Abstract
Background: Recent military conflicts have resulted in numerous extremity injuries requiring complex orthopaedic reconstructive procedures, which begin with a thorough débridement of all contaminated and necrotic tissue in the zone of injury. The site of injury is also the site of healing, and we propose that débrided muscle tissue contains cells with robust reparative and regenerative potential.
Methods: Débrided muscle from soldiers who had sustained traumatic open extremity injuries was collected during surgical débridement procedures at Walter Reed Army Medical Center. With modifications to a previously described stem-cell-isolation protocol, mesenchymal progenitor cells were harvested from traumatized muscle, enriched, expanded in culture, and exposed to induction media for osteogenesis, adipogenesis, and chondrogenesis.
Results: The isolated mesenchymal progenitor cells stained positive for cell-surface markers (CD73, CD90, CD105), which are characteristic of adult human mesenchymal stem cells. Histological identification of lineage-specific markers demonstrated the potential of these cells to differentiate into multiple mesenchymal lineages. Reverse transcription-polymerase chain reaction analysis confirmed multilineage mesenchymal differentiation at the gene-expression level.
Conclusions: To our knowledge, the present report provides the first description of mesenchymal progenitor cell isolation from traumatized human muscle. These cells may play an integral role in tissue repair and regeneration and merit additional investigation as they could be useful in future cell-based tissue-engineering strategies.
Clinical Significance: Mesenchymal progenitor cells isolated from war-traumatized tissues have the potential for applications in cell-based tissue engineering. Elucidating the cellular mechanisms regulating their differentiation activities may lead to the development of novel treatments for musculoskeletal trauma and pathological healing responses, such as heterotopic ossification.
Débridement of contaminated and devitalized tissue is the first step in the surgical treatment of open extremity injuries. This event is often part of a process comprising serial débridements over the span of several days to fully assess the viability of the remaining tissue. The treatment of time-of-war injuries in casualties from Operation Iraqi Freedom and Operation Enduring Freedom has highlighted the importance of this process in determining the long-term success of orthopaedic treatment of traumatic extremity injuries and has allowed the examination of the cellular and molecular characteristics of débrided tissues.
Penetrating trauma results in substantial bone and soft-tissue loss due to the primary injury and the débridement process. As a projectile or blast wave penetrates the skin, it transfers its kinetic energy to the surrounding structures, which include bone, muscle, tendon, cartilage, and fat1. This energy is absorbed in the form of heat, mechanical stress, and chemical stress, and it initiates a number of events, including cell necrosis, apoptosis, and inflammation1,2. While much of the initial damage is largely the result of necrosis and can be seen within the first twenty-four hours, delayed tissue death may result from induced programmed cell death or vascular compromise and may not be apparent for several days after the initial event3. Thus, the serial tissue débridement protocol is necessary to avoid premature wound closure and to minimize the amount of retained devitalized tissue. After débridement, the tissues are reassessed and definitive treatment is planned. The degree and nature of tissue loss determine the need for tissue-grafting or tissue substitutes that are often derived from allograft or synthetic sources.
After fracture fixation and closure or coverage of open wounds, revision and reconstructive surgery is frequently required to restore the function of the injured extremity. In many instances, this may require bone and soft-tissue augmentation, lysis of adhesions (about joints and along tendons), and/or ligament reconstruction. In most cases, revision surgery stems from a need to repair or replace absent, damaged, or deranged tissues such as articular cartilage, tendon, and/or bone with use of autograft, allograft, bioengineered tissue replacement, or prosthetic materials and devices. Unfortunately, these options for tissue repair or replacement are limited by the inability of the implant to fully integrate and subsequently remodel. In addition, the inferior structural, biomechanical, and biochemical properties of the implant as compared with normal human tissue prevent full restoration of the structure-function relationship. However, as the burgeoning field of tissue engineering develops, additional options may become available.
An essential component of all tissue- engineering construct designs is a readily available, viable, and plastic cell source. Many sources of multipotent progenitor cells (e.g., bone marrow, trabecular bone, adipose tissue, umbilical cord blood, and synovial tissue), which yield cells that have varying degrees of regenerative potential and that can be expanded in vitro, have been described4-8. However, these tissue types may not be readily available as a source of autologous multipotent cells at the time of musculoskeletal trauma. Therefore, the goal of the present study was to assess the availability of regenerative cells in débrided tissues that are potentially applicable for tissue engineering. Our specific aims were (1) to isolate viable cells from tissues obtained at the time of débridement surgery, (2) to determine whether the cells express surface markers that are characteristic of multipotent progenitor cells, and (3) to assess the regenerative potential of these cells by testing their ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro.
Materials and Methods
Tissue Procurement
With institutional review board approval from Walter Reed Army Medical Center and informed patient consent, tissue specimens were obtained from patients who had sustained traumatic extremity injury during Operation Iraqi Freedom and Operation Enduring Freedom. These patients presented to Walter Reed Army Medical Center approximately three to seven days after the injury and underwent serial débridement and irrigation procedures until the wounds were determined to be clinically acceptable for definitive orthopaedic treatment. The amount and nature of débrided tissue was surgeon-dependent and was based on trauma surgery principles of circumferential removal of all grossly contaminated, apparently necrotic, and nonviable tissue along with a thin margin of healthy-appearing tissue. This procedure was repeated at each surgical encounter until only healthy tissue remained, and cells typically were harvested from muscle tissue obtained during the second or third serial débridement.
Cell Isolation
The protocol for extracting muscle-derived multiprogenitor cells was based on a modification of previous work in isolating mesenchymal stem cells that was performed in our laboratory4. Fat, fascia, other connective tissue, and necrotic tissue were dissected away from the healthy margin of the débrided muscle sample. Approximately 0.5 cc of the remaining muscle tissue was processed for cell extraction. The tissue was washed three times in Hanks' Balanced Salt Solution (Gibco, Carlsbad, California) and then was extensively minced in a 10-cm culture dish containing Dulbecco's Modified Eagle Medium (Gibco) and 3× penicillin/streptomycin/Fungizone (Gibco) until it could pass through the tip of a 25-mL serological pipette (Falcon; BD Biosciences, San Jose, California). The minced tissue was transferred to a 50-mL conical vial with digestion medium containing Dulbecco's Modified Eagle Medium, 3× penicillin/streptomycin/Fungizone, and 0.5 mg/mL collagenase type 2 (Worthington Biochemical, Lakewood, New Jersey). The tissue slurry was agitated gently at 37°C for two hours, and the resulting digest was filtered through a 40-μm cell strainer (Falcon), pelleted by means of centrifugation, resuspended in growth medium (Dulbecco's Modified Eagle Medium with 10% fetal bovine serum; Gibco) and 5× penicillin/streptomycin/Fungizone, and then plated onto tissue culture polystyrene (150-cm2 flask; Falcon). The cells were incubated at 37°C in a 5% CO2-humidified cell incubator for two hours and then were extensively washed with Hanks' Balanced Salt Solution before fresh growth medium was added with 3× penicillin/streptomycin/Fungizone. Once multiprogenitor cell colony forming units were observed, the concentration of penicillin/streptomycin/Fungizone was lowered to 1×. Cell confluence was obtained after approximately two weeks. The cell cultures were routinely passaged at 80% to 90% confluence and split 1:4.
Adult human bone marrow-derived mesenchymal stem cells were isolated as described previously4 with use of bone marrow obtained from the medullary canal of long bones from patients undergoing elective total hip replacement. The cells were then washed and plated onto tissue culture polystyrene.
Immunophenotyping
Mouse anti-CD29 monoclonal IgG (clone Ha2/5), mouse anti-CD44 monoclonal IgG (clone IM7), mouse anti-CD105 monoclonal IgG (clone 35), and mouse anti-CD146 monoclonal IgG (clone P1H12) antibodies and phytoerythrin-conjugated mouse anti-CD45 monoclonal IgG (clone TU116), mouse anti-CD73 monoclonal IgG (clone AD2), mouse anti-CD90 monoclonal IgG (clone 5E10), and mouse anti-CD105 monoclonal IgG (clone 35) antibodies were obtained from BD Biosciences (San Jose, California). All antibodies were reactive against human antigens. Donkey anti-mouse IgG conjugated with fluorescein isothiocyanate were obtained from Jackson ImmunoResearch (West Grove, Pennsylvania). Testing with negative and positive controls confirmed the specificity of these antibodies.
Cells used for staining were cultured in growth medium on glass coverslips for fourteen days during the second or third passage. They were washed once with Hanks' Balanced Salt Solution and then were fixed in 3% phosphate-buffered paraformaldehyde for twenty minutes. Fixed cells were first blocked in 2% bovine serum albumin (Sigma-Aldrich, St. Louis, Missouri) for thirty minutes and then were incubated with the respective primary antibodies in phosphate-buffered saline solution (diluted 1:100) with 1% whole donkey IgG for two hours at room temperature or overnight at 4°C and then with fluorescein isothiocyanate-conjugated secondary antibodies (diluted 1:100) and DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, Carlsbad, California; diluted 1:10,000) in phosphate-buffered saline solution for thirty minutes. The coverslips were then mounted to slides with VECTASHIELD (Vector Laboratories, Burlingame, California) and viewed with a Zeiss 510 Meta confocal laser scanning microscope (Carl Zeiss Microimaging, Thornwood, New York).
During the second passage, approximately 250,000 cells were plated in a 150-cm2 cell-culture flask for flow cytometric analysis. When the cultures were approximately 80% confluent, the cells were rinsed once with Hanks' Balanced Salt Solution and then were lifted off the surface with 0.25% trypsin and were transferred to a 50-mL centrifuge tube. The tube was centrifuged for five minutes at 200 g, the supernatant was aspirated, and the pellet was resuspended in (fluorescence-activated cell-sorting) buffer (0.1% bovine serum albumin and 0.01% sodium azide in Hanks' Balanced Salt Solution). Next, 100 μL of the cell suspension was aliquoted into fluorescence-activated cell-sorting tubes, and the phytoerythrin-conjugated antibodies (CD14, CD73, CD90, CD105, and an isotype control; BD Biosciences) were added to each tube at a 1:50 dilution. The cells were incubated in the dark at 4°C for forty minutes, washed once in fluorescence-activated cell-sorting buffer, and resuspended in 100 μL of fresh fluorescence-activated cell-sorting buffer. The fluorescent intensity profiles of the cells were analyzed by means of fluorescence-activated cell-sorting with use of a FACSCalibur flow cytometer (BD Biosciences).
Cell Differentiation
Differentiation assays were performed on cells during the second or third passage with use of previously described induction conditions9. Monolayer cultures of multiprogenitor cells were plated at an initial density of 5000 cells/cm2 for osteogenesis or 40,000 cells/cm2 for adipogenesis and were treated with either (1) osteogenic induction medium, consisting of growth medium supplemented with 10-mM β-Glycerol phosphate (Sigma-Aldrich, St. Louis, Missouri), 50 mg/mL ascorbic acid (Sigma-Aldrich), 10-nM 1,25-di-hydroxyvitamin D3 (BIOMOL International, Plymouth Meeting, Pennsylvania), and 0.01 μM dexamethasone (DEX; Sigma-Aldrich); or (2) adipogenic induction medium, consisting of growth medium supplemented with 0.5-mM 3-Isobutyl-1-methylxanthine (IBMX; Acros Organics, Geel, Belgium), 1 μM DEX, and 1 mg/mL insulin (Sigma-Aldrich) for as long as twenty-eight days. Chondrogenic differentiation was performed in high-density pellets containing 2.5 × 105 cells per pellet, cultured in chondrogenic medium containing Dulbecco's Modified Eagle Medium supplemented with 1% ITS (BD Biosciences, San Jose, California), 10 ng/mL transforming growth factor-β (TGF-β3; R&D Systems, Minneapolis, Minnesota), and 0.1-mM DEX for twenty-one days. Cells in monolayer were fixed after twenty-one days and were stained with use of a kit for alkaline phosphatase activity (Sigma-Aldrich), or Oil Red O (Sigma-Aldrich) for intracellular lipid droplets. Cells were fixed and stained with alizarin red (Rowley Biochemical Institute, Danvers, Massachusetts) for evidence of a mineralized matrix after twenty-eight days. Chondrogenic pellets were fixed after twenty-one days, dehydrated, embedded in paraffin, sectioned, and stained with alcian blue (Rowley) for sulfated glycosaminoglycans.
Gene-expression assays were performed by culturing the cells for twenty-one days in the defined induction conditions. The differentiated cells were lysed and RNA was extracted with TRIzol (Invitrogen). The mRNA was isolated with RNeasy Mini columns (Qiagen), the RNA concentrations were estimated on the basis of A260, and RNA quality corresponded to an A260/280 value of at least 1.8. One microgram of each RNA sample was reverse-transcribed with SuperScript III for qRT-PCR (Invitrogen) with use of Oligo(dT). The expression levels of osteogenic, adipogenic, and chondrogenic genes (see Table I for gene primers) were assessed with conventional polymerase chain reaction with use of Platinum Taq (Invitrogen) following the manufacturer's protocol. The primers were designed with MacVector (MacVector, Cary, North Carolina) with use of the nucleotide sequences from Entrez Gene (National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland) and have previously been verified to amplify the target genes10. The polymerase chain reaction products were analyzed electrophoretically on a DNA 1000 LabChip with use of the Agilent bioanalyzer (Agilent Technologies, Santa Clara, California), and visual representations of the cDNA concentrations were created from the electropherograms with the bioanalyzer software.
TABLE I.
Primer Sequences (5′ to 3′) for Gene-Expression Analysis by Reverse Transcription Polymerase Chain Reaction, Annealing Temperatures, Number of Polymerase Chain Reaction Cycles Performed, and Expected Size of Each Polymerase Chain Reaction Product
| Gene* | Primer Sequence | Annealing Temperature | No. of Cycles Performed | Product Size (no. of base pairs) |
|---|---|---|---|---|
| GAPDH | ||||
| Forward | CAAGGCTGAGAACGGGAAGC | 58°C | 26 | 194 |
| Reverse | AGGGGGCAGAGATGATGACC | |||
| COL1A2 | ||||
| Forward | GGCTCCTGCTCCTCTTAGCG | 58°C | 26 | 132 |
| Reverse | CATGGTACCTGAGGCCGTTC | |||
| CBFA1/RUNX2 | ||||
| Forward | ACTGGGCCCTTTTTCAGA | 53°C | 26 | 317 |
| Reverse | GCGGAAGCATTCTGGAA | |||
| ALP | ||||
| Forward | TCAGAAGCTCAACACCAACG | 55°C | 26 | 199 |
| Reverse | GTCAGGGACCTGGGCATT | |||
| Osteocalcin | ||||
| Forward | GCCTTTGTGTCCAAGC | 52°C | 28 | 315 |
| Reverse | GGACCCCACATCCATAG | |||
| PPARγ2 | ||||
| Forward | TGAATGTGAAGCCCATTGAA | 53°C | 26 | 161 |
| Reverse | CTGCAGTAGCTGCACGTGTT | |||
| LPL | ||||
| Forward | GAGATTTCTCTGTATGGCACC | 52°C | 26 | 276 |
| Reverse | CTGCAAATGAGACACTTTCTC | |||
| FABP4 | ||||
| Forward | TGGGCCAGGAATTTGACGAAGT | 59°C | 24 | 215 |
| Reverse | TCAACGTCCCTTGGCTTATGCT | |||
| SOX9 | ||||
| Forward | ACATCTCCCCCAACGCCATC | 58°C | 34 | 173 |
| Reverse | TCGCTTCAGGTCAGCCTTGC | |||
| COL2A1 | ||||
| Forward | GGAAACTTTGCTGCCCAGATG | 58°C | 34 | 167 |
| Reverse | TCACCAGGTTCACCAGGATTGC | |||
| Aggrecan | ||||
| Forward | TGCGGGTCAACAGTGCCTATC | 58°C | 36 | 182 |
| Reverse | CACGATGCCTTTCACCACGAC |
GAPDH = Glyceraldehyde 3-phosphate dehydrogenase. ALP = Alkaline phosphatase. PPARγ2 = peroxisome proliferator-activated receptors-γ2. LPL = Lipoprotein lipase. FABP4 = fatty acid binding protein 4.
Results
With use of the cell-isolation protocol described here, we were able to efficiently isolate, culture, and expand cells from traumatized tissues obtained at the time of débridement. After initial processing and digestion, 7.1 ± 6.4 million cells were harvested per gram of muscle tissue, and 26.4% ± 3.6% of the harvested cells were plastic-adherent. We found that extensive rinsing after two hours of plating was important to decrease contamination by non-adherent cells. In our initial cohort, we collected twelve specimens of débrided muscle tissue from ten individuals (including two individuals with injuries of multiple extremities). Of the initial twelve cell populations, two were lost to contamination within the first three days of in vitro expansion. The remaining ten populations (which were harvested from eight different patients) were assayed for their phenotype and multidifferentiation potential.
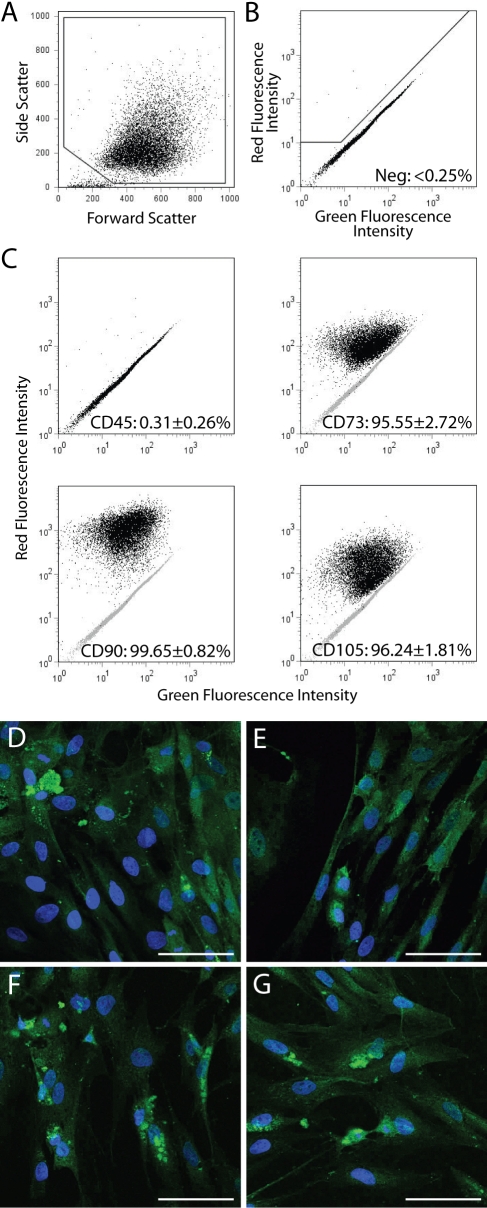
The cells that were adherent within two hours eventually assumed an elongated and spindle-shaped morphology, which was similar to that of bone-marrow-derived mesenchymal stem cells (Fig. 1). Adherent cells began to proliferate within twenty-four hours, and, within approximately two weeks, these cells achieved 80% to 90% confluence. The multiprogenitor cells were positive for CD73, CD90, and CD105 and were negative for CD45 on flow cytometry (Fig. 2, C), and the multiprogenitor cells stained positively for CD29, CD44, CD105, and CD146 (Fig. 2, D through G), which is a cell-surface epitope profile that is characteristic of adult mesenchymal stem cells11.
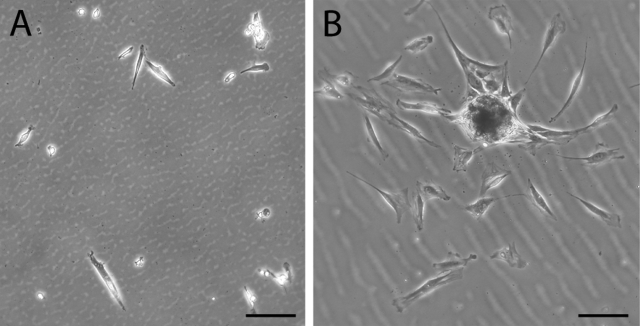
Fig. 1.
Phase contrast microscopy showing multipotent cells obtained as adherent cells twenty-four hours after plating. Traumatized muscle-derived multiprogenitor cells (A) and bone marrow-derived mesenchymal stem cells (B) were plated, and, after two hours, the cultures were washed extensively with phosphate-buffered saline solution. Cells were visualized with phase contrast microscopy after twenty-four hours. The morphology of many of the muscle-derived cells is spindle-shaped and elongated, similar to that of bone marrow-derived mesenchymal stem cells. Bar = 100 μm.
Fig. 2.
A through G: Immunophenotyping of multiprogenitor cells derived from traumatized muscle. A: Whole multiprogenitor cells were identified and gated on the basis of their size (forward scatter) and granularity (side scatter). B: Multiprogenitor cells stained with a non-immunogenic isotype control were used to define the gate for positive phytoerythrin (red fluorescence) staining. C: Cells were stained with phytoerythrin-conjugated antibodies raised against CD45, CD73, CD90, and CD105. The average percentage of positive cells for each marker in ten multiprogenitor cell populations is listed in each panel. D through G: Multiprogenitor cells were stained with fluorescein isothiocyanate-conjugated antibodies raised against (D) CD29, (E) CD44, (F) CD105, and (G) CD146. Confocal laser scanning microscopy revealed positive green cell-surface staining for all four markers. Bar = 50 μm.
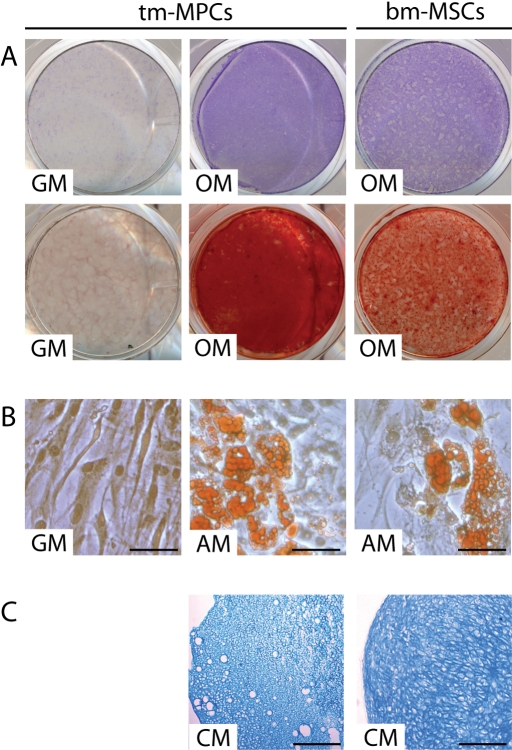
Culturing in inductive media used for mesenchymal stem cell osteogenesis, adipogenesis, and chondrogenesis resulted in sustained differentiation of the traumatized muscle-derived multiprogenitor cells. Monolayer culture was used for osteogenesis and adipogenesis, whereas pellet culture was used for chondrogenesis12. After four weeks in osteoinductive medium, there was histological evidence of increased alkaline phosphatase activity, and matrix mineralization could be detected by staining with alizarin red in the multiprogenitor cell cultures (Fig. 3, A). Oil Red O staining of adipogenic cultures of multiprogenitor cells showed evidence of intracellular lipid droplet accumulation, consistent with an adipocyte phenotype (Fig. 3, B). Finally, alcian blue staining of histological sections of the multiprogenitor cell pellet cultures demonstrated the presence of a sulfated proteoglycan-rich extracellular matrix characteristic of cartilage (Fig. 3, C). Both small, round chondrocytes and large, hypertrophic-like chondrocytes were seen embedded in the sulfated proteoglycan-rich matrix. Staining intensity decreased toward the central pellet region, most likely as a result of decreased nutritional perfusion as the pellet increased in size13. The histological features of osteogenic, adipogenic, and chondrogenic differentiation in the traumatized muscle-derived multiprogenitor cells were similar to those in bone-marrow-derived mesenchymal stem cells, and they were consistent in all ten multiprogenitor cell populations.
Fig. 3.
A, B, and C: Histological analysis of differentiated multiprogenitor cells derived from traumatized muscle. Traumatized muscle-derived mulitprogenitor cells (tm-MPCs) were cultured in growth medium (GM), osteogenic induction medium (OM), adipogenic induction medium (AM), or in three-dimensional pellet cultures with chondrogenic medium (CM). Bone marrow-derived mesenchymal stem cells (bm-MSCs) were used as positive controls for osteogenic, adipogenic, and chondrogenic differentiation. A: Multiprogenitor cells cultured in osteogenic induction medium exhibited enhanced alkaline phosphatase activity (Fast Blue BB; top) and mineralized matrix (alizarin red; bottom). B: Multiprogenitor cells cultured in adipogenic induction medium developed intracellular lipid droplets that stained positively with Oil Red O. Bar = 50 μm. C: Histological sections of the multiprogenitor cell pellet cultures showed positive matrix staining with alcian blue, suggesting the presence of sulfated proteoglycan-rich extracellular matrix. Bar = 500 μm.
Reverse transcription polymerase chain reaction analysis of traumatized muscle-derived multiprogenitor cells undergoing differentiation demonstrated expression of lineage-specific genes for osteoblasts and adipocytes (Fig. 4, A). Gene-expression analysis was performed on three populations of multiprogenitor cells from different patients, and the results shown are characteristic of all three populations. Multiprogenitor cells cultured in osteogenic medium expressed core binding factor a1 (CBFA1/RUNX2), and multiprogenitor cells cultured in adipogenic medium expressed PPARγ2 (peroxisone proliferator-activated receptor γ2), key regulatory genes for osteogenic and adipogenic commitment, respectively. Expression of alkaline phosphatase and osteocalcin, genes necessary for osteoblastic function, was observed after osteogenic differentiation. PPARγ2 was also expressed strongly in multiprogenitor cells under osteogenic conditions. Adipogenic differentiation of multiprogenitor cells resulted in the upregulation of lipoprotein lipase (LPL) and fatty acid binding protein 4 (FABP4). Similarly, multiprogenitor cells cultured under chondrogenic conditions expressed SOX9 and aggrecan, indicating chondrogenic commitment. The expression of the collagen type II (COL2A1), which is cartilage-specific, increased from Day 7 to Day 21 of the culture period (Fig. 4, B).
Fig. 4.
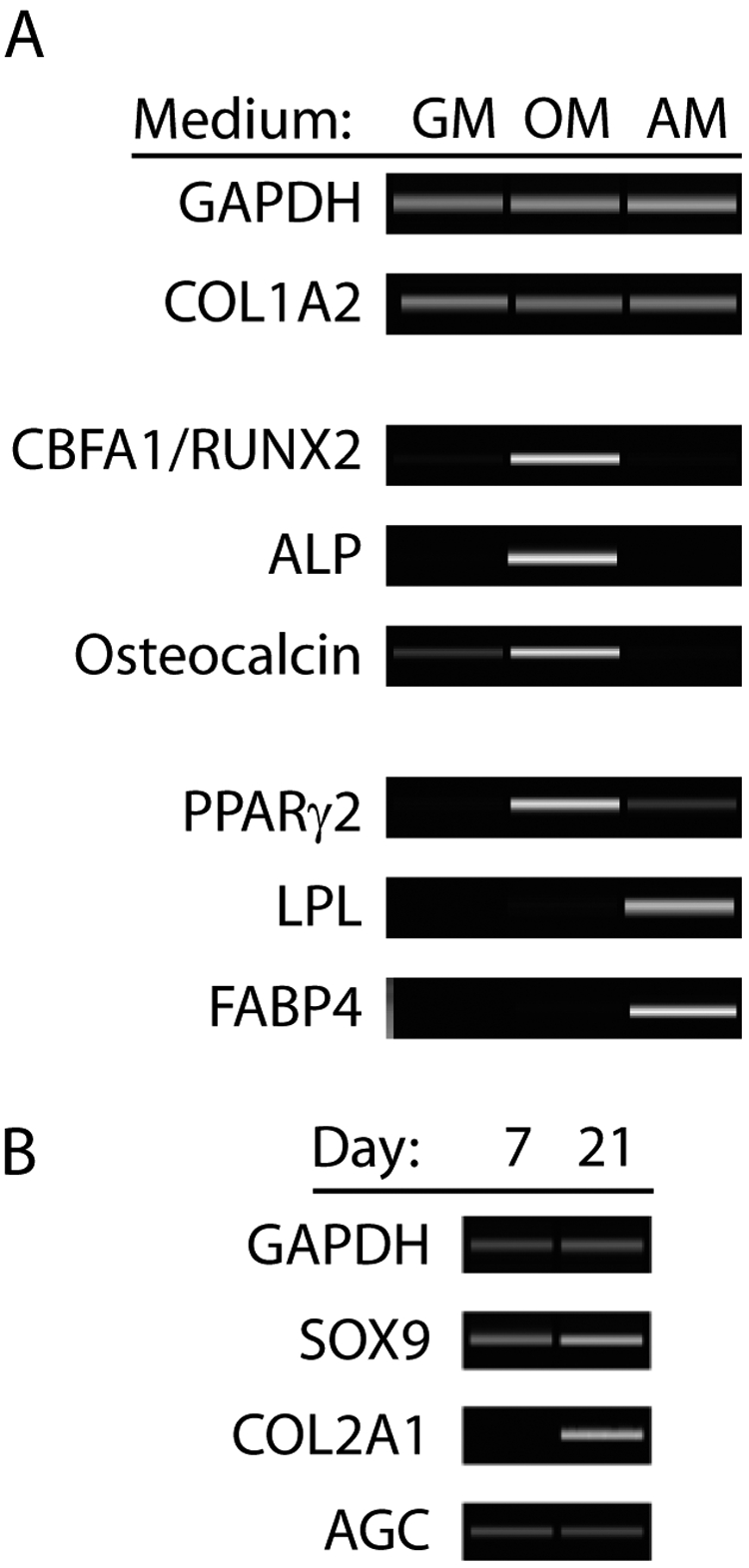
A and B: Gene-expression analysis of differentiation of traumatized muscle-derived multiprogenitor cells. A: Traumatized muscle-derived multiprogenitor cells were cultured in growth medium (GM), osteogenic induction medium (OM), or adipogenic induction medium (AM) and then were lysed and RNA-extracted. Gene-expression profiles were then analyzed with use of reverse transcription polymerase chain reaction. Cells cultured in osteogenic induction medium showed upregulated expression of CBFA1/RUNX2, alkaline phosphatase (ALP), and osteocalcin, characteristic of osteoblastic phenotype. Cells cultured in adipogenic induction medium exhibited upregulated expression of PPARγ2, lipoprotein lipase (LPL), and fatty acid binding protein 4 (FABP4), characteristic of adipocytic phenotype. B: Pellet cultures of multiprogenitor cells in chondrogenic medium expressed the chondrogenic genes SOX9, COL2A1, and aggrecan (AGC). The expression of COL2A1 was upregulated from Day 7 to Day 21, suggesting chondrocyte maturation. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was analyzed as a control for RNA loading.
Discussion
The overall goal of the present study was to determine whether débrided muscle contains multipotent cells that may be applicable in future cell-based tissue-engineering strategies. Tissues obtained at the time of surgery underwent a modified stem cell isolation protocol that yielded viable cells expressing markers characteristic of mesenchymal stem cells11,14. These multiprogenitor cells were culture-expanded and exhibited multipotentiality (adipogenic, osteogenic, and chondrogenic) on appropriate induction. These results indicate that débrided muscle is a readily available source of multiprogenitor cells, which may be used in the initial reparative process or in future reconstructive operations in combination with appropriate tissue-engineering biomaterial scaffolds.
Several aspects of our findings support the postulate of using débrided muscle obtained from trauma wounds as a source of cells for clinically relevant tissue-engineering and regeneration applications. First, the present study was performed not on animal models but with use of human tissues and cells derived from traumatized muscle, and the findings thus have direct implications for translation into clinical investigation. Second, the histological evidence of differentiation was compared with differentiated bone-marrow-derived mesenchymal stem cells, a well-characterized cell type with known multiple differentiation potential. Finally, multiple assays were performed to verify the multipotent differentiation activities of the traumatized muscle-derived multiprogenitor cells. Specifically, the differentiation assays were corroborated by the expression of corresponding adipogenic, osteogenic, and chondrogenic lineage-specific genes. On the basis of these findings, we have verified that muscle-derived multiprogenitor cells have the potential to differentiate into osteoblasts, adipocytes, and chondrocytes.
One substantial caveat related to the present study is that multiprogenitor cells derived from traumatized muscle still require substantial characterization in terms of their origin within the body and their relationship to better-characterized stem cell types. The multiprogenitor cells may originally reside in the nontraumatized muscle tissue in a quiescent state (i.e., as pericytes)15, or they may have migrated from the bone marrow to the site of injury in response to wound-healing signals16,17. Initial plating of the multiprogenitor cells yielded a greater number of tissue-adherent cells than is typically reported for progenitor cell populations, which may have been due to a lower overall cellularity and a higher percentage of multiprogenitor cells relative to other erythroid or mononuclear cell types in traumatized muscle compared with other sources. General characteristics of the multiprogenitor cells, such as the associated cell-surface markers, prolonged culture-expansion capabilities, and multidifferentiation potential, are characteristic features of mesenchymal stem cells11,18-20. However, one difference in the gene-expression profile of differentiated multiprogenitor cells has been noted in the present study. PPARγ2, which is an indicator of adipogenic differentiation in bone-marrow-derived mesenchymal stem cells, is also upregulated by osteogenic induction of traumatized muscle-derived multiprogenitor cells. The effect of PPARγ2 on the multiprogenitor cells does not appear to be anti-osteogenic as there is strong evidence from the histological and gene-expression findings that cells underwent osteogenic induction. In fact, the regulatory pathways governing PPARγ2 activity can modulate its anti-osteogenic function21 and may represent a tissue-specific feature of regenerative cells that are present in muscle tissue. These observations underscore the need for further investigation of the traumatized muscle-derived multiprogenitor cells to better characterize their regenerative properties and function within the injured tissue.
Surgical débridement of open wounds is a medical and surgical necessity. Although removal of tissue from wounds that are characterized by substantial tissue loss is counterintuitive, it is essential for definitive treatment, wound closure, and proper healing22-24. The results of the present study suggest that this waste tissue may possess cellular building blocks that might be useful in future treatment and tissue-regeneration strategies. Although multiprogenitor cells have been theorized to occupy traumatized muscle and their presence has been demonstrated in animal models, to our knowledge, the present report is the first to describe and characterize these cells in human tissues. Qu-Petersen and colleagues described the presence of a population of progenitor cells obtained from skeletal muscle in a mouse model that exhibited characteristics similar to mesenchymal stem cells25. Those authors isolated the cell population of interest with use of a preplating technique, which is a method that selects for the least-adherent cell population after a series of six serial platings. With use of this cell preplating method, we were unable to obtain multiprogenitor cells from human tissue samples, and the cells that were obtained differed significantly from the multiprogenitor cells described here. Instead, in the present study, we selected the most adherent cells two hours after initial plating and expanded the isolated cells in a culture medium identical to that used for bone marrow-derived mesenchymal stem cells.
The traumatized muscle-derived multiprogenitor cells are of particular interest in our patient population given the high prevalence of heterotopic ossification following blast trauma-induced injuries26. The osteogenic potential of these cells suggests their possible role in the pathological processes that result in ectopic bone formation22. Accordingly, it would be of interest to study how these progenitor cells respond to biochemical factors in the wound-healing environment during the regenerative process. The mechanism of osteogenic differentiation of the multiprogenitor cells may provide insight into the development of heterotopic ossification in this traumatized tissue. Identifying the initial events committing these multiprogenitor cells into an osteogenic lineage may prove to be instrumental in developing novel, therapeutic strategies to prevent heterotopic ossification.
In summary, we have demonstrated that traumatized muscle tissue contains multipotent progenitor cells that can be harvested and expanded in vitro. Therefore, this cell type may potentially be used in future applications for delayed reconstructive efforts or in cell-based tissue-engineered constructs for bone, tendon, cartilage, and fat. Multipotent adult stem cells are already being employed for orthopaedic reconstructive procedures; for example, bone-marrow aspirates have been used to augment bone defects, and intraoperative isolation systems have been used to augment fracture fixation and spine fusions with additional mesenchymal stem cells16,27,28. With further development of more advanced isolation techniques, traumatized muscle-derived multiprogenitor cells may become readily available at the time of surgery, without the need for additional invasive cell-harvesting procedures, for use in engineered constructs and delayed reconstruction following injury. Additional studies to identify the exact source (or sources) and the regenerative potential (in vitro and in vivo) of the traumatized muscle-derived multiprogenitor cells are needed tofacilitate their effective use in the treatment of extremity trauma and to gain insights into the improvement of clinical outcomes. 
Acknowledgments
Note: Confocal microscopy images were collected at the NIAMS Light Imaging Section with the help of Kristien Zaal, and fluorescence-activated cell-sorting analysis was performed at the NIAMS Flow Cytometry Section with the assistance of James Simone. Additionally, portions of this work were performed at the Naval Surgical Research Laboratory in the National Naval Medical Center.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Military Amputee Research Program at Walter Reed Army Medical Center (PO5-A011) and by the Intramural Research Program at the NIH, NIAMS (Z01 AR41131). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Cartilage Biology and Orthopaedic Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland, and Walter Reed Army Medical Center, Washington, DC
References
- 1.Jussila J. Measurement of kinetic energy dissipation with gelatine fissure formation with special reference to gelatine validation. Forensic Sci Int. 2005;150:53-62. [DOI] [PubMed] [Google Scholar]
- 2.Jussila J, Kjellstrom BT, Leppaniemi A. Ballistic variables and tissue devitalisation in penetrating injury—establishing relationship through meta-analysis of a number of pig tests. Injury. 2005;36:282-92. [DOI] [PubMed] [Google Scholar]
- 3.Williams AJ, Hartings JA, Lu XC, Rolli ML, Tortella FC. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J Neurotrauma. 2006;23:1828-46. [DOI] [PubMed] [Google Scholar]
- 4.Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245-56. [DOI] [PubMed] [Google Scholar]
- 5.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060-9. [DOI] [PubMed] [Google Scholar]
- 6.Flynn A, Barry F, O'Brien T. UC blood-derived mesenchymal stromal cells: an overview. Cytotherapy. 2007;9:717-26. [DOI] [PubMed] [Google Scholar]
- 7.Boquest AC, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2:319-29. [DOI] [PubMed] [Google Scholar]
- 8.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, Ichinose S, von der Mark K, Sekiya I. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689-96. [DOI] [PubMed] [Google Scholar]
- 9.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980-2. [DOI] [PubMed] [Google Scholar]
- 10.Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24:1707-18. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-49. [DOI] [PubMed] [Google Scholar]
- 12.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599-609. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JA Jr, Li WJ, Bailey LO, Hudson SD, Lin-Gibson S, Anseth KS, Tuan RS, Washburn NR. Encapsulated chondrocyte response in a pulsatile flow bioreactor. Acta Biomater. 2007;3:13-21. [DOI] [PubMed] [Google Scholar]
- 14.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384-92. [DOI] [PubMed] [Google Scholar]
- 15.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165-75. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-90. [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7. [DOI] [PubMed] [Google Scholar]
- 19.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang Y, Cui P, Chen W, Gao P, Zhang H. Quantitative study of tissue-engineered cartilage with human bone marrow mesenchymal stem cells. Arch Facial Plast Surg. 2005;7:7-11. [DOI] [PubMed] [Google Scholar]
- 21.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376-84. [DOI] [PubMed] [Google Scholar]
- 22.Granick M, Boykin J, Gamelli R, Schultz G, Tenenhaus M. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen. 2006;14 Suppl 1:S1-10. [DOI] [PubMed] [Google Scholar]
- 23.Gregory P, Sanders R. The management of severe fractures of the lower extremities. Clin Orthop Relat Res. 1995;318:95-105. [PubMed] [Google Scholar]
- 24.Jacob E, Erpelding JM, Murphy KP. A retrospective analysis of open fractures sustained by U.S. military personnel during Operation Just Cause. Mil Med. 1992;157:552-6. [PubMed] [Google Scholar]
- 25.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-86. [DOI] [PubMed] [Google Scholar]
- 27.Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;432:242-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38 Suppl 1:S75-80. [DOI] [PubMed] [Google Scholar]