Abstract
Background: Early diagnosis is a challenge in the treatment of degenerative disc disease. A noninvasive biomarker detecting functional mechanics of the disc is needed. T1ρ-weighted imaging, a spin-lock magnetic resonance imaging technique, has shown promise for meeting this need in in vivo studies demonstrating the clinical feasibility of evaluating both intervertebral discs and articular cartilage. The objectives of the present study were (1) to quantitatively determine the relationship between T1ρ relaxation time and measures of nucleus pulposus mechanics, and (2) to evaluate whether the quantitative relationship of T1ρ relaxation time with the degenerative grade and glycosaminoglycan content extend to more severe degeneration. It was hypothesized that the isometric swelling pressure and compressive modulus would be directly correlated with the T1ρ relaxation time and the apparent permeability would be inversely correlated with the T1ρ relaxation time.
Methods: Eight cadaver human lumbar spines were imaged to measure T1ρ relaxation times. The nucleus pulposus tissue from the L1 disc through the S1 disc was tested in confined compression to determine the swelling pressure, compressive modulus, and permeability. The glycosaminoglycan and water contents were measured in adjacent tissue. Linear regression analyses were performed to examine the correlation between the T1ρ relaxation time and the other measured variables. Mechanical properties and biochemical content were evaluated for differences associated with degeneration.
Results: A positive linear correlation was observed between the T1ρ relaxation time on the images of the nucleus pulposus and the swelling pressure (r = 0.59), glycosaminoglycan content per dry weight (r = 0.69), glycosaminoglycan per wet weight (r = 0.49), and water content (r = 0.53). No significant correlations were observed between the T1ρ relaxation time and the modulus or permeability. Similarly, the T1ρ relaxation time, swelling pressure, glycosaminoglycan content per dry weight, and water content were significantly altered with degeneration, whereas the modulus and permeability were not.
Conclusions: T1ρ-weighted magnetic resonance imaging has a strong potential as a quantitative biomarker of the mechanical function of the nucleus pulposus and of disc degeneration.
Clinical Relevance: Several in vivo studies have previously demonstrated the clinical feasibility of using T1ρ-weighted imaging to evaluate both intervertebral discs and articular cartilage. Its application for the diagnosis of disc degeneration looks promising.
The function of the intervertebral disc is mechanical; it supports and distributes large loads, permits spine motion, and dissipates energy. The swelling pressure of the nucleus pulposus is a particularly important property. Pressurization within the nucleus pulposus is generated by the negatively charged proteoglycans that are entrapped within a loose type-II-collagen network and create a fixed charge density, attracting and binding water. Pressurization of the nucleus pulposus enables the disc to absorb and transmit the compressive spinal loads, maintains disc height, and prevents large deformations within the low-load neutral zone1-3. During the early stages of disc degeneration, the proteoglycans and associated glycosaminoglycans in the nucleus pulposus break down, reducing the pressure within the nucleus pulposus4-7.
A major challenge in treating degenerative disc disease is the need to diagnose the disorder early and accurately. Early-stage diagnosis is essential to a positive prognosis, particularly as emerging treatment modalities aimed at restoring mechanical function, such as nucleus pulposus replacement, total disc replacement, cell or growth factor injection, or gene therapy, are translated to the clinical setting. A noninvasive biomarker detecting functional mechanics of the disc would facilitate early-stage diagnosis.
Conventional magnetic resonance imaging techniques are not sensitive enough to detect early stages of disc degeneration8. Other techniques, including delayed gadolinium-enhanced and sodium magnetic resonance imaging, may have sufficient sensitivity but there are also substantial limitations with regard to implementation of these methods9-11. T1ρ-weighted imaging is a spin-lock magnetic resonance imaging technique that shows promise as a noninvasive biomarker, as several in vivo studies have demonstrated the clinical feasibility of evaluating both intervertebral disc and articular cartilage with this modality12-15. The spin-lock technique was first employed by Redfield in 1955 and allows for a lower Larmor frequency16. The lower Larmor frequency increases the sensitivity of T1ρ-weighted imaging, permitting the detection of low-frequency physicochemical interactions between water and extracellular matrix molecules17,18. In articular cartilage, the T1ρ relaxation time is inversely correlated with proteoglycan content19,20. With degeneration of articular cartilage, the proteoglycan content decreases, the matrix molecules and water become less restricted, and the T1ρ relaxation time increases. The T1ρ relaxation time was also found to be inversely correlated with the compression modulus of articular cartilage in an interleukin-1β-treated degeneration model20. In contrast, the T1ρ relaxation time in human discs was demonstrated to be directly correlated with both the degenerative grade and the sulfated-glycosaminoglycan content18, although the study was limited to a maximum degenerative grade of 3 (out of 5) and limited to sixteen disc samples. The potential for correlations between the mechanical function and T1ρ-weighted imaging measurements of the intervertebral disc remains unknown. If they are correlated, T1ρ-weighted imaging could serve as a biomarker for mechanical function of the disc.
The objectives of this study were twofold: (1) to quantitatively determine the relationship between T1ρ relaxation time and the mechanics of the nucleus pulposus, and (2) to evaluate whether the quantitative relationship of the T1ρ relaxation time with the degenerative grade and the glycosaminoglycan content extends to more severe degeneration. With human disc degeneration, the swelling pressure, compressive modulus, and proteoglycan content decrease4,21,22, the permeability increases21, and the T1ρ relaxation time decreases18. Therefore, it was hypothesized that the isometric swelling pressure and compressive modulus would be directly correlated with the T1ρ relaxation time and the apparent permeability would be inversely correlated with the T1ρ relaxation time.
Materials and Methods
Eight fresh-frozen cadaver lumbar spine sections (mean age of the donors at the time of death, 51.8 years; range, fifteen to seventy-nine years) were imaged on a 1.5-T whole-body clinical magnetic resonance scanner (Sonata; Siemens Medical Solutions, Malvern, Pennsylvania) as previously described18. Briefly, a series of sagittal plane T2 and T1ρ-weighted images were acquired with a field of view of 28 × 28 cm, a slice thickness of 4 mm, an acquisition matrix of 256 × 256, an echo time of 3000 msec, a repetition time of 12 msec, a spin-lock pulse time of 15 to 75 msec in five increments of 15 msec each, and a spin-lock pulse amplitude of 500 Hz. The T1ρ values were calculated, with use of a custom-written MATLAB (The MathWorks, Natick, Massachusetts) program, on a pixel-by-pixel basis by fitting intensity data of the spin-lock images at each spin-lock pulse time to the following exponential function: 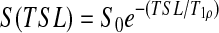 (Fig. 1). Mean T1ρ values were calculated from a manually selected 5-mm-diameter circular region of interest in the center of the nucleus pulposus. The T2-weighted images were used to assess each disc's degenerative grade according to the classification system described by Pfirrmann et al.23. Non-degenerated discs were defined as discs with a grade of <2.5 (fifteen discs), and degenerated discs were defined as discs with a grade of ≥2.5 (eighteen discs).
(Fig. 1). Mean T1ρ values were calculated from a manually selected 5-mm-diameter circular region of interest in the center of the nucleus pulposus. The T2-weighted images were used to assess each disc's degenerative grade according to the classification system described by Pfirrmann et al.23. Non-degenerated discs were defined as discs with a grade of <2.5 (fifteen discs), and degenerated discs were defined as discs with a grade of ≥2.5 (eighteen discs).
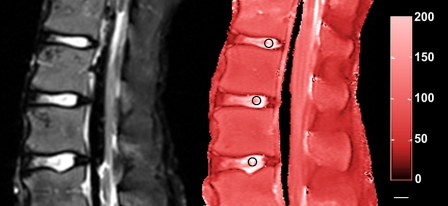
Fig. 1.
Representative T2-weighted (left) and T1ρ-weighted (right) images of the spine of a fifteen-year-old male donor. The average degenerative grade is 1.43. The 5-mm-diameter circular regions of interest where the T1ρ relaxation times were determined are denoted by the black circles. Scale bar (small, horizontal white bar) = 10 mm. The numbers on the right of the red bar indicate the T1ρ relaxation time.
The L1 through S1 discs were isolated with sharp dissection at the superior and inferior end plates. Seven discs were too degenerated to dissect and test, resulting in thirty-three discs for testing. A 7-mm-diameter punch-biopsy specimen was removed from the center of the nucleus pulposus. A freezing-stage (model BFS-30; Physitemp, Clifton, New Jersey) sledge microtome (model SM2400; Leica, Nussloch, Germany) was then used to section each sample to a uniform thickness. Thickness was measured in triplicate with use of a micro-laser sensor (LM10 Micro Laser Displacement Sensor; Aromat, New Providence, New Jersey). The average thickness (and standard deviation) of the thirty-three samples was 2.55 ± 0.14 mm. The samples were frozen at −20°C prior to testing.
A 4.37-mm-diameter circular punch was used to remove cylindrical samples from the sectioned 7-mm-diameter biopsy specimen of each disc. The samples were mechanically tested in confined compression as previously described21. The sample was placed into the test chamber, and the porous platen was lowered at 10 μm/sec with a linear stepper motor until a contact load of 0.20 N was reached. The chamber was then filled with 0.15-M phosphate buffered solution. After a five-minute wait period, a 1% compressive strain at a rate of 0.25 μm/sec was applied, followed by a three-hour hold. The isometric swelling pressure was determined from the equilibrium stress at the end of that three-hour hold. Following the isometric swelling test, a 5% compressive strain at a rate of 0.25 μm/sec was applied, followed by a two-hour hold to measure relaxation. The data acquired during the stress relaxation test were analyzed with use of the linearly elastic, isotropic biphasic theory24. Compressive modulus was calculated from a direct analysis of the relaxation data, and apparent permeability was calculated from a fit to a forward finite-difference approximation of the biphasic theory21,25.
Following testing, adjacent thickness-matched nucleus pulposus tissue from the original 7-mm specimens were used to determine water and sulfated-glycosaminoglycan content. The wet weight of each tissue sample was first recorded in triplicate. Samples were then lyophilized, dry weight was recorded in triplicate, and the percentage water content was calculated. To determine sulfated-glycosaminoglycan content, 5-μL aliquots were pipetted into a ninety-six-well plate and were analyzed with a microplate reader (Synergy HT Multi-Mode Microplate Reader; BioTek Instruments, Winooski, Vermont) and the 1,9-dimethylmethylene blue assay26. The sulfated-glycosaminoglycan content was normalized to dry weight and wet weight.
Sample size was based on a power analysis, with a power of 0.8 and a level of significance of 0.05, in which we used data from our previous study demonstrating significant differences in sulfated-glycosaminoglycan content with degeneration21. Linear regression analyses were performed among the following variables: degeneration (degenerative grade, age, and T1ρ relaxation time), mechanical characteristics (swelling pressure, compressive modulus, and apparent permeability), and biochemical composition (water content and sulfated-glycosaminoglycan content) with use of GraphPad Prism software (GraphPad Software, San Diego, California). A significance level of 0.05 was used. The correlation was considered strong when the r value was >0.7, moderate if it was ≥0.5 but <0.7, and weak if it was ≤0.527. Analysis of variance with a Bonferroni post hoc test was also used to determine significant differences in mechanical characteristics and biochemical composition between non-degenerated and degenerated discs.
Results
Twenty-six discs were imaged, tested mechanically, and analyzed biochemically. An additional seven discs were imaged and analyzed biochemically but were too severely degenerated to be tested mechanically. The degenerative grades for the thirty-three discs ranged from 1.3 to 4.7, with a mean grade (and standard deviation) of 2.7 ± 1.0. (1.9 ± 0.5 for the fifteen non-degenerative discs and 3.6 ± 0.7 for the eighteen degenerated discs) (Table I). The imaging and biochemistry data for sixteen of the thirty-three discs have been previously reported18.
TABLE I.
Correlations Between T1ρ Relaxation Times and Mechanical and Biochemical Parameters in Non-Degenerated and Degenerated Discs
| Parameter | Non-Degenerated Discs* | Degenerated Discs* | Correlation with T1ρ Relaxation Time (R) |
|---|---|---|---|
| Degenerative grade | 1.9 ± 0.5 | 3.6 ± 0.7† | −0.83† |
| T1ρ relaxation time (msec) | 124 ± 38 | 40 ± 18† | |
| Swelling pressure (MPa) | 0.142 ± 0.077 | 0.048 ± 0.037† | 0.59‡ |
| Compressive modulus (MPa) | 1.42 ± 0.69 | 1.04 ± 0.62 | 0.20 |
| Apparent permeability (×10-15 m4/N-s) | 0.47 ± 0.26 | 0.71 ± 0.34 | −0.25 |
| Sulfated-glycosaminoglycan content (μg/mg) | |||
| Per dry weight | 457 ± 193 | 269 ± 142† | 0.69‡ |
| Per wet weight | 87 ± 24 | 70 ± 28 | 0.49‡ |
| Water content (%) | 79.8 ± 6.9 | 71.6 ± 8.4† | 0.53‡ |
The values are given as the mean and standard deviation.
P < 0.05 for the difference between the non-degenerated and degenerated discs.
P < 0.05 for the significance of the correlation.
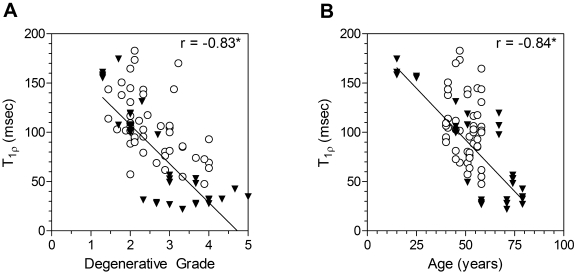
T1ρ relaxation times ranged from 22 to 195 msec (Fig. 1). The average imaging time was approximately thirty-five minutes. The T1ρ relaxation time was strongly correlated with the degenerative grade (r = −0.83, p < 0.05) and age (r = −0.84, p < 0.05) (Fig. 2), and it was significantly higher for the fifteen non-degenerated discs than it was for the eighteen degenerated discs (Fig. 3, A; Table I).
Fig. 2.
Correlation between T1ρ relaxation time and degenerative grade (A) and between T1ρ relaxation time and age (B). The asterisks denote significance (p < 0.05). The T1ρ relaxation times in this in vitro study (filled triangles) were within the range of values in a previous in vivo study of ten asymptomatic forty to sixty-year-old subjects (open circles)13.
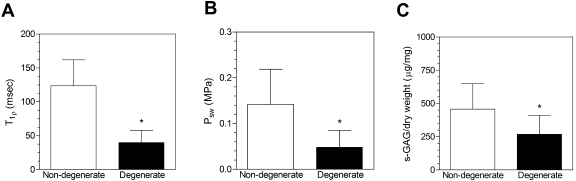
Fig. 3.
Comparison of T1ρ relaxation time (A), swelling pressure (Psw) (B), and sulfated-glycosaminoglycan content (s-GAG) per dry weight between non-degenerated and degenerated discs (C). The asterisks denote significance (p < 0.05). The biochemistry and T1ρ data for sixteen of the thirty-three discs were previously reported18.
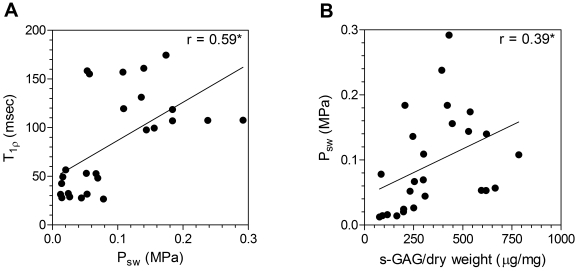
The swelling pressure as measured with confined compression testing was directly correlated with the T1ρ relaxation time (r = 0.59, p < 0.05) (Fig. 4, A; Table I) and the sulfated-glycosaminoglycan content per dry weight (r = 0.39, p < 0.05) (Fig. 4, B). Additionally, the swelling pressure in the thirteen non-degenerated discs in which it was tested was nearly three times as high as that in the thirteen degenerated discs in which it was tested (Fig. 3, B; Table I). The compression modulus and apparent permeability were not correlated with the T1ρ relaxation time (modulus: r = 0.20, p > 0.05; permeability: r = −0.25, p > 0.05) and did not differ significantly between the non-degenerated and degenerated discs (Table I).
Fig. 4.
Correlation between T1ρ relaxation time and swelling pressure (Psw) (A) and between swelling pressure and sulfated-glycosaminoglycan content (s-GAG) per dry weight (B). The asterisks denote significance (p < 0.05).
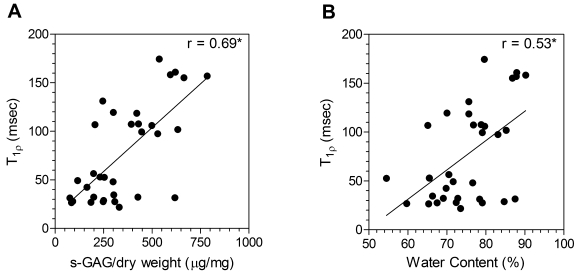
Biochemical assays showed the sulfated-glycosaminoglycan content per dry weight to be correlated with the T1ρ relaxation time (r = 0.69, p < 0.05) (Fig. 5, Table I) and to be 70% higher in the non-degenerated discs (p < 0.05) (Fig. 3, C; Table I). The T1ρ relaxation time was moderately correlated with the water content (r = 0.53, p < 0.05) (Fig. 5, Table I) and the sulfated-glycosaminoglycan content per wet weight (r = 0.49, p < 0.05). The water content was 11% higher in the non-degenerated discs (p < 0.05) (Table I), whereas the sulfated-glycosaminoglycan content per wet weight did not differ significantly between the non-degenerated and degenerated discs (p > 0.05) (Table I).
Fig. 5.
Correlation between T1ρ relaxation time and sulfated-glycosaminoglycan content (s-GAG) per dry weight (A) and between T1ρ relaxation time and water content (B). The asterisks denote significance (p < 0.05).
Discussion
This study demonstrated that, as we had hypothesized, isometric swelling pressure is directly correlated with findings on T1ρ-weighted imaging. However, the compressive modulus and permeability were not correlated with the T1ρ values, which was not consistent with our hypotheses. Overall, the findings suggest that T1ρ-weighted magnetic resonance imaging may be a valuable quantitative biomarker of nucleus pulposus mechanical function and proteoglycan composition for the diagnosis of disc degeneration. The linear correlation between swelling pressure and T1ρ relaxation time was the primary finding of this study. The mechanical function of the nucleus pulposus depends on its swelling pressure, the mechanism through which the disc supports axial loads. Although the primary utility of T1ρ-weighted magnetic resonance imaging will remain the detection of early degenerative changes in swelling pressure and proteoglycan content, this study extended the previous observation that T1ρ relaxation time is correlated with disc proteoglycan content over a broader range of degeneration and a larger sample size18. However, across the spectrum of degeneration, there may be several other biochemical and structural changes within the disc that affect the T1ρ relaxation time or that are not detected by T1ρ-weighted imaging, so the application of this biomarker should not be extrapolated beyond the specific properties quantified in this study. The lack of correlations of T1ρ relaxation time with permeability and compression modulus were not surprising considering previous observations of moderate-to-weak correlations between proteoglycan content and these mechanical properties21. With degeneration, the modulus and permeability of the nucleus pulposus changed by 50% in a previous study21. In this study, these properties were not significantly affected by degeneration, although their values and trends with degeneration were consistent with those in previous reports21. In contrast, the swelling pressure changes by a factor of two to three with degeneration. Thus, although T1ρ-weighted imaging may not be sensitive enough to detect changes in modulus and permeability with degeneration, these properties are probably not as important in the degenerative process as is swelling pressure.
An intriguing observation is that, while disc and articular cartilage contain similar constituents and undergo similar degenerative processes, these two tissues have opposite correlations between T1ρ relaxation time and proteoglycan content. The correlation is positive in disc tissue and negative in articular cartilage19,20. The strong direct correlation of T1ρ relaxation time with proteoglycan content in the disc suggests that, as proteoglycan content decreases with degeneration, the water and extracellular matrix molecules within the nucleus pulposus become more restricted, resulting in a lower T1ρ relaxation time (and also a lower swelling pressure). However, in articular cartilage, as the proteoglycan decreases with degeneration the water and extracellular matrix molecules become less restricted, resulting in a higher T1ρ relaxation time. While T1ρ relaxation time is believed to be primarily related to interactions between water and proteoglycans in the tissue, it is not known how other macromolecules may also contribute to T1ρ relaxation. The mechanisms driving the opposing T1ρ-relaxation-time responses associated with disc and articular cartilage degeneration may be related to compositional differences during the degenerative processes18. Furthermore, it is likely that intrinsic differences between these tissues with regard to structural and functional properties, such as the containment of the nucleus pulposus and the increased fibrosis and cross-linking with degeneration, can be attributed to the opposing responses in terms of T1ρ relaxation time. Specific factors that lead to the differences in correlations observed for disc and articular cartilage remain to be determined.
While this study was limited to cadaver human spines, the measured T1ρ relaxation times were consistent with the values measured in vivo in ten asymptomatic forty to sixty-year-old subjects13. Several preliminary studies have demonstrated this feasibility of in vivo T1ρ-weighted imaging of articular cartilage28-30 and the spine13,14. This study was also limited to two-dimensional magnetic resonance scans acquired at the midsagittal section of the disc, with which it would be difficult to register locations across sequential imaging sessions in an in vivo longitudinal study. Work to develop a pulse sequence to acquire three-dimensional T1ρ maps is ongoing31. While T2-weighted images were used to determine the degenerative grades, we did not attempt to correlate T2 relaxation time with disc mechanics or composition in this study. T1ρ has a larger dynamic range than T2, which will be needed to translate this modality into clinical use13,18.
Correlations in this study were not adjusted for intercorrelation among discs within the same spine. Although some changes may be similar from one adjacent disc to the next, the discs were treated as independent levels within the spine. With the addition of an adjustment for intercorrelations within the same spine, the correlations of T1ρ relaxation time with the degenerative grade, age, and swelling pressure remain significant (degenerative grade: r = −0.83, p < 0.05; age: r = −0.73, p < 0.05; swelling pressure: r = 0.65, p < 0.05). Sulfated-glycosaminoglycan content per dry weight also remains significantly correlated with swelling pressure (r = 0.38, p < 0.05). The adjusted correlations of T1ρ relaxation time with sulfated-glycosaminoglycan content per dry weight or water content are not significant. Increasing the number of spines to account for this adjustment may result in significant correlations. Thus, adjustment of the correlations did not affect the main finding of this study.
In conclusion, this study demonstrated a positive linear correlation between the findings on T1ρ-weighted imaging of the nucleus pulposus and both swelling pressure and proteoglycan content. Thus, T1ρ-weighted imaging has potential as a quantitative biomarker for mechanical function of the nucleus pulposus and disc degeneration. Future work should include a longitudinal in vivo study of symptomatic patients to build on the previous in vivo study of normal volunteers13. The application of T1ρ-weighted magnetic resonance imaging could also be investigated as a guide for selection of treatment, to quantify the state of the nucleus pulposus following herniation and/or discectomy, and to evaluate emerging technologies developed to treat early degeneration, including prosthetic replacement or biological and cell-based therapies. 
Acknowledgments
Note: The authors acknowledge Chenyang Wang for assistance in imaging, Dr. Marco Cannella and Dr. Michele Marcolongo for providing some disc tissue, and Dr. Marcolongo, Dr. Makarand Risbud, and Dr. Irving Shapiro for their helpful discussions.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant AR 050052) and the National Football League Charities (Medical Research Grant). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
References
- 1.Johannessen W, Vresilovic EJ, Wright AC, Elliott DM. Intervertebral disc mechanics are restored following cyclic loading and unloaded recovery. Ann Biomed Eng. 2004;32:70-6. [DOI] [PubMed] [Google Scholar]
- 2.Guerin HAL, Elliott DM. Structure and properties of soft tissues in the spine. In: Kurtz SM, Edidin AA, editors. Spine technology handbook. San Diego: Elsevier Academic Press; 2006. p 35-62.
- 3.Urban JP, Maroudas A. The chemistry of the intervertebral disc in relation to its physiological function and requirements. Clin Rheum Dis. 1980;6:51-76. [Google Scholar]
- 4.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-14. [DOI] [PubMed] [Google Scholar]
- 5.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198-205. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22:145-57. [DOI] [PubMed] [Google Scholar]
- 8.Morgan S, Saifuddin A. MRI of the lumbar intervertebral disc. Clin Radiol. 1999;54:703-23. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim MA, Haughton VM, Hyde JS. Enhancement of intervertebral disks with gadolinium complexes: comparison of an ionic and a nonionic medium in an animal model. AJNR Am J Neuroradiol. 1994;15:1907-10. [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim MA, Jesmanowicz A, Hyde JS, Estkowski L, Haughton VM. Contrast enhancement of normal intervertebral disks: time and dose dependence. AJNR Am J Neuroradiol. 1994;15:419-23. [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39:697-701. [DOI] [PubMed] [Google Scholar]
- 12.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15 Suppl 3:338-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenkrantz G, Li X, Han ET, Newitt DC, Crane JC, Link TM, Majumdar S. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging. 2006;24:1001-7. [DOI] [PubMed] [Google Scholar]
- 15.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17:1135-46. [DOI] [PubMed] [Google Scholar]
- 16.Redfield AG. Nuclear magnetic resonance saturation and rotary saturation in solids. Phys Rev. 1955;98:1787-1809. [Google Scholar]
- 17.Regatte RR, Akella SV, Wheaton AJ, Borthakur A, Kneeland JB, Reddy R. T 1 rho-relaxation mapping of human femoral-tibial cartilage in vivo. J Magn Reson Imaging. 2003;18:336-41. [DOI] [PubMed] [Google Scholar]
- 18.Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheaton AJ, Casey FL, Gougoutas AJ, Dodge GR, Borthakur A, Lonner JH, Schumacher HR, Reddy R. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20:519-25. [DOI] [PubMed] [Google Scholar]
- 20.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54:1087-93. [DOI] [PubMed] [Google Scholar]
- 21.Johannessen W, Elliott DM. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine. 2005;30:E724-9. [DOI] [PubMed] [Google Scholar]
- 22.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179-87. [DOI] [PubMed] [Google Scholar]
- 23.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873-8. [DOI] [PubMed] [Google Scholar]
- 24.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73-84. [DOI] [PubMed] [Google Scholar]
- 25.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927-34. [DOI] [PubMed] [Google Scholar]
- 26.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 27.Devore JL. Probability and statistics for engineering and the sciences. 3rd ed. Pacific Grove, CA: Brooks-Cole; 1995.
- 28.Wheaton AJ, Borthakur A, Kneeland JB, Regatte RR, Akella SV, Reddy R. In vivo quantification of T1rho using a multislice spin-lock pulse sequence. Magn Reson Med. 2004;52:1453-8. [DOI] [PubMed] [Google Scholar]
- 29.Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, Reddy R. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741-9. [DOI] [PubMed] [Google Scholar]
- 30.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229:269-74. [DOI] [PubMed] [Google Scholar]
- 31.Niyogi S, Witschey W, Wang C, Borthakur A, Reddy R. SLIPS: a novel method for rapid three-dimensional spin-locked imaging. Poster presented at the Joint Annual Meeting of the International Society for Magnetic Resonance in Medicine and the European Society for Magnetic Resonance in Medicine and Biology; 19-25 May 2007; Berlin, Germany.