of the LC system. Two types of impurities can be present in the standard. The first type are inert materials that have no absorbance at the wavelength of analytical interest for the standard. Standard spectrometry can be used to measure the total absorbance at the wavelength of interest, and thus, by calculation against known absorptivities, eliminate the inertmaterials. The second type of impurities are those that absorb at the wavelength of interest and thus add to the total absorbance, resulting in an artificially high estimate of purity when only spectrophotometric absorbance is used for purity determination. The total peak area of a liquid chromatogram at the wavelength of interest will be proportional to the total spectrophotometric absorbance and the all-trans-β-carotene peak area as a proportion of the total area will represent the proportion of the absorbing content that is the all-trans-β-carotene. Thus, both the spectrophotometric and the LC measurements of purity can be made and multiplied by each other to obtain the actual purity of the standard at the time of injection for calibration of the instrument. The purity of standards found by the various laboratories is shown in Table 2. As can be seen, significant differences in purity were obtained by each of the various laboratories participating in the collaborative study.
Table 2.
β-Carotene standard purity determinations
| Laboratory | Spectrophotometric purity, % |
LC purity, % | Total purity at time of injection for calibration, % |
|---|---|---|---|
| 1 | 88.33 | 91.42 | 80.75 |
| 2 | 97.90 | 97.50 | 95.45 |
| 3 | 94.70 | 98.80 | 93.76 |
| 4 | 95.84 | 93.04 | 89.17 |
| 5 | 86.40 | 98.07 | 84.73 |
| 6 | 104.8 | 95.53 | 100.12 |
| 7 | 95.00 | 97.30 | 92.30 |
| 8 | 96.55 | 98.28 | 94.89 |
| 9 | 98.84 | 96.84 | 95.72 |
| 10 | 94.59 | 97.75 | 92.46 |
| 11 | 87.40 | 97.80 | 85.48 |
Use of Control Solutions
Because of the susceptibility of carotenes to isomerization, one means of ensuring consistent calibrations is to purposefully isomerize the all-trans-β-carotene to an equilibrium mixture of carotenes that is known to be stable for an extended period of time if properly stored. Modern LC instruments are of sufficient stability to operate over extended periods of time without recalibration; however, checks need to be made to ensure that the calibration has not shifted, or that the instrument has not become malfunctional over time. When analyzed with each batch assayed by LC, the stable control solution [prepared as in B(q)] readily checks the performance of the LC system without the need to recalibrate the unit with each analytical run performed. Examples of the chromatographic analysis of the control sample using Systems A and B are shown in Figures 2005.07A and 2005.07B.
Linearity Study
At the onset of the study, each laboratory verified the expected linear response range of its LC detector under conditions of LC System A using the following procedure: Dissolve 10 mg all-trans-β-carotene in 5 mL dichloromethane in a 100 mL volumetric flask. Add 50 mL ethanol and reflux at 80°C in a water bath for 2 h. Add ca 40 mL dichloromethane, bring to room temperature, and dilute to volume with dichloromethane to give a solution of 100 μg/mL. Dilute this solution with a mixture of dichloromethane—ethanol (1 + 1) as shown in Table 3 to obtain β-carotene concentrations of 100–0.05 μg/mL and inject 20 μL aliquots onto the LC system.
Table 3.
Linearity study results
| Laboratory | Linear range found, μg/mL |
Correlation coefficient |
|---|---|---|
| 1 | 0.05–100 | 0.9996 |
| 2 | 0.05–100 | 1.0000 |
| 3 | 0.05–100 | 0.9991 |
| 4 | 0.05–101.2 | 0.9980 |
| 5 | 0.05–20 | 0.9998 |
| 6 | 0.047–94 | 1.0000 |
| 7 | 0.038–75.7 | 1.0000 |
| 8 | 0.04–77.4 | 0.9998 |
| 9 | 0.060–119.4 | 0.9993 |
| 10 | 0.05–100 | 1.0000 |
| 11 | 0.05–100 | 1.0000 |
The results of the linearity studies are shown in Table 3. Laboratories obtaining satisfactory linear response ranges were allowed to continue with the study. The linearity check was to be repeated if, during the study, the LC detector lamp was replaced or if the detector was serviced. No laboratory reported a need to redo the linearity study.
Data Analysis
AOAC INTERNATIONAL provided guidance for the statistical evaluation of the data by supplying a spreadsheet template/calculator [AOAC Statistical Software, updated by Joanna Lynch, Cornell University, Ithaca, NY (2001)]. Results obtained from the 11 laboratories were entered in the spreadsheet and the statistical results are shown in Tables 2005.07A and 2005.07B.
Table 2005.07B.
Interlaboratory study resutls for the determination of trans-β-carotene
| Sample No. |
Sample matrix | Unit | Mean | sr | sR | RSDr,% | RSDR,% | r | R | HorRat values |
|---|---|---|---|---|---|---|---|---|---|---|
| 101, 114 | Softgel with β-carotene and lycopene present | mg/capsule | 0.360 | 0.0281 | 0.0552 | 7.81 | 15.35 | 0.0787 | 0.155 | See Table 2005.07D |
| 102, 120 | Capsule, β-carotene from carrot oil | mg/g | 0.288 | 0.015 | 0.037 | 5.39 | 12.91 | 0.043 | 0.104 | 1.89 |
| 103, 118 | Multivitamin tablet | mg/g | 0.31 | 0.06 | 0.07 | 19.25 | 22.76 | 0.167 | 0.197 | 3.37 |
| 104, 112 | Softgel, β-carotenen in soy oil | mg/capsule | 14.8 | 1.05 | 1.36 | 7.09 | 9.20 | 2.93 | 3.80 | See Table 2005.07D |
| 105, 109 | Softgel, β-carotene from carrot oil | mg/capsule | 6.23 | 0.535 | 0.572 | 8.58 | 9.19 | 1.50 | 1.60 | See Table 2005.07D |
| 106, 110 | Placebo tablet | mg/g | 0 | NAa | NA | NA | NA | NA | NA | NA |
| 107, 119 | Beadlets used as raw material | mg/g | 152 | 11 | 19 | 7.1 | 12.7 | 30 | 54 | 4.8 |
| 108, 116 | Multivitamin/multimineral tablet | mg/g | 0.432 | 0.015 | 0.026 | 3.36 | 5.93 | 0.041 | 0.072 | 0.92 |
| 111, 121 | Softgel, β-carotene in fish oil | mg/capsule | 0.0359 | 0.0035 | 0.011 | 9.81 | 31.0 | 0.0098 | 0.0312 | See Table 2005.07D |
| 113, 117 | Hard tablet | mg/g | 38.2 | 2.7 | 3 | 7.07 | 7.82 | 7.57 | 8.37 | 2.39 |
| 115, 122 | Softgel with vitamins A and E present | mg/capsule | 14.33 | 0.832 | 0.832 | 5.81 | 5.81 | 2.33 | 2.33 | 1.53 |
| 2–1, 2–4 | Ultracarotenodi softgel | mg/capsule | 15.50 | 0.257 | 0.868 | 1.66 | 5.60 | 0.720 | 2.43 | See Table 2005.07D |
| 2–2, 2–3 | Carotenoid softgel | mg/capsule | 2.58 | 0.031 | 0.228 | 1.20 | 8.84 | 0.0867 | 0.693 | See Table 2005.07D |
NA = Not applicable.
For the softgel samples listed in Tables 4 and 5, the standard deviation and %RSD results contain the analytical variation and the variation of the amount of contents within the softgel. The %RSDR values were later adjusted for the variation of the weight of the contents inside the capsules and the adjusted values are shown in Tables 2005.07C and 2005.07D. Adjustment of the results was accomplished by subtraction of the RSD of the capsule contents from the overall RSD (with the %RSDR of the capsule contents adjusted for the 3 capsules used in the analysis):
Table 4.
Total β-carotene raw data
| Laborator |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID |
Unit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Avg. |
| 101 | mg/dose | 0.539 | 0.477 | 0.575 | 0.485 | 0.535 | 0.472 | 0.611 | 0.506 | 0.609 | 0.563 | 0.485 | 0.533 |
| 102 | mg/g | 0.459 | 0.531 | 0.427 | 0.395 | 0.588 | 0.425 | 0.450 | 0.510 | 0.459 | 0.544 | 0.497 | 0.480 |
| 103 | mg/g | 0.431 | 0.553 | 0.479 | 0.239 | 0.872 | 0.499 | 0.510 | 0.740 | 0.612 | 0.617 | 0.607 | 0.560 |
| 104 | mg/dose | 17.5 | 16.3 | 16.83 | 13.29 | 16.12 | 15.29 | 14.91 | 16.63 | 8.98a | 14.88 | 15.6 | 15.1 |
| 105 | mg/dose | 6.94 | 6.6 | 6.475 | 4.80 | 8.07 | 6.38 | 6.25 | 5.47 | 6.81 | 6.47 | 6.4 | 6.43 |
| 106 | mg/g | 0.0 | 0.0 | 0.0 | 0.0 | 0.001 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 107 | mg/g | 227 | 191 | 187.7 | 160.04 | 244 | 156.4 | 177.38 | 216.37 | 222 | 193 | 192 | 197 |
| 108 | mg/g | 0.532 | 0.467 | 0.505 | 0.496a | 0.584a | 0.528 | 0.503 | 0.54 | 0.549 | 0.527 | 0.497 | 0.521 |
| 109 | mg/dose | 7.14 | 6.51 | 6.38 | 6.33 | 8.13 | 6.37 | 8.31 | 6.58 | 7.21 | 6.40 | 6.38 | 6.88 |
| 110 | mg/g | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 111 | mg/dose | 0.0560 | 0.0644 | 0.0610 | 0.0506 | 0.0708 | 0.0568 | 0.0460 | 0.0563 | 0.0517 | 0.0463 | 0.0584 | 0.0562 |
| 112 | mg/dose | 16.7 | 16.3 | 14.49 | 15.64 | 16.00 | 15.91 | 12.70 | 15.68 | 17.27a | 16.92 | 15.9 | 15.8 |
| 113 | mg/g | 47.9 | 50.4 | 46.42 | 47.98 | 55.5 | 44.85 | 41.19 | 51.56 | 30.6a | 49.5 | 46.8 | 46.6 |
| 114 | mg/dose | 0.518 | 0.49 | 0.592 | 0.408 | 0.5988 | 0.485 | 0.555 | 0.517 | 0.541 | 0.508 | 0.521 | 0.521 |
| 115 | mg/dose | 15.5 | 15.0 | 14.32 | 14.69 | 18.75b | 14.78 | 13.34 | 15.41 | 13.67 | 14.93 | 15.1 | 15.0 |
| 116 | mg/g | 0.532 | 0.467 | 0.519 | 1.539a | 0.71a | 0.507 | 0.443 | 0.57 | 0.567 | 0.507 | 0.523 | 0.626 |
| 117 | mg/g | 56.8 | 49.9 | 48.89 | 45.38 | 50.4 | 48.06 | 47.62 | 52.81 | 52.8a | 47.5 | 50 | 50.0 |
| 118 | mg/g | 0.44 | 0.603 | 0.513 | 0.493 | 0.448 | 0.458 | 0.50 | 0.46 | 0.489 | 0.625 | 0.557 | 0.508 |
| 119 | mg/g | 203 | 193 | 189.9 | 187.5 | 212 | 152.6 | 177.0 | 212.7 | 228 | 197 | 189 | 195 |
| 120 | mg/g | 0.474 | 0.481 | 0.549 | 0.514 | 0.722 | 0.46 | 0.437 | 0.55 | 0.55 | 0.569 | 0.542 | 0.532 |
| 121 | mg/dose | 0.0580 | 0.0644 | 0.0630 | 0.0488 | 0.0706 | 0.0513 | 0.0575 | 0.0446 | 0.0494 | 0.0517 | 0.0544 | 0.0558 |
| 122 | mg/dose | 15.1 | 15.1 | 15.31 | 15.05 | 15.80b | 14.53 | 15.87 | 15.50 | 16.84 | 14.93 | 14.7 | 15.3 |
| 2–1 | mg/dose | 17.2 | 16.8 | 15.49 | 17.76 | 16.99 | 15.75 | 15.97 | 17.75 | 18.35a | 18.00 | 16.4 | 17.0 |
| 2–2 | mg/dose | 3.81 | 3.34 | 3.65 | 3.07 | 4.05 | 3.17 | 3.54 | 3.60 | 4.05 | 3.77 | 3.29a | 3.58 |
| 2–3 | mg/dose | 3.78 | 3.31 | 3.66 | 3.03 | 3.59 | 3.12 | 3.49 | 3.67 | 4.02 | 3.80 | 3.55a | 3.55 |
| 2–4 | mg/dose | 17.2 | 16.8 | 15.49 | 17.76 | 16.99 | 15.75 | 15.97 | 17.75 | 18.35a | 18.00 | 16.1 | 16.4 |
Cochran outlier.
Single Grubbs outlier.
Table 5.
all-trans-β-carotene raw data
| Laboratory |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID |
Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Avg. |
| 101 | mg/dose | 0.371 | 0.312 | 0.381 | 0.331 | 0.456 | 0.305 | 0.396 | 0.352 | 0.443 | 0.353 | 0.328 | 0.366 |
| 102 | mg/g | 0.248 | 0.271 | 0.342 | 0.22a | 0.376 | 0.268 | 0.272 | 0.28 | 0.294 | 0.278 | 0.267 | 0.283 |
| 103 | mg/g | 0.208 | 0.314 | 0.337 | 0.174 | 0.455 | 0.294 | 0.2756 | 0.42 | 0.346 | 0.345 | 0.389 | 0.323 |
| 104 | mg/dose | 16.56 | 15.60 | 16.31 | 12.73 | 11.91 | 14.67 | 14.29 | 15.94 | 8.53a | 14.24 | 14.9 | 14.2 |
| 105 | mg/dose | 6.475 | 6.340 | 6.288 | 4.586 | 6.561 | 6.090 | 5.974 | 5.261 | 6.507 | 6.205 | 6.13 | 6.04 |
| 106 | mg/g | 0.00 | 0.00 | 0.00 | 0.00 | 0.001 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 107 | mg/g | 181 | 154 | 149.7 | 126.1 | 178 | 115.0 | 144.1 | 169.6 | 174 | 149 | 150 | 154 |
| 108 | mg/g | 0.449 | 0.397 | 0.429 | 0.455a | 0.45 | 0.437 | 0.413 | 0.44 | 0.455 | 0.425 | 0.418 | 0.433 |
| 109 | mg/dose | 6.73 | 6.27 | 6.23 | 6.02 | 6.21 | 6.10 | 7.63 | 6.30 | 6.96 | 6.11 | 6.13 | 6.43 |
| 110 | mg/g | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 111 | mg/dose | 0.0210 | 0.0348 | 0.0618b | 0.0360 | 0.0407 | 0.0334 | 0.0301 | 0.0338 | 0.0393a | 0.0282 | 0.0347 | 0.0360 |
| 112 | mg/dose | 15.91 | 15.70 | 14.23 | 14.96 | 13.46 | 15.35 | 12.15 | 15.06 | 16.53a | 16.22 | 15.2 | 15.0 |
| 113 | mg/g | 37.9 | 40.9 | 37.37 | 37.69 | 40.6 | 35.13 | 31.57 | 40.99 | 20.6a | 38.9 | 36.9 | 36.2 |
| 114 | mg/dose | 0.351 | 0.326 | 0.388 | 0.251 | 0.496 | 0.325 | 0.366 | 0.352 | 0.372 | 0.308 | 0.347 | 0.353 |
| 115 | mg/dose | 14.66 | 14.50 | 14.05 | 14.08 | 14.82 | 14.21 | 12.80 | 14.82 | 13.13 | 14.35 | 14.4 | 14.2 |
| 116 | mg/g | 0.441 | 0.402 | 0.448 | 1.439a | 0.46 | 0.419 | 0.369 | 0.47 | 0.464 | 0.414 | 0.437 | 0.524 |
| 117 | mg/g | 44.9 | 40.5 | 39.05 | 35.11 | 34.7 | 37.55 | 37.40 | 41.73 | 41.7a | 36.4 | 39.4 | 38.9 |
| 118 | mg/g | 0.211 | 0.339 | 0.385 | 0.271 | 0.267 | 0.269 | 0.272 | 0.27 | 0.266 | 0.348 | 0.357 | 0.296 |
| 119 | mg/g | 160 | 155 | 151.8 | 146.8 | 139 | 111.49 | 138.19 | 166.42 | 181 | 153 | 147 | 150 |
| 120 | mg/g | 0.253 | 0.262 | 0.306 | 0.271a | 0.36 | 0.268 | 0.232 | 0.29 | 0.299 | 0.291 | 0.302 | 0.285 |
| 121 | mg/dose | 0.0220 | 0.0350 | 0.071b | 0.0333 | 0.0411 | 0.0301 | 0.0329 | 0.0335 | 0.0270a | 0.0316 | 0.033 | 0.0357 |
| 122 | mg/dose | 14.42 | 14.60 | 14.85 | 14.40 | 13.16 | 14.04 | 14.46 | 14.93 | 16.095 | 14.34 | 14.09 | 14.5 |
| 2–1 | mg/dose | 15.76 | 15.90 | 15.46 | 16.91a | 14.33 | 15.05 | 14.36 | 16.40 | 17.63a | 16.56 | 15.4 | 15.8 |
| 2–2 | mg/dose | 2.65 | 2.34 | 2.76 | 2.49 | 2.85a | 2.31 | 2.60 | 2.46 | 3.04 | 2.48 | 2.63 | 2.60 |
| 2–3 | mg/dose | 2.624 | 2.320 | 2.846 | 2.496 | 2.473a | 2.263 | 2.631 | 2.512 | 3.093 | 2.521 | 2.6 | 2.58 |
| 2–4 | mg/dose | 15.86 | 15.80 | 16.12 | 16.35a | 13.66 | 15.54 | 14.39 | 16.23 | 9.987a | 16.64 | 15.5 | 15.1 |
Cochran outlier.
Single Grubbs outlier.
Table 2005.07C.
Interlaboratory study results for the determination of total β-carotene
| Sample No. | Original RSDR, %a |
RSDR, % of capsule contentsb |
Net RSDSR, % |
Mean capsule wt, g |
C (g β-carotene/g capsule) |
Expected RSDR |
Adjusted HorRat value |
|---|---|---|---|---|---|---|---|
| 101, 114 | 9.88 | 0.54 | 9.57 | 0.7586 | 0.000695 | 5.98 | 1.60 |
| 104, 112 | 7.60 | 1.23 | 6.89 | 0.3339 | 0.04696 | 3.17 | 2.17 |
| 105, 109 | 12.04 | 2.33 | 10.69 | 0.2554 | 0.02604 | 3.46 | 3.09 |
| 111, 121 | 13.56 | 1.61 | 12.63 | 1.1225 | 4.99E-05 | 8.88 | 1.42 |
| 115, 122 | 6.33 | 2.65 | 4.80 | 0.2353 | 0.06366 | 3.03 | 1.59 |
| 2–1, 2–4 | 4.87 | 1.52 | 3.35 | 0.5173 | 0.0327 | 3.35 | 1.19 |
| 2–2, 2–3 | 9.10 | 0.50 | 8.81 | 0.5613 | 0.00632 | 4.29 | 2.06 |
The Horwitz ratio (HorRat) is defined as the ratio of the reproducibility relative standard deviation (%RSDR) to the expected %RSDexp. An adjusted HorRat value must now be calculated using the adjusted reproducibility relative standard deviation(%ARSDR).
Also, the HorRat values calculated by the AOAC spreadsheet require the data to be expressed on a w/w basis. Since the results for the capsules are expressed as mg β-carotene/capsule, further computational steps are required to generate the adjusted HorRat values. The procedure to calculate the adjusted HorRat values is described as follows and the relevant calculations and adjusted HorRat values are shown in Tables 2005.07C and 2005.07D.
The adjusted HorRat value is defined as the ratio of the adjusted reproducibility relative standard deviation (%ARSDR) to the expected %RSDexp. The %ARSDR values were calculated as described above.
The %RSDexp is defined by the Horwitz equation
where C is the weight of the analyte per weight of sample calculated by dividing the grams of β-carotene per softgel by the average softgel weight (in grams). The calculated values of C are shown in Tables 2005.07C and 2005.07D along with the adjusted HorRat values.
This collaborative study demonstrated that the analytical method for the determination of β-carotene in dietary supplements performed well. These supplements also contained carotenoids, vitamins, and minerals. Interferences were well-resolved, including samples containing elevated levels of α-carotene, which were analyzed by using a second LC system.
Following is a compilation of the additional components that were present in the dietary supplements used in this study. Interferences by these components were not observed at the levels typically found in dietary supplements. Vitamins: A (as retinal acetate), B1, B2, B3, B5, B6, B7, B9, B12, C, D, E, K. Minerals: B, Ca, Cr, Cu, Fe, I, K, Mg, Mn, Mo, Ni, Se, Si, V, Zn. Carotenoids: cis- and trans-α-carotenes, astaxanthin, lutein, lycopene, zeaxanthin.
Raw data received from the laboratories are shown in Tables 4 and 5. Statistical evaluation to determine Cochran and Grubbs outliers was performed by using a data processing program for collaborative studies [AOAC Statistical Software, updated by Joanna Lynch, Cornell University, Ithaca, NY (2001)] supplied by AOAC. Data determined to be outliers were removed from further evaluations and are designated in the tables.
The statistical analysis of the softgel sample results presented some interesting challenges in separating the true analytical variation from the variability of the softgel contents and shell weight. By expressing the results as mg β-carotene per softgel, and by determining the variation in the softgel contents weight, the true analytical variability was determined. The adjusted statistics demonstrates the applicability of the method in the analysis of softgel supplements.
The levels of β-carotene reported in samples 107 and 119 (raw material beadlets) varied substantially between the laboratories as evidenced by the resulting high HorRat values. Visual microscopic examination showed that individual beadlets were composed of agglomerates of starch-like granules containing varying amounts of other material (presumed to be β-carotene). The beadlets varied in size and in the amount of granules. The Study Directors believe that this wide range of agglomerate sizes is a major source of the high analytical variability observed in the β-carotene results. They also believe the analytical method is performing very well but is greatly influenced by the variability of the β-carotene within the subsampled beadlets analyzed.
Hard tablet samples containing β-carotene were also examined microscopically and were found to contain agglomerates resembling the beadlets examined above. The variability of the beadlets is still apparent microscopically and further reflected in the analysis of the hard tablets but the grinding and homogenizing of the tablets prior to shipment lessened the effect. Nevertheless, the inherent variability in the amount of β-carotene in the beadlets contributes to the overall variation and must be recognized as such. Additional research will be necessary to extend the applicability of the method to beadletted and beadlet-containing tablets.
Conclusions
Eleven laboratories representing 4 countries successfully completed the interlaboratory study. Collaborators were able to quantitate trans-β-carotene and total β-carotene in dietary supplements in both tablet and softgel form. The method performance on a beadlet raw ingredient was unsatisfactory and it is recommended that the study on this matrix be continued to explore more appropriate subsampling techniques.
Recommendations
It is the recommendation of the Study Directors that AOAC INTERNATIONAL adopt Determination of β-carotene in Supplements by Reversed-Phase High Pressure Liquid Chromatography as Official First Action.
Acknowledgments
Sneh Bhandari, Silliker Laboratories (Chicago Heights, IL) Mai Huong Bui, Swiss Vitamin Institute (Epalinges, Switzerland)
David Ji, Analytical Laboratories (Anaheim, CA)
Erik Konings and Piet Maas, Netherlands Food and Product Safety Authority/Inspectorate for Health Protection and Veterinary Public Health (Den Bosch, The Netherlands)
Roy Lewis, Excelsior Laboratories (Wilmington, DE)
Helen Parish and Jennie Wang, SRI International (Menlo Park, CA)
Brett Post, Medallion Laboratories (Minneapolis, MN)
Joseph Schierle, DSM Nutritional Products, Ltd (Basel, Switzerland)
Darryl Sullivan, Covance Laboratories (Madison, WI)
George Ware, U.S. Food and Drug Administration (Atlanta, GA)
April Taylor and David Woollard, Agriquality (Aukland, New Zealand)
Tom Wu, Advanced Botanical Consulting & Testing, Inc. (Tustin, CA)
Footnotes
The recommendation was approved by the Methods Committee on Dietary Supplements as First Action. See “Official Methods Program Actions,” (2005) Inside Laboratory Management, July/August issue.