Abstract
Background
Recently, the Teno FixTM device has been detailed in the literature. Conventional stranded cruciate repair requires splinting to protect the sutures from excessive loading, and then, active motion is strongly limited leading to a possible incomplete functional recovery.
Materials and methods
The authors report on their experience in treating 21 patients presenting primary flexor tendon injuries within the digital sheath in zone 2, in all fingers (including the thumb), at an average follow-up of 16 (range: 6–26) months.
Results
There were, according to Strickland and Glogovac criteria: 12 excellent; 6 good; 3 fair.
Conclusions
This new device is practical clinically and can effect strong tendon repairs that withstand early active finger motion, but the best indication is to treat only selected cases of sharp flexor tendon lesions in zone 2. Using this technique it is possible to achieve a quick functional recovery and early return to work.
Keywords: Flexor tendon injuries, New device, Metallic suture, Early mobilization
Introduction
Recently, the Teno FixTM device (Tenofix) has been reported in the literature [1] and the implantation technique is fully explained [2]. This metallic device has been proposed as a practical clinical tool, able to carry out strong tendon repairs effectively, that withstand early active finger motion.
Established treatments, like conventional four-stranded cruciate repair, require splinting or pull-out systems to protect the sutures from excessive loading and dehiscence and, in so doing, the active motion is strongly limited for several weeks; this factor may lead to an incomplete functional recovery. Actually, it will be advisable to mobilize the tendon with either passive or active flexion, at the earliest, to prevent adhesions and contractures and the eventual need for tenolysis [3–6].
The authors report on their experience in treating 21 patients presenting primary flexor tendon injuries within the digital sheath in zone 2, using this intratendinous metallic anchoring device.
Materials and methods
Device and operative procedures
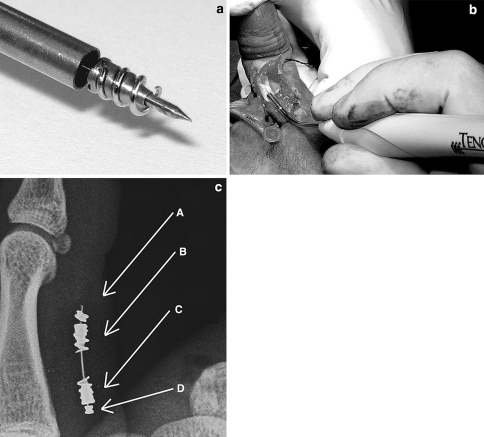
The Tenofix (Ortheon Medical, Winter Park, FL, USA) is a stainless steel device (ASTM F138–00) composed of two intratendinous anchor-coil complexes (2.0 mm in diameter and 4.0 mm in length) joined by a multifilament 2-0 stainless steel suture. The anchor-coil complex is composed of a spiraling cork-screw-like coil around a hollow spindle core (Fig. 1a). Two delivery devices are available, one for the proximal anchor and one for the distal anchor.
Fig. 1.
a The device is composed of two intratendinous anchor-coil complexes joined by a multifilament stainless steel suture. The anchor-coil complex is composed of a spiraling cork-screw-like coil around a hollow spindle core. b Two delivery devices, one for the proximal end, and one for the distal end, are provided; they are assembled with a delivery tube, containing the anchor-coil complex, and a handle. c A radiogram showing the main components of the system: a stainless steel wire connecting the distal (B) and proximal (C) anchor-coil complexes, with a preassembled stop-bead (D) and an opposite stop-bead (A), which are crimped intraoperatively once the device has been put in place
Surgical technique
Under brachial plexus block anesthesia, after the skin has been incised according to Bruner, the injured tendon stumps are exposed and a longitudinal palmar split is made about 10 mm away from the cut edge (about the same exposure which is required for traditional suture), to accommodate the obturator and the delivery tube, which contains the anchor-coil complex (Fig. 1b). Both proximal and distal stumps can be chosen as the first point of entry, depending on the clinical need. Once the complex has been gently twisted in place, a straight needle with the stainless steel suture and an attached stop-bead is passed through the hole in the core until the bead comes into contact with the complex. The stainless steel suture is passed into the second complex and into a stop-bead allocated into a preloaded crimping instrument using a 22-gauge needle. The tendon stumps are gently redirected into the tendon sheath and under the pulleys and placed together under proper tension until they slightly overlap. Finally, the stop-bead is crimped and the excessive stainless steel suture is cut (Fig. 2). The longitudinal tenotomies are sutured by a buried knot, while a continuous epitendinous 6-0 nylon suture at the edge of the stumps completes the repair.
Fig. 2.
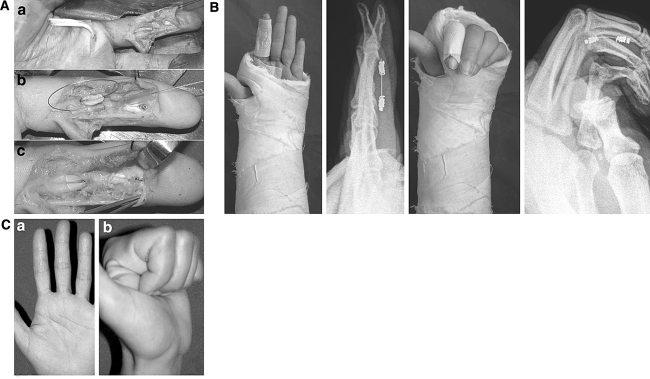
a FDP injury at zone 2A: the proximal stump was retrieved proximally (A). The anchor and the metallic suture were used to reroute the proximal stump into the digital canal by a TeflonTM guide (B). The suture was performed preserving the A3 pulley (C). b Full extension and full flexion were allowed postoperatively. c Clinical result at 4 weeks
Clinical cases
The study has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. All the patients gave their informed consent.
Exclusion criteria
Patients were selected according to the following exclusion criteria. Flexor tendon primary repair (within 12 h from injury) was performed with Tenofix on 21 patients [14 males, 7 females; mean age 32 (range: 18–46) years] presenting complete tendon transection by a sharp blade injury in flexor digital sheath zone 2; the subdivision zones defined by Tang [7–9] were followed. Patients with injuries of both flexor tendons or associated injuries (of the vessels or joints) or fractures were excluded. Because of the diameter of the anchors, children as well as adult patients presenting lesions in comparably small-sized fingers (like, most often, the little finger) were excluded from the study.
Four kinds of lesions were included:
Complete transection of the flexor digitorum profundus (FDP) tendon without concomitant lesion of the flexor digitorum superficialis (FDS) tendon, occurring at zone 2B and 2C.
Complete transection of the FDP tendon without concomitant lesion of the FDS tendon, occurring at zone 2A.
Complete transection of the FDS alone, occurring at zone 2D.
Complete transection of the flexor pollicis longus (FPL) tendon, occurring at zone 2.
Regarding the surgical repair of complete transection of the FDS alone occurring at zone 2D (point c-), the authors proposed surgical intervention in young and active patients; the reason is that even if finger motion remains normal when only the FDP is present, the cumulative grip strength is reduced.
Assessment
Results were assessed after a mean of 16 (range: 6–26) months of follow-up.
Clinical end points assessed included a comparison in range of active motion, according to Strickland [5, 6], of the DIP and PIP joints of the finger (Strickland modified Total Active Motion: SmTAM) as well as a recording of the linear measurement of pulp-to-distal-palmar-crease distance [10].
We adopted the Strickland and Glogovac criteria in the documentation of outcome of flexor tendon repair in zones 1 and 2 because we found these criteria (in fact a modified TAM method) to be more practical than TAM where, precisely, only fingers whose total range of active motion is the same as that of the contralateral hand can be rated as excellent. In fact, a varying degree of joint stiffness is always present after tendon repair so that patients gaining an excellent TAM score are rare. With the SmTAM an excellent functional status requires a sufficiently ample total range of active motion, but not necessarily similar to that of the contralateral side [9].
Data about the patients
Lesions were treated on the thumb (3 patients), index (11 patients), third (3 patients), fourth (1 patient) and little (3 patients) fingers. The study reports the complete experience of the authors and no patient was lost to follow-up before an end result was determined.
The most attainable active digital flexion to the palm was allowed from the first day postoperative, while extension was limited by placing the hand in a plaster with both the wrist and metacarpophalangeal (MCP) joints flexed at 30° until the 14th day postoperation; then the plaster and the skin suture were removed. A dorsal splint in neutral wrist position was applied until the 28th day for limiting wrist extension, while complete active motion of all the fingers was allowed (apart from flexion against resistance and forced passive extension).
Outpatient ward clinical controls were made weekly during the first month, then at 2, 6, 12 and 24 months. Anterior and lateral X-ray films, on flexion and extension, were made on the 14th and 60th day post-op, to evaluate the actual sliding and eventual gapping of the tendon, recordable by the position of stainless steel markers placed both proximal and distal to the intratendinous anchors of the device.
Results
Data about the patients
Regarding the 21 patients under study, the results according to SmTAM were as follows: 12 excellent; 6 good; 3 fair at an average follow-up of 16 (range: 6–26) months.
Complications
Three patients had unsatisfactory results and in two of them the device was removed. All these three patients had a fair score when evaluated by SmTAM.
The first patient fell accidentally after surgery provoking a forced hyperextension and the eventual rupture of tendon around the anchors; the device did not rupture but was removed.
The second patient required the removal of the device because of a low-grade sepsis that gave persistent pain; the use of Tenofix was probably badly indicated in this patient, which presented a torn and poorly vascularized wound at the time of operation.
The third patient was extremely uncooperative and did not follow any rehabilitation program.
In the two cases of removal, a traditional four-stranded degradable cruciate repair was performed.
Assessment (see Table 1)
Table 1.
Statistical analysis: data for age and follow-up are reported as a mean and range; all the others are reported as a mean and standard deviation
| Cases | Sex F/M | Mean age (years) | SmTAM (%) | Pulp-to-palm (mm) | Follow-up (months) | Functional recovery (days) | Return to work (days) | |
|---|---|---|---|---|---|---|---|---|
| FDP injuries at zone 2B & C | 6 | 3/3 | 31 (19–41) | 78 (±19) | 15 (±14) | 12 (6–26) | 33 (±4) | 42 (±12) |
| FDP injuries at the border 2A | 5 | 3/2 | 32 (24–38) | 70 (±16) | 21 (±11) | 19 (14–25) | 28 (±6) | 34 (±6) |
| FDS injuries at zone 2D | 4 | 1/3 | 35 (24–41) | 92 (±8) | 6 (±7) | 18 (14–24) | 26 (±4) | 32 (±10) |
| FPL injuries at zone 2 | 3 | 0/3 | 28 (18–45) | 92 (±3) | 2 (±2) | 17(10–24) | 21 (±0) | 21 (±7) |
| Cumulative results | 18 | 7/11 | 32 (18–45) | 81 (±16) | 12 (±12) | 16 (6–26) | 28 (±6) | 34 (±12) |
Complete transection of the FDP tendon without concomitant lesion of the FDS tendon, occurring at zone 2B and 2C Six patients (3 females and 3 males),of a mean age of 31 (range: 19–41) years were treated with a mean follow-up of 12 (range: 6–26) months. The mean SmTAM was 78 (±19 SD)%, with two excellent results in treatment of lesions of the index finger. The mean pulp-to-palm distance achieved was 15 (±14 SD) mm. Adequate functional recovery was obtained after a mean of 33 (±4 SD) days, while they returned to work after a mean of 42 (±12 SD) days.
Complete transection of the FDP tendon without concomitant lesion of the FDS tendon, occurring at zone 2A Five patients (3 females and 2 males), of a mean age of 32 (range: 24–38) years were treated with a mean follow-up of 19 (range: 14–25) months. The mean SmTAM was 70 (±16 SD)%: treatment of the index finger had excellent SmTAM; other fingers had a good SmTAM. The mean pulp-to-palm distance achieved was 21 (±11 SD) mm. Adequate functional recovery was obtained after a mean of 28 (±6 SD) days, while return to work occurred after a mean of 34 (±6 SD) days. Use of the device in this zone is strongly related to the exposure available and the dimensions of the site for each patient (Fig. 2).
Complete transection of the FDS alone, occurring at zone 2D Four patients (1 female and 3 males), with a mean age of 35 (range: 24–41) years were treated with a mean follow-up of 18 (range: 14–24) months. The mean SmTAM was 92 (±8 SD)% and all the treatments were for the index finger. The mean pulp-to-palm distance achieved was 6 (±7 SD) mm. Adequate functional recovery was obtained after a mean of 26 (±4 SD) days, while return to work occurred after a mean of 32 (±10 SD) days.
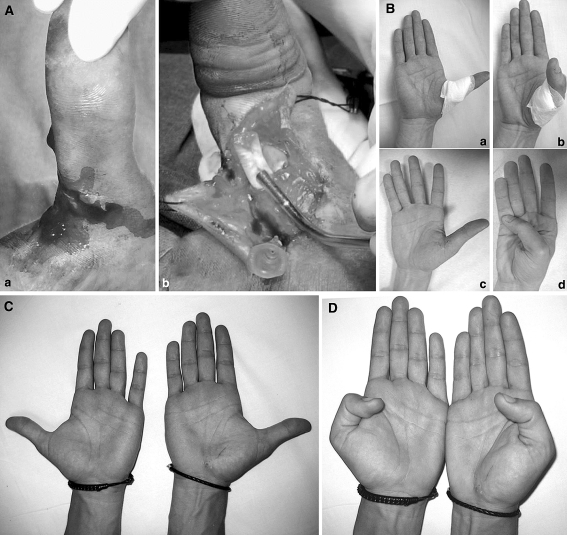
Complete transection of the flexor pollicis longus (FPL) tendon, occurring at zone 2 Three patients (3 males), with a mean age of 28 (range: 18–45) years were treated with a mean follow-up of 17 (range: 10–24) months. Treatments of injuries of the flexor pollicis longus at zone 2 had excellent SmTAM, namely 92 (±3 SD)%. The mean pulp-to-palm distance achieved was 2 (±2 SD) mm and adequate functional recovery was obtained after a mean of 21 (±0 SD) days, while return to work occurred after a mean of 21 (±7 SD) days (Fig. 3).
Fig. 3.
a FPL injuries at zone 2 (A); the proximal anchor is about to be inserted by the delivery tube which contains the anchor-coil complex (B). b Full extension and full flexion have been documented after 2 weeks, when skin sutures were removed (A–B), after 3 weeks (C–D). c Full extension has been documented after 91 weeks (21 months) in a bilateral comparison. d Full flexion has been documented after 91 weeks (21 months) in a bilateral comparison
Radiological findings
Dynamic X-ray films in full flexion and full extension showed that no blockage of the repaired tendon occurred under the pulleys; this proved to be true also for the A4 pulley in distal zone 2A lesions, even if at this level there is more limited space in comparison with more proximal pulleys.
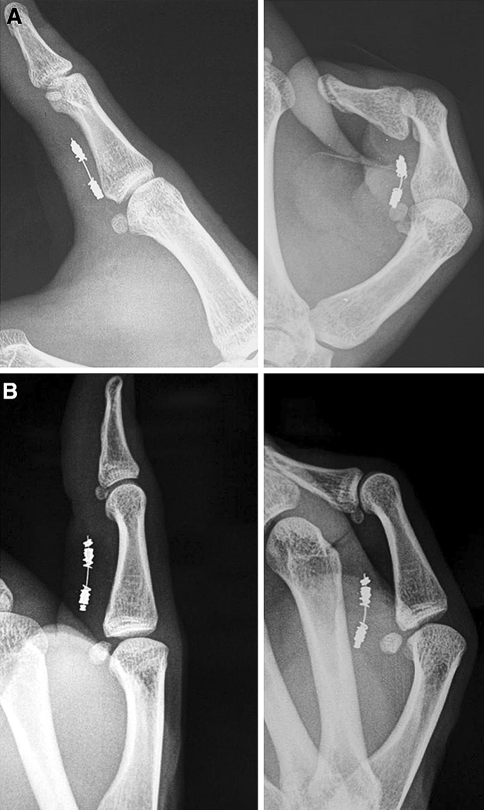
Dynamic X-ray films in full flexion and full extension also showed the effective tightening of the tendon suture in the longer term (Fig. 4a, b). Actually, it has to be noted that in 14 cases of the series, the X-ray control after 2 months showed that the anchors have slid around the steel core, closer to the junction site (Fig. 5), than in the beginning; this has been interpreted as a sign of effective tendon scaring, which involves a physiological contracture of the stump tissue, but another possibility may include the displacement of the anchors through repeated contact with the pulley edges; anyway, the latter hypothesis seems to be not supported by cases where anchors were not displaced even after 91 weeks (21 months) (Fig. 4b).
Fig. 4.
a Dynamic X-ray films, in full extension and full flexion, showed the effective gliding of the tendon suture at 3 weeks. b Dynamic X-ray films, in full extension and full flexion of the same patient after 91 weeks (21 months), showed no variation in gliding
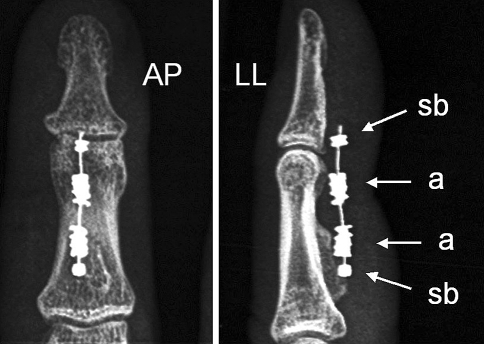
Fig. 5.
In the majority of cases the X-ray control at 2 months showed the anchors (a) were closer than at the beginning, having slid around the steel core toward the junction site, as noted by the increased distance from the stop-bead (sb); this has been interpreted as a sign of effective tendon scaring, which involves a physiological contracture of the tissue of the stumps
Discussion
The rationale for the use of Tenofix
Treatment of flexor tendon injuries at fingers and recovery of an adequate digital performance following tendon interruption still represent difficult challenges to the orthopedic and hand surgeons [9, 13]. Several different viewpoints persist with regard to the technique of repair, the management of flexor tendon sheath and the postoperative mobilization protocol of the injured finger [3–5, 7, 21]. Many proposed techniques which are adequate in tensile strength are technically demanding and require excessive tendon manipulation and increase the tendon bulk with a high number of strands that cross the repair site [8, 16, 17, 19]. Furthermore, modifying the diameter and shape of tendon cross-section is a cause of vascular impairment and fibrotic adhesions [11].
Achieving excellent results from flexor tendon repair still remains to be difficult. Attention to the details of suture technique and rehabilitation regime is important, but it is also true that significant future improvements are likely to come through new devices and materials able to prevent early gap formation and to allow full function as soon as possible. Strickland wrote in 1995: “the most effective method of restoring strength and excursion to repaired tendons involves the use of a strong, gap resistant suture technique followed by the application of controlled motion stress” [12].
The objectives of the ideal tendon repair can be summed up as follows: simple and reproducible execution; high resistance from the beginning; small occupancy of the tendon sheath; negligible gap; respect of vascularization, and very early mobilization. It is the opinion of the authors that tendon repair with the Tenofix device seems to satisfy these conditions and presents some advantages in comparison with traditional sutures.
In a recent article, a strength analysis and comparison of Tenofix with the widely used “modified Kessler” suture technique has been conducted on cadaver Achilles tendons [14]. Evaluation of the repairs consisted of tensile strength testing and measurement of the gap formation and peak stressed. Results showed that in the Tenofix repairs the gap formation stress was 67% of the peak tensile stress, in comparison to 29% of the peak stress in the modified Kessler suture. This has been correlated to the elastic property of the synthetic Kessler repair and the initial tightening of the suture around the tendon fibers. The Tenofix system is nonelastic and it is fully tensioned during installation; this leads to a better gap-resistant repair.
In another cadaveric study, where FDP tendons were studied [1] comparing the Tenofix with four-strand 3-0 cruciate suture, energy absorbed up to 2 mm gap was significantly greater for Tenofix, irrespective of the presence or absence of a 5-0 circumferential suture; anyway the load required to create the gap was not different. This kind of suture can then be able, with an unique core passage, to cope with the widely described problems of gapping [15–19].
A final note should be made on the fact that data reported in this paper support the indication, not previously described in the literature, to use the Tenofix device in lesions of the thumb.
Advantages
The authors noted that it is peculiar to this device that the intratendinous complex inserted in the tendon belly allows the tendon collagen fibers, entangled between the coil and the core, to maintain their physiological elongation, without the excessive twisting and/or constriction, which may occur with the ordinary stranded suture. This is a clear biological advantage in comparison with the vascular interference that can be produced by a traditional suture. Furthermore, the appearance of a well demarcated acellular zone has been evidenced in tied knots, apparently without any correlation with vascularity, casting a shadow on the trend for multistrand locking flexor tendon suture repairs [20].
It is widely known that early mobilization techniques following tendon repair within the digital sheath have improved the final end results. Experimental studies have shown that immediate mobilization favors healing, stimulating at the same time the regeneration of the tendon and the remodeling of the scar [21]. Clinical studies on controlled active motion following primary flexor tendon repair showed best functional results using the methods of earliest active finger mobilization [3–5]. It is an advantage of the Tenofix device to allow an active motion from the first day, with a partial limitation of full extension until the 14th day; this ensures short-term healing and functional recovery.
Disadvantages
Because of the diameter of the anchors, the device is not applicable to children and adult patients presenting lesions in comparably small-sized fingers, like the little finger. For the risk of infective involvement due to the presence of a metallic device, in our opinion, it is not indicated in treatment of dirty lesions and generally in complex multiple lesions. For want of a good quality of the tissue of the stumps receiving the anchors, the device is not indicated in case of fraying cutting lesions. Finally, the need of a full compliance of the patient with the rehabilitation program suggests avoiding the use of the device in the uncooperative patient. All these are clear disadvantages that prevent the use of Tenofix in those clinical occurences.
A comparison with previous reports using other repairs
A review of the literature on the repair of flexor tendon injuries in zone 2 shows that Tenofix results are in the same range of the best clinical series [9] when SmTAM and percentage of rupture are considered. In Table 2 results reported in the literature, from clinical series where flexor tendon injuries in zone 2 were evaluated by Strickland and Glogovac criteria (SmTAM), are grouped together and compared with our series; the mean values are superimposable.
Table 2.
Reports from the literature which assessed results by the Strickland and Glogovac criteria in patients treated with multistranded sutures: they are compared with the present serie treated with Tenofix
| Authors | Digits | Year of publication | % of excellent-good results | % of rupture |
|---|---|---|---|---|
| Cullen et al. | 38 | 1989 | 78 | 6 |
| Thang and Shi | 54 | 1992 | 80 | |
| Silfverskiod et al. | 55 | 1994 | 90 | 4 |
| Sandow and McMahon | 23 | 1996 | 78 | 0 |
| Baktir et al. | 88 | 1996 | 81 | 5 |
| Kitsis et al. | 87 | 1998 | 88 | 6 |
| Cumulative | 57.5 | 83 | 4 | |
| Rocchi et al. | 21 | 86 | 4 |
Anyway, it is worth highlighting that Tenofix appears to have a shorter time for functional recovery and for resuming the working activity: i.e., an average of about 30 days in comparison with an average of 60 days from previous reports on multistranded repair [3, 4].
Long-term problems (20 years)
They are, obviously, not known yet, because this is a short to middle term follow-up study. Anyway, it is known that the two most important complications of conventional flexor tendon repair are adhesions and dehiscence, which often lead to incomplete functional recovery [21, 22]; they seem to have been avoided in this clinical series by using this new device.
Adhesion formation can be limited first of all by increasing the excursion of the repaired tendon throughout the healing period: this is possible using different systems of protection of the suture like the widely known splints of Kleinert, Duran and others [13]. More easily, the use of the Tenofix device allows the finger immediate active motion with a simple short-time splinting at 30° of flexion. Furthermore, it does not determine modification of the tendon shape, the so-called “bulging”, nor any reduction of tendon sliding, and its axial disposition does not involve the deep surface of tendon in the suture, respecting the vascular perfusion provided by the vincula vessels. The reparation with this device does not directly involve the extremities of the cut tendon and eliminates knots from the junction site, so that no material interposition is present in the place of fibroblast proliferation. This characteristic also facilitates the closure of the epitenon, which acts like a barrier to the ingrowth of extratendon adhesions, and the reestablishment of the stump's continuity, helping the restoration of tendon metabolism [13, 22].
Despite its increased tensile strength, a stainless steel suture is not usually employed in flexor tendon repair because of its difficult handling and knot tying [15, 16].
Different from the traditional methods, the strength of the repair using Tenofix is not proportional to the numbers of strands that cross the repair site. The absence of multistrands and ligatures around the tendon represents a great advantage in terms of tightness, since it has been demonstrated that flexor tendon repairs usually rupture at the suture knots [17].
Finally, our findings confirm that the strength at 2 months is assured by the tendon healing rather than the device and this accords with the studies that state that between 3 and 6 weeks post-tendon repair, the suture becomes secondary to tendon healing as the primary provider of tensile strength to the tendon wound [22].
Final considerations
Based on preliminary results of using Tenofix, we think this new device is practical clinically and can effect strong tendon repairs that withstand early active finger motion and appear to ensure a quicker recovery after surgery. However, in our series, for three patients, treatment with Tenofix was not the right choice owing to giving us results that led to a reoperation rate not lower than those present in recent reports of primary flexor tendon repairs in zone 2. We conclude that there is an indication to treat with Tenofix, selected cases of sharp flexor tendon lesions in zone 2 which, by this technique, may achieve a faster functional recovery and early return to work.
Conflict of interest statement
Authors do not have any financial relationship with the manufacturer of the device and have the full control of all primary data and agree to allow the journal to review their data if requested.
References
- 1.Su BW, Protopsaltis TS, Koff MF, et al. The biomechanical analysis of a tendon fixation device for flexor tendon repair. J Hand Surg. 2005;30A:237–245. doi: 10.1016/j.jhsa.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Su BW, Solomons M, Barrow A, et al. Device for zone-II flexor tendon repair: a multicenter, randomised, blinded, clinical trial. J Bone Joint Surg. 2005;87A:923–935. doi: 10.2106/JBJS.C.01483. [DOI] [PubMed] [Google Scholar]
- 3.Kitsis CK, Wade PJ, Krikler SJ. Controlled active motion following primary flexor tendon repair: a prospective study over 9 years. J Hand Surg. 1998;23B:344–349. doi: 10.1016/s0266-7681(98)80055-7. [DOI] [PubMed] [Google Scholar]
- 4.Peck FH, Bucher CA, Watson JS. A comparative study of two method of controlled mobilization of flexor tendon repairs in zone 2. J Hand Surg. 1998;23B:41–45. doi: 10.1016/s0266-7681(98)80216-7. [DOI] [PubMed] [Google Scholar]
- 5.Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg. 1980;5A:537–543. doi: 10.1016/s0363-5023(80)80101-8. [DOI] [PubMed] [Google Scholar]
- 6.Strickland JW. Flexor tendon surgery. Part 1: primary flexor tendon repair. J Hand Surg. 1989;14B:261–272. doi: 10.1016/0266-7681_89_90079-x. [DOI] [PubMed] [Google Scholar]
- 7.Tang JB, Shi D. Subdivision of flexor tendon “no man’s land” and different treatment methods in each sub-zone. A preliminary report. Chin Med J. 1992;105:60–68. [PubMed] [Google Scholar]
- 8.Tang JB, Shi D, Gu YQ, Chen JC, Zhou B. Double and multiple looped suture tendon repair. J Hand Surg. 1994;19B:699–703. doi: 10.1016/0266-7681(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 9.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clinic. 2005;21:199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Boyes JH. Flexor tendon grafts in the fingers and thumb: an evaluation of end results. J Bone Joint Surg. 1950;32A:489–499. [PubMed] [Google Scholar]
- 11.Strickland JW. Flexor tendon repair. Hand Clinic. 1985;1:55–68. [PubMed] [Google Scholar]
- 12.Strickland JW. Flexor Tendon Injuries: I. Foundation of treatment. J Am Acad Orthop Surg. 1995;3:44–54. doi: 10.5435/00124635-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg. 2000;25A:214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 14.Lewis N, Quitkin HM. Strength analysis and comparison of the Teno Fix tendon repair system with the two-strand modified Kessler repair in the Achilles tendon. Foot Ankle Int. 2003;24:857–860. doi: 10.1177/107110070302401109. [DOI] [PubMed] [Google Scholar]
- 15.Robertson GA, Al-Quattan MM. A biomechanical analysis of a new interlock suture for flexor tendon repair. Journal of Hand Surg. 1992;17B:92–93. doi: 10.1016/0266-7681(92)90020-3. [DOI] [PubMed] [Google Scholar]
- 16.Wade PJ, Wetherell RG, Amis AA. Flexor tendon repair: significant gain in strength from the Halsted peripheral suture technique. J Hand Surg. 1989;14B:232–235. doi: 10.1016/0266-7681_89_90135-6. [DOI] [PubMed] [Google Scholar]
- 17.Trail IA, Powell ES, Noble J. The mechanical strength of various suture techniques. J Hand Surg. 1992;17B:89–91. doi: 10.1016/0266-7681(92)90019-x. [DOI] [PubMed] [Google Scholar]
- 18.Seradge H. Elongation of the repair configuration following flexor tendon repair. J Hand Surg. 1983;8A:182–185. doi: 10.1016/s0363-5023(83)80012-4. [DOI] [PubMed] [Google Scholar]
- 19.Barrie KA, Tomak SL, Cholewicki J. Effect of suture locking and suture calibre on fatigue strength of flexor tendon repairs. J Hand Surg. 2001;26A:340–346. doi: 10.1053/jhsu.2001.22926. [DOI] [PubMed] [Google Scholar]
- 20.Wong JKF, Cerovac S, Ferguson MWJ, McGrouther DA. The Cellular effect of a single interrupted suture on tendon. J Hand Surg. 2006;31B:358–367. doi: 10.1016/j.jhsb.2006.03.162. [DOI] [PubMed] [Google Scholar]
- 21.Gelberman RH, Woo SL, Amiel D, Horibe S, Lee D. Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg. 1990;15A:69–77. doi: 10.1016/s0363-5023(09)91108-x. [DOI] [PubMed] [Google Scholar]
- 22.Ketchum LD. Suture materials and suture techniques used in tendon repair. Hand Clinic. 1985;1:43–53. [PubMed] [Google Scholar]