Abstract
The aim of this research was to validate transcription magnetic resonance (MR) imaging (MRI) for gene transcript targeting in acute neurological disorders in live subjects. We delivered three MR probe variants with superparamagnetic iron oxide nanoparticles (SPION, a T2 susceptibility agent) linked to a phosphorothioate-modified oligodeoxynucleotide (sODN) complementary to c-fos mRNA (SPION-cfos) or β-actin mRNA (SPION-β-actin) and to sODN with random sequence (SPION-Ran). Each probe (1 μg Fe in 2 μl) was delivered via intracerebroventricular infusion to the left cerebral ventricle of male C57Black6 mice. We demonstrated SPION retention, measured as decreased T2* signal or increased R2* value (R2*=1/T2*). Animals that received the SPION-β-actin probe exhibited the highest R2* values, followed (in descending order) by SPION-cfos and SPION-Ran. SPION-cfos retention was localized in brain regions where SPION-cfos was present and where hybrids of SPION-cfos and its target c-fos mRNA were detected by in situ reverse transcription PCR. In animals that experienced cerebral ischemia, SPION-cfos retention was significantly increased in locations where c-fos mRNA increased in response to the ischemic insult; these elevations were not observed for SPION-β-actin and SPION-Ran. This study should enable MR detection of mRNA alteration in disease models of the central nervous system.
Keywords: cardiac arrest, immediate early genes, oxidative stress, nanotechnology, signal transduction
Brain damage resulting from cardiac arrest and stroke is a major cause of mortality and disability in the United States. Although the brain may repair itself, elevation of immediate early genes has been known to be positively related to expression of matrix metalloproteinase-9, a precursor of brain edema (1–5), a major cause of stroke-induced death in humans. The ability to detect alterations in endogenous gene transcription in live subjects can be pivotal for early intervention after an ischemic episode. Contrast-enhanced magnetic resonance (MR) imaging (MRI) permits real-time imaging, tracking of pathophysiological changes, and longitudinal studies at both the cellular and molecular levels (6–8).
Single-stranded complementary DNA and RNA have been used to report gene transcription in molecular biology based on Watson and Crick's double helix theory (Fig. 1A), and previous studies have shown that brain cells retain phosphorothioate-modified oligo DNA (sODN) in vivo (9, 10). Although at high doses (≥40 nmol/kg), sODNs with sequences complementary to mRNA (antisense sODNs) produce transient gene knockdown effects (11–13), equal doses of sense-sequence control sODNs and low doses of antisense sODNs do not affect gene expression in the brain (14, 15). Some investigators (16–18) have shown that liposome can facilitate sODN fusion to the cell membrane and thus enhance cerebral sODN retention.
Figure 1.
Schematic diagram of in vivo transcription MRI.
The goal of this study is to establish whether MR contrast agents function as suitable labels for nucleic acid probes to report gene transcription in the brains of live animals (Fig. 1B, C). The target transcript in this study is c-fos mRNA, an immediate early gene transcript that encodes the Fos peptide, an essential component of activator protein-1 and a neuronal transcription regulator that activates the expression of many genes including nerve growth factor (17, 19, 20). In normal resting neurons, c-fos mRNA is minimally expressed (is less abundant), but neuronal activities, stress, and cerebral ischemia can elevate its expression to levels at least 1 to 2 orders of magnitude higher than resting levels (21, 22). Conversely, β-actin mRNA is constitutively expressed at high levels and is not significantly elevated by cerebral ischemia; because its expression remains relatively stable, β-actin can be reliably used as a control (23). We aimed to determine whether SPION-cfos binds to its target mRNA in vivo, and whether SPION-sODN with sequence complementary to intracellular mRNA would report its gene transcript in the central nervous system. This MR probe will allow longitudinal studies of a wide range of disease models at both the cellular and molecular levels.
Materials and Methods
Probe preparation for SPION-NeutrAvidin and MRI Probes has been described previously. Briefly, all sODNs are with biotin attached to either the 3′ or 5′ end (24, 25). To trace cell uptake of sODN, we labeled the 3′-OH terminus of sODN-cfos with digoxigenin-dUTP (dig-dUTP), a DNA marker, generating 5′biotin-sODN-3′dig with the addition of terminal transferase (Roche Applied Sciences, Indianapolis, IN, USA). The 5′biotin-sODN-3′dig was purified using a dextran column for biotinylated oligo DNA (Roche Applied Sciences) and stored at −20°C (17). We mixed biotinylated sODN-cfos-3′dig (20 parts biotinylated sODN-cfos and one part biotinylated sODN-cfos-3′dig) just before use.
All probe conjugates were constructed with the same lot of activated SPION to minimize composition variations in the proportions of iron, dextran, and reaction intermediates. We incubated activated SPION with sODN-3′biotin at a ratio of 120 pmol SPION (720 nmol Fe) to 0.5 nmol sODN-3′biotin for 20 min at room temperature and then at 4°C. The conjugates were filtered in a Micron column (YM30, Millipore, Bedford, MA, USA) before use, within 24 h of conjugation. The resulting conjugate of activated SPION and sODN is referred to as SPION-sODN, or more specifically SPION-cfos, SPION-β-actin, or SPION-Ran, according to the sequence of the linked sODN. The distribution of sODN-cfos-dig was indirectly examined with FITC-labeled immunoglobin G (IgG) against digoxigenin (17).
Surgical procedures
All procedures and animal care practices adhered strictly to AAALAC, Society for Neuroscience, and institutional guidelines for experimental animal health, safety, and comfort. See Table 1 for protocol.
TABLE 1.
 | |
| 1. Transcription MRI | 2. Molecular Biological Assays |
|
|
MR probe delivery
Male C57Black6 mice (24±3 g, Taconic Farm) were anesthetized with ketamine (70 mg/kg ip) and xylazine (12 mg/kg ip), and the contrast conjugate was delivered to the brain at a dose of 1 μg per mouse (120 pmol SPION per kg body wt.) via ICV infusion (24, 25). Unless power calculation called for a greater number of animals, each study was repeated with at least three animals in each treatment group.
Bilateral carotid artery occlusion
Five hours after the ICV infusion, we induced cerebral ischemia by occluding the carotid arteries in two animals randomly chosen from a group of four. Briefly, the carotid arteries on both the left and right sides of the neck were exposed and occluded for 30 min (26). The other two animals in the group were given a sham operation (the same surgical procedure except for actual execution of artery occlusion) that also lasted 30 min. The animals were returned to the same cage, where they remained until MRI scanning on day 2.
Molecular biology assay
Postmortem tissue preparation
Animals were anesthetized with ketamine plus xylazine, as described in tissue preparation (24, 25).
SPION-cfos binding assay using in situ RT to cDNA
Our binding assay involved procedures adapted from in vitro reverse transcription (RT) (27). We used RNase ZAP decontamination solution (Ambion, Austin, TX, USA), at room temperature unless otherwise specifically indicated, to ensure that all procedures were carried out in an RNase-free environment. We prepared 20 μm sections of brain tissue and stored them at −80°C. The frozen brain samples were removed from the freezer and dried overnight in a vacuum and then sequentially treated with 4% PFA (20 min) and three washings with RNase-free phosphate-buffered saline (pH 7.2). Samples were dehydrated in ethanol (50, 70, 95, and 100%) and Pronase (Biomeda, Foster City, CA, USA, 10 min, 37°C), washed in 0.2% glycine/PBS, and then treated with DNase I (Invitrogen Life Technologies, Carlsbad, CA, USA; 0.1 U/μl, 37°C, 10 min) to remove all genomic DNA (verified by PCR results showing the absence of amplifiable nuclear gene (28). After washing the sample in RNase-free water and dehydrating it with 100% ethanol (wash and dry), we used reverse transcriptase (Invitrogen Life Technologies) to reverse transcribe c-fos mRNA in the absence of DNA primer at 37°C for 90 min. The sample was washed and dried as before.
We used PCR to amplify and detect cDNA produced by the SPION-cfos binding assay. We first preheated brain samples on a hot plate at 95°C while adding the PCR reaction mix (20 mM Tris-HCl, pH 8.4, 50 mM KCl, 300 μM each of a pair of upstream and A18 primers, 1.5 mM MgCl2, 20 μM dNTP, 1 μM FITC-dUTP, and 1 U TaqDNA polymerase (Invitrogen LT). The upstream PCR primer (5′-gcaactgagaagccaaga-3′) had a sequence matching positions 151–168 of c-fos mRNA (29); the sequence of the downstream A18 primer (5′-catcatggtcgtggtttg-3′) was complementary to positions 276–294. The negative control reaction contained all substrates except the PCR primers. We immediately transferred the samples to a thermocycler (the GeneAmp In Situ PCR system 1000, Applied Biosystems, Foster City, CA, USA) preset at 55°C and sealed the reaction chamber with AmpliCover (Applied Biosystems) and a clamp. Amplification, consisting of 25 cycles of 45 s at 94°C, 1 min at 55°C, and 1 min at 68°C, followed by 10 min at 72°C, was started after samples had been preheated at 95°C for 3 min. PCR was terminated at 4°C. After PCR amplification, we treated all samples with Mung Bean nuclease (Promega, San Diego, CA, USA) to remove excess single-stranded primers (37°C, 10 min). The amplified cDNA (containing FITC) was directly observed using a mercury light source (17). The RT-PCR procedure we describe is RNA dependent, as evidenced by nullified amplification when DNase I was replaced with RNase A before reverse transcription.
Gene expression using in situ hybridization
We treated one group of C57Black6 mice with 30 min of transient cerebral ischemia and then with 120 min of reperfusion. A second group received a sham operation of the same duration. We used complementary RNA (cRNA) probes for in situ hybridization to image the gene transcript where it is naturally expressed, as has been described previously (22, 27, 30).
Gene expression using in situ RT-PCR
Samples were prepared for the in situ RT-PCR experiment as for the binding assay except that no SPION-sODNs were infused beforehand. The procedures for reverse transcription in situ were likewise the same as described for the binding assay, except that total mRNA was reverse transcribed in the presence of oligo (dT)15 primer. The resulting cDNA was subsequently amplified by PCR with a pair of c-fos-specific primers (forward: 5′-atgggctctcctgtcaac-3′, and reverse: 5′-ggtcattgggatcttgc-3′) and dUTP-dig at a molar ratio of 0.1×. The amplified double-stranded dig-cDNA was 521 base pairs in length and complementary to nucleotides 250–766 of exons 1 through 4 in the c-fos mRNA (27). We used alkaline phosphatase-antidig IgG (Roche Applied Science, Mannheim, Germany) to detect dig-cDNA and stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolin (BCIP/NBT) for alkaline phosphatase (Biomeda). To validate our sample-handling techniques, we amplified β-actin mRNA in adjacent samples from the same animal when any brain sample failed to give visible cfos mRNA signal in the in situ hybridization assay.
MRI acquisition
In vivo MRI
In vivo image acquisition was performed with a 9.4 Tesla MRI scanner (Bruker Avance System, Bruker Biospin MRI) immediately after infusion (<30 min) and 7 h and 1 and 2 days after infusion (24, 25). Gradient echo (GE) images of constant repetition time (TR) and incremental echo spacing (TE) were acquired at each time point along the axial direction. Acquisition parameters were as follows: TR = 500 ms, TE = 3, 4, 6, 8 and 10 ms, flip angle = 30, twenty 0.5 mm slices, 15 × 15 mm field of view (FOV) and 128 × 128 pixels.
Brain metabolic disturbance
We acquired diffusion-weighted imaging (DWI) and average diffusion coefficients (ADC) with the following sequence: TR/TE = 3000/27ms; b = 154, 1160 s/mm2; 180 × 180 μm2 inplane resolution, and 1 mm slice thickness for assessment of tissue injury (25).
In vitro MR microscopy
We compared the in vivo MRI data 2 days after SPION-sODN infusion and induction of cerebral ischemia. We then euthanized the animals and extracted their brains for postmortem MR microscopy examination. We immersed the whole mouse brains in FC-40, a perfluoro compound solution to eliminate background proton signals, and imaged the brain samples using a 1 cm volume coil in a vertical bore 14 Tesla MR imaging system (Bruker-Avance System). We acquired high-resolution T2*-weighted images to delineate brain structures and visualize SPION presence (FLASH sequence, TR/TE=50/18 ms, resolution 50×50×100 μm3 or 40 μm isotropic, flip angle 20 degrees) (31).
Data analysis
We performed image analysis using MRVision MRI image analysis software (MRVision, Winchester, MA, USA), MAT-LAB, and in-house software as described (24).
Results
We aimed to determine the reporter characteristics of SPION-sODN for gene transcription analysis in live subjects. We selected to assess SPION retention in the contralateral hemisphere for statistical analysis because that area is characterized as having the least blooming-related T2* signal reduction (e.g., signal spillover) and fewest T1 effects caused by high SPION concentration near the infusion site and in the ventricular space where signal reductions may not be related to probe retention and therefore transcription. In addition, air-tissue interfaces of the ear and trachea may contribute irrelevant signal reduction (25). To quantitatively analyze SPION retention, we obtained T2* (ms) or average R2* (R2*=1/T2*) values (s−1) from the region of interest (ROI) encompassing most of the contralateral somatosensory cortex (SSC) of five contiguous brain slices (−1.4 to 0.6 mm from the bregma) in each animal that received either SPION-cfos or SPION-Ran (Fig. 2A). We chose to analyze R2* values because they change positively with elevation of iron oxide (24). We demonstrated that 1 μg dose of SPION-cfos (40 μg Fe/kg) was retained by the brain with a peak R2* value of 40 s−1 in the mouse contralateral SSC 1 day after ICV infusion (Fig. 2A); R2* decreased toward baseline values at day 2. This dose was high enough for signal detection but low enough not to produce excess noise in the MR image. No significant R2* elevation was observed when SPION-Ran was infused. Figure 2B shows that at 7 h, SPION-Ran was retained at low levels in the hippocampus and cortex posterior to the infusion site. At a dose of 80 μg Fe/kg, SPION-cfos was demonstrated to have greater retention but with a peak time of more than 3 days (25). Given this longer peak time for the 80 μg Fe/kg dose, we chose to use the 40 μg Fe/kg infusion dose of SPION-sODN for our current studies.
Figure 2.

Temporal profiles of SPION retention. An extended window for MR detection is represented by significant elevation of in vivo R2* profile (mean and ses of mean, sem) in the contralateral SSC (see outlines on anatomic images in right panel) of animals that received SPION-cfos (A) compared to baseline (preinfusion) using t test. Dynamic changes in R2* profile of animals that received SPION-Ran are also demonstrated (A and B). Uptake and clearance of SPION-cfos in ROI are indicated by significant elevation at 1 day and a return to baseline on day 2. Sample size is indicated below graph. Some animals were euthanized at end of MR scanning on day 1 for postmortem histological and molecular biological assays (Figs. 4 and 5). Example of postmortem T2* images and R2* maps are shown in Figs. 3 and 6 below.
Location of sODN-cfos-FITC or SPION-cfos-FITC after delivery
Because SPION has a quenching effect on FITC when bound to sODN (as SPION-sODN), we infused SPION-cfos-digoxigenin and showed its localization by its immunoreactivity to IgG against digoxigenin at 1 day (25). We show here that sODN of SPION-cfos retention was localized in the neuronal formation of C57Black6 mouse brains (Fig. 3 A, B), where we also observed T2* signal reduction in MRI (Fig. 3C). We observed positive dig-immunoreactivity in the cortical nuclei of all cell layers except layer I (Fig. 3B). Again, sODN is colocalized with iron oxide in concert with SPION retention. Our results indicate that at 40 – 80 μg Fe/kg, the linkage between a sODN and a SPION contrast agent remains intact for at least 1 day.
Figure 3.
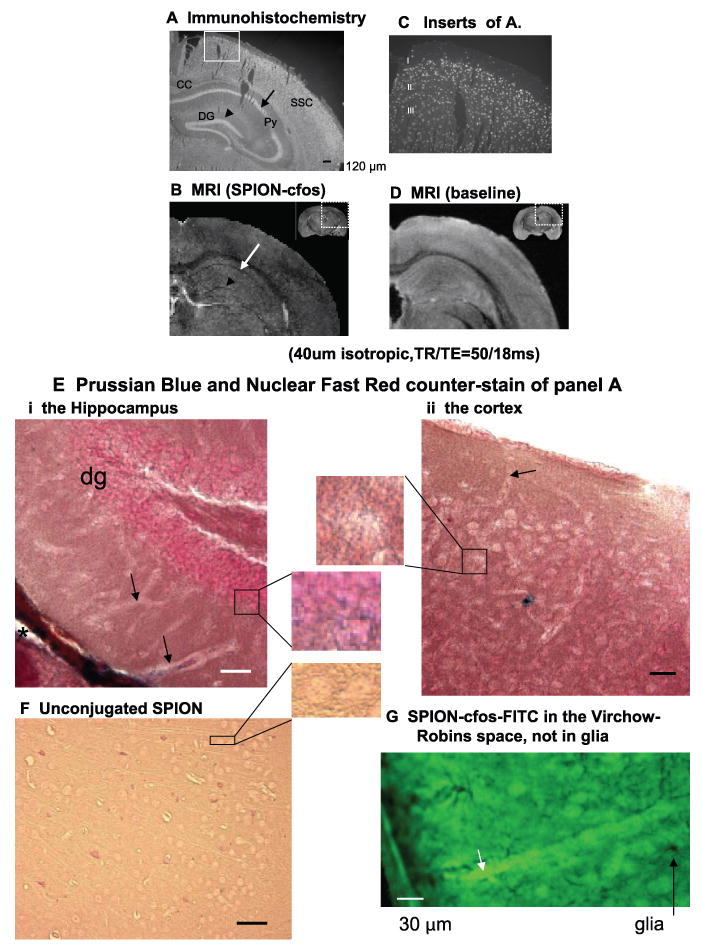
Histology of intracellular sODN-cfos correlates with MR signal reduction in postmortem brains: SPION-cfos-dig was delivered by ICV infusion; postmortem brain sections were obtained 1 day postinfusion. A) Immunohistochemistry with FITC-IgG against dig. B) Nuclear FITC signal from an inset of A. Reduced MR signal caused by the presence of SPION, imaged in vitro at 14T (TR/TE=55/18 ms, 40 μm isotropic, α = 20 degrees, scan time=24 h). D) Preinfusion baseline MRI. C and D both show contralateral hemisphere. E, F) Prussian Blue and Nuclear fast red counterstain in 100 μm-thick brain sections from animals also shown in A (Ei & Eii) or animals that received unconjugated SPION (F, cortex). G) The presence of SPION-cfos-FITC in the Virchow-Robins space (short arrow) is shown in cortical section (100 μm, BCIP/NBT staining after alkaline-phosphotase-IgG treatment against glial fibrillary acidic protein, long arrow).
This same 40 – 80 μg Fe/kg dose of unconjugated SPION (without linked sODN) was neither retained in the brain after infusion (24, 25) nor showed MR signal reduction, as in baseline MRI (Fig. 3D). SPION retention in the contralateral SSC of animals that received SPION-cfos was slightly elevated but significantly higher than baseline at 1 day (Fig. 2A). This modest elevation also correlated with MR T2* signal reduction (Fig. 3C) compared to baseline samples (Fig. 3D). Reduced MR signal in animals that received SPION-cfos was no longer present on day 2 as predicted in Fig. 2A. Iron oxide was present in the ventricular wall, in the Virchow-Robins space, in neurons of the dentate gyrus of the hippocampus, and in the cortex (Fig. 3E, inset). No iron oxide staining was evident in animals infused with unconjugated SPION (Fig. 3F, inset). In animals infused with fluorescein isocyanate (FITC)-labeled SPION-cfos, we demonstrated that SPION-cfos-FITC is present in the Virchow-Robins space but not in the glia, as shown in Fig. 3G.
In vivo c-fos mRNA targeting of SPION-cfos
To determine whether elevation of R2* values in live animal brains correlates positively with the presence of SPION-cfos, we investigated 1) the targeting ability, and 2) the location of SPION-cfos in mouse brains after delivery. We aimed to demonstrate whether intracellular SPION-cfos forms a specific heteroduplex with c-fos mRNA in vivo (c-fos mRNA targeting). Figure 4A schematically depicts our targeting assay, wherein hybridization between target and probe serves to prime RT of cDNA and obviate the need for conventional RT primer. The resulting cDNA facilitates subsequent PCR amplification using additional and c-fos-specific primers in the presence of FITC-labeled substrates. We examined the priming of two MR probes (SPION-Ran and SPION-cfos) 1 day after ICV infusion (n=3 each). We observed amplification of c-fos mRNA in brain samples from animals infused with SPION-cfos (Figs. 4B, C) but not when PCR did not include specific c-fos primers (Fig. 4D); we did not observe similar amplification in control animals that received the random-sequence SPION-Ran (Fig. 4E, F). We present the cortex and hippocampus regions in Fig. 4 both because these regions are easily identified under a microscope and because the hippocampus, located in the ventricle posterior to the infusion site, is not physically damaged by probe delivery. FITC signal was present in both the nucleus and the surrounding cytoplasm (Fig. 4B). Our results show that 1) the infusion procedure does not destroy transcripts of cerebral c-fos mRNA, and 2) excess SPION-sODN (random or cfos not hybridized to target mRNA) was not present during PCR amplification. Taken together, these results indicate that SPION-cfos delivered to the mouse brain forms a hybrid with intracellular cfos mRNA, a prerequisite for transcription MRI; SPION-Ran does not.
Figure 4.

SPION-cfos binds to its endogenous target in vivo. A) Schematic representation of the SPION-cfos binding assay. Postmortem brain samples were obtained 1 day after infusion (n=4). In situ reverse transcription was carried out on each section, with infused SPION-sODN serving as primer, followed by PCR with specific primers for c-fos or β-actin. B–F) Fluorescent images of cortex and dentate gyrus from brains infused with SPION-cfos (B, C) or SPION-Ran (E, F) after in vivo RT-PCR. D) Fluorescent image of the dentate gyrus acquired in control brain without PCR primers; asterisks represent autofluorescence in the tissue. Addition of β-actin-specific primers during PCR resulted in no amplification (not shown), suggesting specificity of SPION-cfos binding and signal amplifications. Autofluorescent signal observed in negative control is typically attributed to non-neural cells located on ventricular or vascular walls (D).
Toxicity of SPION-cfos retention
We present results from DWI experiments aimed at investigating the potential deleterious effects of the iron-containing probe in a fragile cellular environment such as cerebral ischemia. Because formation of edema and necrosis following cerebral ischemia-reperfusion is related to the presence of reactive oxygen species (ROS) and nitric oxide (32, 33) and because edema can be detected noninvasively and very early with MRI (34, 35), we are interested in exploring the development of edema or necrosis in animals previously infused with SPION-cfos. In this investigation, we employed a model of global cerebral ischemia involving bilateral carotid artery occlusion (BCAO) in C57Black6 mice (26). If artery occlusion is limited to 30 min (n>4), this model induces oxidative stress and DNA fragmentation without gross brain damage (36). However, BCAO in excess of 60 min does induce brain edema, determined by ADC below the threshold value, in the bilateral cortex and striatum (25). Global cerebral ischemia-based induction of blood–brain barrier leakage appears to be biphasic in nature (37). We hypothesize that brain edema and/or necrosis will develop after 30-minute artery occlusion in animals previously infused with SPION-cfos.
Our studies involved SPION-cfos infusion before BCAO and measurement of DWI and ADC at three specific time points (see Table 1 for protocol). We observed no apparent DWI hyperintensity or gross tissue damage in the brain either 4 h after ICV (Figure 5Ai) or immediately after BCAO (Fig. 5Aii), including peak SPION-cfos retention (24 h post-ICV delivery or 20 h postocclusion, Fig. 5Aiii). The ADC showed no significant differences between respective regions in the left and right hemispheres in the cortex and striatum of the same brain at any of the three time points (>0.05, t test, Fig. 5Bi, ii, iii). Here we show that SPION-cfos infusion to the brain does not enhance metabolic disturbance induced by global cerebral ischemia of 30 min.
Figure 5.

Minimal neurotoxicity in animals that received SPION-cfos and BCAO: Diffusion-weighted images (A) and ADC maps (B) were obtained from animals according to protocol (Fig. 2) 4 h after ICV but immediately before BCAO (i) and at two time points (ii, iii) at 30 min and 20 h after BCAO. Threshold ADCs (×10−4) were 5.5, 4.7, and 5.9 for the cortex, stiratum and hippocampus, respectively. We did not observe hyperintense DWI in A nor did we see ADC values below the threshold in these 3 regions (n=4). Toxicity testing using 2,3,5-triphenyltetrazolium chloride (TTC) did not reveal necrosis.
Application in animals with acute neurological disorders
Intracellular c-fos mRNA is activated to a high level in the brain after global cerebral ischemia. We aimed to show that SPION-cfos retention (per MR maps) is related to intracellular mRNA levels in the brains of live animals. To better illustrate SPION retention with statistical analysis, we adapted a set of criteria to analyze SPION uptake by 1) measuring regional R2* values in R2* maps for quantitative analysis of MR images, and 2) comparing MR assessments to in situ hybridization and in situ RT-PCR results. We also included assessment of two controls with and without cellular targets (SPION-β-actin and SPION-Ran, respectively). We detected ischemia-induced SPION retention at 2 days postinfusion. The rationale for the 2 day delay between operation and imaging was based on two previous observations: 1) SPION retention in animals that received SPION-cfos at a dose of 1 μg per mouse peaks at 1 day postinfusion and then returns to baseline levels in the contralateral SSC, and 2) if intracellular mRNA is elevated after cerebral ischemia, intracellular hybrids of mRNA-sODNs remain in the brain cells for at least 1 day (14), while unbound and excess SPION-cfos is no longer detectable as shown in Fig. 2A.
Identification of in vivo transcription hotspots for c-fos mRNA
Figure 6A, B shows cerebral R2* maps of SPION-cfos and SPION-β-actin, respectively, in mice with and without cerebral ischemia treatment. Using a computer-generated scale representative of R2* intensities, we noted visible R2* differences in the cortex, hippocampus, and hypothalamus in all groups of animals. In general, R2* maps in the lower half of the brain revealed elevated values in live animals, possibly from noise unrelated to transcription but due to interference from the air-surface interface of the ear and trachea (24). To eliminate such noise, we constructed subtraction maps (cerebral ischemia group minus sham operation group). We identified ischemia-induced R2* elevations in the cortex and hippocampus as hotspots (Fig. 6A). We observed no elevation in the subtraction maps of SPION-β-actin relative to those of SPION-cfos (Fig. 6B), except in a few spots near the amygdala and/or piriform cortices (upward arrows) and the contralateral cortex. We observed no such elevation in the SPION-Ran group (not shown). The hotspots depicted in Fig. 6A allowed us to explore possible elevation of gene transcription for c-fos mRNA.
Figure 6.

Transcription MRI after cerebral ischemia detected in live animals: SPION-sODNs were infused to both sham-operated and ischemia-induced animals; MRI was acquired 2 days afterward. R2* maps after sham operation (SO) and cerebral ischemia (CI) are shown. Scale of R2* intensity is 0 to 90 s−1. A, B) Average R2* maps from two MR slices at −1.4 to −0.9 mm (Bregma) from live animals in the SPION-cfos (A) and SPION-β-actin (B) groups (n≥3 per group). Subtraction maps of the 2 groups (CI–SO, overlaid on corresponding anatomical images of a normal mouse) show that cerebral ischemia induced SPION retention. Subtraction map minimized possible interference from the ear canals of the mouse, which may have been produced at the location of the amygdala and piriform cortices (at MR slices −1.4 and −0.9 mm mm to Bregma). Expression of c-fos and β-actin mRNA in animals without SPION-sODN infusion and with or without cerebral ischemia (plus 1 h of reperfusion) is detected using in situ hybridization with 33P-labeled complementary RNA probes. Corresponding autoradiograms (RNA maps) are shown adjacent to R2* maps (C, D). No subtraction mRNA map was generated, because we could not align samples between groups as the postmortem brains are not in the same shape in the confinement of the skull.
Differences in R2* values by statistical analysis
We obtained mRNA maps in autoradiographs using in situ hybridization and a radioactive probe for complementary RNA (cRNA). If precautions are taken to ensure that mRNA is not destroyed (27, 30), this conventional assay is a sensitive molecular method for assessment of postmortem samples. A representative autoradiograph is displayed adjacent to the corresponding R2* maps (Fig. 6). RNA maps acquired 1 h after 30-minute cerebral ischemia showed elevated levels of c-fos mRNA, but not β-actin mRNA (Fig. 6C, D), in a general agreement with the R2* maps shown in Figs. 6A, B, respectively. We observed no cfos-mRNA in sham-operated group (Fig. 6C). Nevertheless, the mRNA maps from c-fos in the cerebral ischemia group matches those of R2* subtraction maps (Fig. 6A), because RNA map from sham-operation group has no signal. We observed mRNA elevation in the cortex and hippocampus in the R2* maps and mRNA map (Fig. 6 A, C).
To support our observations of hotspots following cerebral ischemia, we calculated the mean R2* values in the contralateral SSC of individual animals infused with SPION-cfos. R2* values in this ROI were significantly higher (Fig. 7), by roughly 18 s−1 (or 60%), in animals treated with cerebral ischemia than in sham-operated animals. However, we observed no significant change in R2* when comparing sham-operated and cerebral ischemia-induced animals after SPION-β-actin or SPION-Ran infusion. This observation is in line with the known stability of β-actin mRNA in response to stress conditions.
Figure 7.

Statistical analysis of SPION retention (R2* values) for c-fos and β-actin mRNA. Mean R2* values in the ROI of contralateral SSC of 5 MR slices 2 days after SPION-sODN infusion from all the animals given cerebral ischemia or sham operation, were compared for MR contrast enhancement.
MRI of mRNA transcripts in the hippocampus-MR image comparisons
The defined formation of neurons in the hippocampus (38) was the primary factor in our decision to adopt a series of semiquantitative analyses on the hippocampus of postmortem brains using high resolution 14 Tesla MR Microscopy. We compared in vitro MR images and in situ RT-PCR images of the hippocampus to strengthen the validation of our MRI method of tracking specific mRNA transcript levels. Our acquisition of 3D T2*-weighted images of in vitro brains focused on the contralateral hippocampus (Fig. 8A, B). Visible but relatively modest T2* signal reduction was found in the hippocampus of sham-operated animals two days after SPION-cfos infusion (Fig. 8A). This finding is consistent with the temporal profile of R2* values at day 2 (Fig. 2A), and comparable to MRI results at the preinfusion baseline, Fig. 3D. After induction of cerebral ischemia, T2* signal reduction was clearly enhanced in the pyramidal cell layer, including the CA1 and CA3 regions, and in the dentate gyrus of the mouse hippocampus (Fig. 8B). It should be mentioned that we did note some non-specific T2* signal reduction along the ventricular wall between the cortex and hippocampus in all brains (Fig. 8A).
Figure 8.
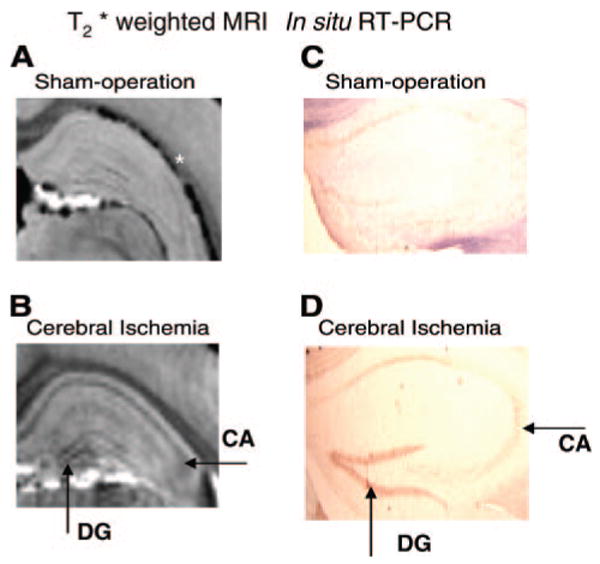
Transcription MRI using MR Microscopy is as sensitive as in situ RT-PCR. We show validation of c-fos mRNA expression using MRI and in situ RT-PCR. Expression of endogenous c-fos mRNA in mouse brains 2 days after infusion of SPION-cfos and either sham operation (A, C) or cerebral ischemia (B, D) was detected using high-resolution 3D 14 Tesla MRI (A, B) or in situ RT-PCR (C, D). (T2*-weighted image: 3D FLASH, TR/TE=50/18ms, 50×50×100 μm3, flip angle=20 degree) Arrows show SPION-cfos retention in DG and CA neuronal formation of hippocampus. Hippocampus of contralateral hemisphere is shown from 1 representative mouse in each group (n=4 each).
Verification of MRI using RT-PCR
We developed an in situ RT-PCR method to overcome the typically poor resolution provided by in situ hybridization. In this study, which aimed to show localization of endogenous c-fos mRNA, we did not infuse animals with SPION-cfos. We reverse-transcribed total cDNA using (dT)15 primer and then amplified c-fos cDNA in the presence of c-fos-specific primers. Although baseline mRNA expression is typically extremely low in normal brains, trace amounts of c-fos mRNA can be seen in the CA1 of the pyramidal cell layer and, to a lesser extent, in the DG (Fig. 8C). Cerebral ischemia greatly augments c-fos mRNA expression, specifically in the DG, CA1, and CA3 formations (Fig. 8D). Together, the results of our studies demonstrate the sensitivity and specificity of the SPION-cfos probe for imaging cerebral mRNA transcripts. Our observations in MR microscopy at 14 T (Fig. 8B) are similar to that the PCR results depicted in Fig. 8D (20-cylces of PCR represents an amplifications of transcripts by 6 orders of magnitude). We can thus conclude that MR microscopy is sensitive enough to detect differences in gene transcription without requiring amplification. In this manuscript, we have demonstrated here that DNA-RNA binding in advanced molecular assay (Figs. 1A and 4) is the basis for the MR detection of mRNA expression in mouse brain after acute global cerebral ischemia.
Discussion
We developed an MRI technique that utilizes novel contrast probes labeled with reporter sODNs. This method offers the flexibility of in vivo or in vitro MRI to study endogenous gene transcription in the mouse brain after cerebral ischemia. We have reported that the uptake of SPION-sODN is different from unconjugated SPION in living subjects (24, 25); we now demonstrate binding or targeting evidence of this probe using in situ RT-PCR (Fig. 4). Therefore, the sequence of our SPION-cfos probe mediates the retention of c-fos mRNA-dependent aptamer, the enhancement of MR contrast in mouse brains, and the efficacy of in vivo MRI for reporting mRNA levels. Our probe design and detection methods not only represent unique advances for reporting gene transcription without mRNA purification in system neuroscience, they also overcome some inherent difficulties associated with RNA isolation in conventional assays. We show an absence of toxicity for this probe after uptake in disease environments (Fig. 5). We show this novel MR method is as sensitive as advanced molecular biology using RT-PCR and in situ hybridization (Figs. 6 and 8). We provide here the mechanism of SPION retention is based on target binding (Fig. 4), according to intracellular mRNA levels (Figs. 6–8). Moreover, we used different control probes for constitutively expressed target (SPION-β-actin) in a different disease model (Fig. 7). We compared the retention of probes in various regions of the brain where comparison would be made in our cerebral ischemia model. These are a prerequisite for any new probe in a given disease model to obtain the best time point for MRI.
We now demonstrate the sensitivity and utility of mRNA-targeting probes to report active transcription with MRI after global cerebral ischemia, a condition that induces oxidative stress similar to that in acute and chronic neurological disorders in humans. We selected to evaluate c-fos mRNA transcription because the brain is capable of increasing specific gene transcripts (c-fos, but not β-actin, mRNA) in response to oxidative stress or with neuronal activity in the central nervous system (20, 22, 39, 40). This elevation response may be associated with cerebral repair activities in animal brains under certain conditions such as neurodegenerative and neurological disorders. We chose a cerebral ischemia model of bilateral carotid artery occlusion (BCAO) in C57Black6 mice (25, 41–44) because this model permits occlusion of the carotid arteries outside the brain, and does not require vessel suturing or filament placement inside the brain. Rather, this vessel occlusion model induces global cerebral ischemia by limiting blood flow to the brain while allowing the heart to remain active and supply vital nutrients to other organs, reducing lethality. Although this model induces apoptosis and apparent diffusion coefficient (ADC) reduction after 60 min of global cerebral ischemia (25, 36), it produces no measurable differences in ADC in SPION-cfos-infused animals either before or after 30 min of BCAO, suggesting that within 1 day of the ischemic episode the iron-containing probe did not increase metabolic disturbance by diffusion-weighted imaging (34, 35). Seeking support for the application and utility of this new neuroimaging probe, we used in situ hybridization and in situ RT-PCR, conventional yet advanced molecular biological assays to detect levels of intracellular c-fos mRNA. These assays allowed us to compare mRNA maps to our MR R2* maps.
A reporter probe for transcription detection in live animal brains must satisfy several challenging requirements. The probe must be able to 1) penetrate the blood-brain barrier to reach brain cells for uptake, 2) achieve adequate sensitivity for contrast enhancement, 3) provide an extended window for detection, 4) demonstrate specific target selectivity, and 5) be biodegradable or readily cleared after detection. We have demonstrated the utility of our probe for meeting all but the first of these requirements, with a dose that is lower than that used in humans and other rodents (45, 46), and with no measurable toxicity (Fig. 5). The extended window for MRI with the SPION-cfos probe is proportional to the delivered dose: one day for a 1 μg dose in this study (Fig. 2), and 3 days for a 2 μg dose (25).
The ICV infusion method for delivering probe to the brain cells bypasses the blood-brain barrier to achieve near-uniform distribution of the reporter probe (Fig. 3). Our observations agree with those that have been reported by others (12, 15, 46–49). Our reporter for live animal transcription MRI demonstrates distinct interactions with both the cell membrane (surface interaction) and intracellular mRNA (target interaction). These interactions exhibit some degree of specificity, as illustrated by the retention profiles of SPION-Ran (no retention), SPION-cfos (less retention), and SPION-β-actin (great retention; Fig. 7). We are certain that the interactions we have noted are representative of many others that take place once the conjugated probe reaches the inside of the cell.
Unlike unconjugated SPION and NeutrAvidin (proteins) that represent uncharged molecules and require either receptors or liposome for transduction, charged sODN appears to facilitate surface interaction by its ability to attach to the ventricular walls (17). Unconjugated SPION, either by itself or in mixture with sODN but without linkage, exhibits no such transient retention (24). The specificity of the sODN's membrane interaction is supported by the transient retention of SPION-Ran 7 h after infusion (Fig. 2B). Endocytosis is one mechanism that has been proposed to facilitate the entry of sODN into cells (50). The differences between the molecular mass of SPION-cfos, SPION-β-actin, and SPION-Ran are <1% (a difference of 0.6 kDa in a complex greater than 165 kDa). Enhanced endocytosis and delayed metabolism or exocytosis based on molecular weight may not be likely to support SPION retention. Although unconjugated SPION has been shown to be located in endosomes (8), our data in Fig. 4 support more than entrapment of SPION-cfos in the endosomes; rather, these data demonstrate and support the hybridization of sODN-cfos to cerebral mRNA (14). We propose that the hybridization-based double-helix theory increases the retention of SPION-cfos. This in vivo evidence suggests that SPION-sODN gains access to its target. It is very likely that endosomes may briefly encapsulate SPION-sODN, but it is the charge on sODN that facilitates SPION-sODN to break away from endosomes for intracellular RNA targeting. The ability of SPION-cfos to accomplish transfection without Lipofectin supports this proposed mechanism (24). This intracellular targeting mechanism may extend the window of retention for probes with sequences that match those of gene transcripts, shown by a graded retention of SPION-Ran, SPION-cfos, and SPION-β-actin (Fig. 2B and 7). This mechanism will support the findings of many investigators who have reported that transfection of antisense DNA functions as a gene knockdown agent in the brain.
Moreover, we hypothesize that gene transcript copy number will determine the level of SPION-sODN retention. This hypothesis is supported by the different SPION retention profiles of various MR probes, ranging from no retention for probes with no matching mRNA target (SPION-Ran; Fig. 4), to high retention for probes that target mRNA such as β-actin, which is highly expressed even under normal conditions (Sham-operation groups in Fig. 7). The retention profile of SPION-β-actin also illustrates that the reporting of intracellular mRNA is discriminatory (Figs. 6 and 7). Because SPION-Ran retention remained unchanged before and after BCAO, indiscriminate uptake of SPION-sODN after cerebral ischemia is less likely a contributing mechanism for elevated SPION retention after global cerebral ischemia. Along with molecular biology data, we demonstrate evidence that increased probe retention is related to a change in the number of copies of gene transcript after cerebral ischemia (Figs. 6 and 8). The sequence of SPION-cfos and its homologous binding to its target c-fos mRNA transcript most likely mediate reporter properties for transcription MRI. Therefore, intracellular interaction between SPION-sODN and mRNA will enable reporting transcription in dynamic changes in living animals with acute neurological disorders such as global cerebral ischemia and receptor signal transduction by amphetamine (24).
To enable quantitative MRI detection of gene transcription, one must also consider the clearance of unbound reporter SPION-sODN to ensure proper resolution and probe specificity. The mechanism of SPION-sODN clearance is less clear because the stability of the avidin-biotin linkage in the MR probe may be a factor for SPION-cfos turnover time. The lymphatic system has been suggested as one route of clearance for unconjugated iron oxide (46), but the clearance of sODN may not be the same. Although the presence of SPION-sODN in the Virchow-Robins space supports this proposed clearance pathway, we cannot distinguish incoming probes from outgoing probes. The mechanisms and pathways of clearance and hybridization of SPION-sODN are not yet entirely understood, but will certainly be important for future applications of this method. Once we have a better understanding of these mechanisms, we may be better able to address quantification by serially imaging the same transcript at different time points or by acquiring multiple images of different gene transcripts.
Using ICV delivery, we have shown that elevation of SPION-cfos retention after cerebral ischemia can be specific. Although ICV infusion may not be the ideal method of probe delivery, it may induce cortical spreading depression (51). The ICV route is currently a very efficient means of delivering genes and stem cells for therapeutic use. In experimental therapies that involve animals such as rodents, for which lumbar puncture is not a feasible option, ICV delivery may be the only option for achieving uniform distribution for robust efficacy analysis. This route can be used for probes with therapeutic value, at least in the contralateral cortex, without requiring autopsy or tissue biopsy—exceedingly important for longitudinal study at both the cellular and molecular levels in drug discovery. In summary, we have demonstrated the feasibility of both in vivo and in vitro MRI of intracellular gene expression at the nucleic acid level in the central nervous system. The data we show here suggest that our probe functions with targeting efficacy in a mechanism similar to that of probes used in molecular biology.
Acknowledgments
We thank Drs. H. D'Arceuil and J. Mandeville for consultation, Dr. Jia Q. Ren and S. Nagpal for GFAP staining, Dr. Charng-Ming Liu for providing DNA probes, and N. Eusemann for editing. This project was supported by grants from NINDS (R01NS45845), the NCRR (5P41RR14075), NCI (5T32CA009502), (P01AT002048), the Mental Illness and Neuroscience Discovery (MIND) Institute, and the Martinos Center for Biomedical Imaging.
References
- 1.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
- 2.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Lynch JR, Blessing R, Warner DS, Friedman AH, Laskowitz DT. Serum von Willebrand factor, matrix metalloproteinase-9, and vascular endothelial growth factor levels predict the onset of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51:1128–1134. doi: 10.1097/00006123-200211000-00005. discussion 1134–1125. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- 5.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Back T, Hemmen T, Schuler OG. Lesion evolution in cerebral ischemia. J Neurol. 2004;251:388–397. doi: 10.1007/s00415-004-0399-y. [DOI] [PubMed] [Google Scholar]
- 7.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 8.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. Magnetoden-drimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan NB. Trafficking of intracerebroventricularly injected antisense oligonucleotides in the mouse brain. Antisense Nucleic Acid Drug Dev. 2002;12:353–357. doi: 10.1089/108729002761381320. [DOI] [PubMed] [Google Scholar]
- 10.Shi N, Boado RJ, Pardridge WM. Antisense imaging of gene expression in the brain in vivo. Proc Natl Acad Sci U S A. 2000;97:14709–14714. doi: 10.1073/pnas.250332397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci U S A. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahlestedt C, Golanov E, Yamamoto S, Yee F, Ericson H, Yoo H, Inturrisi CE, Reis DJ. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature. 1993;363:260–263. doi: 10.1038/363260a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Widmayer MA, Zhang B, Cui JK, Baskin DS. Suppression of post-ischemic-induced fos protein expression by an antisense oligonucleotide to c-fos mRNA leads to increased tissue damage. Brain Res. 1999;832:112–117. doi: 10.1016/s0006-8993(99)01459-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu PK, Salminen A, He YY, Jiang MH, Xue JJ, Liu JS, Hsu CY. Suppression of ischemia-induced fos expression and AP-1 activity by an antisense oligodeoxynucleotide to c-fos mRNA. Ann Neurol. 1994;36:566–576. doi: 10.1002/ana.410360405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolliver BK, Sganga MW, Sharp FR. Suppression of c-fos induction in the nucleus accumbens prevents acquisition but not expression of morphine-conditioned place preference. Eur J Neurosci. 2000;12:3399–3406. doi: 10.1046/j.1460-9568.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi A, Furuno T, Kawaura C, Nakanishi M. Membrane fusion plays an important role in gene transfection mediated by cationic liposomes. FEBS Lett. 1998;433:169–173. doi: 10.1016/s0014-5793(98)00837-0. [DOI] [PubMed] [Google Scholar]
- 17.Cui JK, Hsu CY, Liu PK. Suppression of postischemic hippocampal nerve growth factor expression by a c-fos antisense oligodeoxynucleotide. J Neurosci. 1999;19:1335–1344. doi: 10.1523/JNEUROSCI.19-04-01335.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overhoff M, Alken M, Far RK, Lemaitre M, Lebleu B, Sczakiel G, Robbins I. Local RNA target structure influences siRNA efficacy: a systematic global analysis. J Mol Biol. 2005;348:871–881. doi: 10.1016/j.jmb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 21.An G, Lin TN, Liu JS, Xue JJ, He YY, Hsu CY. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann Neurol. 1993;33:457–464. doi: 10.1002/ana.410330508. [DOI] [PubMed] [Google Scholar]
- 22.Gu ZZ, Pan YC, Cui JK, Klebuc MJ, Shenaq S, Liu PK. Gene expression and apoptosis in the spinal cord neurons after sciatic nerve injury. Neurochem Int. 1997;30:417–426. doi: 10.1016/s0197-0186(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 23.Yang K, Mu XS, Xue JJ, Whitson J, Salminen A, dixon CE, Liu PK, Hayes RL. Increased expression of c-fos mRNA and AP-1 transcription factors after cortical impact injury in rats. Brain Res. 1994;664:141–147. doi: 10.1016/0006-8993(94)91964-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu CH, Kim YR, Ren JQ, Eichler F, Rosen BR, Liu PK. Imaging cerebral gene transcripts in live animals. J Neurosci. 2007;27:713–722. doi: 10.1523/JNEUROSCI.4660-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CH, Huang S, Kim YR, Rosen BR, Liu PK. Forebrain ischemia-reperfusion simulating cardiac arrest in mice induces edema and DNA fragmentation in the brain. Mol Imaging. In press. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu PK, Hsu CY, Dizdaroglu M, Floyd RA, Kow YW, Karakaya A, Rabow LE, Cui JK. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Liu PK. Neuronal NOS inhibitor that reduces oxidative DNA lesions and neuronal sensitivity increases the expression of intact c-fos transcripts after brain injury. J Biomed Sci. 2001;8:336–341. doi: 10.1007/BF02258375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu PK, Trujillo JM, Monnat RJ., Jr Spectrum of spontaneous mutation in animal cells containing an aphidicolin-resistant DNA polymerase alpha. Mutat Res. 1993;288:229–236. doi: 10.1016/0027-5107(93)90089-x. [DOI] [PubMed] [Google Scholar]
- 29.Van Straaten F, Muller R, Curran T, Van Beveren C, Verma IM. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci U S A. 1983;80:3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui J, Holmes EH, Liu PK. Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J Neurochem. 1999a;73:1164–1174. doi: 10.1046/j.1471-4159.1999.0731164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natt O, Watanabe T, Boretius S, Radulovic J, Frahm J, Michaelis T. High-resolution 3D MRI of mouse brain reveals small cerebral structures in vivo. J Neurosci Methods. 2002;120:203–209. doi: 10.1016/s0165-0270(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 32.Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res. 1998;53:333–338. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- 33.MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 34.Moseley ME, de Crespigny AJ, Roberts TP, Kozniewska E, Kucharczyk J. Early detection of regional cerebral ischemia using high-speed MRI. Stroke. 1993;24:I60–65. [PubMed] [Google Scholar]
- 35.Liu CH, D'Arceuil HE, de Crespigny AJ. Direct CSF injection of MnCl(2) for dynamic manganese-enhanced MRI. Magn Reson Med. 2004;51:978–987. doi: 10.1002/mrm.20047. [DOI] [PubMed] [Google Scholar]
- 36.Huang D, Shenoy A, Cui J, Huang W, Liu PK. In situ detection of AP sites and DNA strand breaks bearing 3′-phosphate termini in ischemic mouse brain. FASEB J. 2000;14:407–417. doi: 10.1096/fasebj.14.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mossakowski MJ, Lossinsky AS, Pluta R, Wisniewski HM. Abnormalities of the blood-brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. Acta Neurochir Suppl (Wien) 1994;60:274–276. doi: 10.1007/978-3-7091-9334-1_73. [DOI] [PubMed] [Google Scholar]
- 38.Neigh GN, Glasper ER, Kofler J, Traystman RJ, Mervis RF, Bachstetter A, DeVries AC. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in CA1 pyramidal cells and selectively alters acquisition of spatial memory. Eur J Neurosci. 2004;20:1865–1872. doi: 10.1111/j.1460-9568.2004.03649.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu PK, Grossman RG, Hsu CY, Robertson CS. Ischemic injury and faulty gene transcripts in the brain. Trends Neurosci. 2001;24:581–588. doi: 10.1016/s0166-2236(00)01918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 41.Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–1810. doi: 10.1161/01.str.28.9.1805. discussion 1811. [DOI] [PubMed] [Google Scholar]
- 42.Huang CY, Fujimura M, Chang YY, Chan PH. Overexpression of copper-zinc superoxide dismutase attenuates acute activation of activator protein-1 after transient focal cerebral ischemia in mice. Stroke. 2001;32:741–747. doi: 10.1161/01.str.32.3.741. [DOI] [PubMed] [Google Scholar]
- 43.Wu C, Zhan RZ, Qi S, Fujihara H, Taga K, Shimoji K. A forebrain ischemic preconditioning model established in C57Black/Crj6 mice. J Neurosci Methods. 2001;107:101–106. doi: 10.1016/s0165-0270(01)00356-9. [DOI] [PubMed] [Google Scholar]
- 44.Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- 45.Saini S, Sharma R, Baron RL, Turner DA, Ros PR, Hahn PF, Small WC, Delange EE, Stillman AE, et al. Multicentre dose-ranging study on the efficacy of USPIO ferumoxtran-10 for liver MR imaging. Clin Radiol. 2000;55:690–695. doi: 10.1053/crad.2000.0504. [DOI] [PubMed] [Google Scholar]
- 46.Muldoon LL, Varallyay P, Kraemer DF, Kiwic G, Pinkston K, Walker-Rosenfeld SL, Neuwelt EA. Trafficking of superparamagnetic iron oxide particles (Combidex) from brain to lymph nodes in the rat. Neuropathol Appl Neurobiol. 2004;30:70–79. doi: 10.1046/j.0305-1846.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Miller C, He Y, Martel-Pelletier J, Pelletier JP, Di Battista JA. Calphostin C induces AP1 synthesis and AP1-dependent c-jun transactivation in normal human chondrocytes independent of protein kinase C-alpha inhibition: possible role for c-jun N-terminal kinase. J Cell Biochem. 1999;76:290–302. doi: 10.1002/(sici)1097-4644(20000201)76:2<290::aid-jcb12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Chiasson BJ, Hooper ML, Murphy PR, Robertson HA. Antisense oligonucleotide eliminates in vivo expression of c-fos in mammalian brain. Eur J Pharmacol. 1992;227:451–453. doi: 10.1016/0922-4106(92)90167-t. [DOI] [PubMed] [Google Scholar]
- 49.Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardridge WM, Boado RJ. Enhanced cellular uptake of biotinylated antisense oligonucleotide or peptide mediated by avidin, a cationic protein. FEBS Lett. 1991;288:30–32. doi: 10.1016/0014-5793(91)80996-g. [DOI] [PubMed] [Google Scholar]
- 51.Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]



