Abstract
The lipid second messenger sphingosine 1-phosphate (S1P) is a critical mediator of cellular proliferation and survival signals, and is essential for vasculogenesis and neurogenesis. S1P formation is catalysed by sphingosine kinases 1 and 2 (Sphk1 and Sphk2). We have found that the endogenous glycolipid sulfatide (3-O-sulfogalactosylceramide) binds to and inhibits the activity of Sphk2 and the closely related ceramide kinase (Cerk), but not Sphk1. Using sulfatide as a probe, we mapped the lipid binding domain to the N-terminus of Sphk2 (residues 1–175), a region of sequence that is absent in Sphk1, but aligns with a pleckstrin homology domain in Cerk. Accordingly, Sphk2 bound to phosphatidylinositol monophosphates but not to abundant cellular phospholipids. Deleting the N-terminal domain reduced Sphk2 membrane localisation in cells. We have therefore identified a lipid binding domain in Sphk2 that is important for the enzyme’s sub-cellular localisation.
Keywords: Sphingosine 1-phosphate, sulfatide, sphingosine kinase, ceramide kinase, pleckstrin homology
Introduction
The sphingolipids are a family of lipids that play important roles in cell membrane structure and function, and as second messengers in signal transduction processes. The common constituent of all sphingolipids is the long chain base, which in mammals takes the form of 18-carbon sphingosine or dihydrosphingosine. Ceramides are sphingosine with an N-acyl chain of varying length (usually 16–24 carbons). Ceramide and sphingosine may be phosphorylated on their 1-OH, by ceramide kinase (Cerk) and sphingosine kinases 1 and 2 (Sphk1 and Sphk2), respectively. Whilst there is now a considerable body of information concerning the cellular and physiological roles for sphingosine kinases and their product, sphingosine 1-phosphate (S1P), Cerk and ceramide 1-phosphate (Cer1P) have been the subject of research in recent years only [1; 2].
S1P is important for maturation of the vasculature, control of vascular tone, lymphocyte entry into the circulation, inflammatory cell activation, and cellular transformation [2; 3]. Mice carrying a null allele for either Sphk1 or Sphk2 appear to be phenotypically normal, but knockout of both alleles results in a complete loss of S1P synthesis, and embryonic lethality due to vascular and neural defects [4]. At the cellular level, S1P enhances cell survival and proliferation, modulates adhesion molecule expression, and acts as a chemotactic migration signal. The effects of S1P are mediated primarily through its binding to a family of five G-protein coupled receptors (GPCRs), termed S1P1-S1P5, although some of the effects of S1P appear to be mediated through unidentified intracellular targets [2; 3].
Sphk1 is a cytosolic enzyme that is recruited to the plasma membrane following its phosphorylation in response to growth stimuli [5]. Sphk1 is activated by multiple stimuli, including growth factors, PMA, and inflammatory mediators such as TNFα and IgE [2], and is itself oncogenic [5; 6]. Sphk2 localises broadly throughout the cytosol, intracellular membranes, and the nucleus, and is anti-proliferative and pro-apoptotic when overexpressed [7; 8; 9; 10]. Forced expression of Sphk1 in the endoplasmic reticulum or nucleus also inhibits proliferation and promotes apoptosis [7; 10]. Sphk2 is activated in response to EGF [11], stem cell factor, interleukin-3 and IgE [12], and is required for mast cell activation and degranulation in response to IgE [13]. Sphk2 has also been implicated in the induction of p21 in response to chemotherapeutic agents [14]. Phosphorylation of the immunosuppressive prodrug FTY720 by Sphk2 is essential for its induction of peripheral lymphopenia, which is in turn required for successful treatment of autoimmune diseases and acceptance of transplants [3].
Little is known of the factors governing the sub-cellular localisation of Sphk2 and its constitutive association with intracellular membranes. In this report we show that the N-terminal domain of Sphk2 binds to sulfatide and phosphoinositides. This domain appears to be important for correct sub-cellular localisation and may exert allosteric control over enzyme activity.
Materials and Methods
Materials
Avanti Polar Lipids: brain sulfatide, C6-NBD ceramide, C18:0 ceramide, phosphatidic acid, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate; Matreya LLC: brain cerebroside, C16 sulfatide, C2 sulfatide; Sigma Aldrich: ATP, cholesterol, phosphatidylserine, phosphatidylinositol, and cardiolipin; Perkin Elmer: [32P]-ATP, Europium labelled anti-rabbit IgG and DELFIA reagent; SAFC Biosciences: fatty acid free BSA; Echelon Biosciences: SphingoStrips and PIP Strips; Cell culture reagents were purchased from Invitrogen. Other suppliers are indicated in parentheses below.
Molecular biology and production of recombinant proteins
Cloning of human Sphk1, Sphk2, and Cerk cDNAs have been described [15; 16]. The following primers were used to generate truncations of Sphk2, in combination with forward or reverse full length Sphk2 primers: Δ1-77, gaattccggctgcgccccaaacctgaag; Δ1-99, gaattctgcaccctgcgaagccgca; Δ1-172, gaattcacccctgacctgctacctcggc; Δ240-654, ggatcctcaatcccactcactcaggctcagc; Δ176-654, ggatcctcagtcaggggtgatctccccatcc; Δ100-654, ggatcctcagcctgagacctcggccaacgg. PCR products were ligated into pCR-TOPO 2.1 vector (Invitrogen) and sequenced, then cloned as EcoRI fragments into pMAL-c2e (NEB) or pAcGFP-C2 (Clontech). MBP fusion proteins were purified on amylose resin as described in the product manual for pMAL-c2e. After purification, proteins were concentrated to 1–2 mg/mL, dialysed into 20 mM Tris/10 mM KCl, pH 7.4, and stored at −20 °C in 50% (v/v) glycerol. Protein concentration was assayed with BCA reagent (Pierce).
Lipid overlay blots
SphingoStrips were blocked for 1–2 h with Tris Buffered Saline/0.05% Tween-20 (TBST), containing 3% (w/v) fatty acid free BSA. All subsequent incubations and washes were with TBST/3% BSA. Membranes were incubated overnight with 2.5 μg/mL test protein, then washed 4 times over 45 min, and incubated for 2 h with rabbit anti-MBP serum (NEB) at 1:4000 dilution. Membranes were then washed, incubated with anti-rabbit IgG-HRP at 1:10,000 dilution (BioRad), and washed again before developing with ECL Plus (Perkin Elmer). For the PIP Strips, TBS/1% skim milk was used for blocking and incubation steps.
HEK293 cells were transfected in 6-well plates with 6 μL FuGene-6 (Roche) and 2 μg plasmid DNA per well, in OptiMem I serum free medium. Medium was replaced with DMEM/10% FBS growth medium 6 h after transfection. Cells were lysed 24 h after transfection, in 200 μL 20 mM Hepes, pH 7.4, 150 mM NaCl, 0.1 % TritonX-100, 1 mM EDTA, 1 mM DTT, and Complete Protease Inhibitor Cocktail (Roche). Lysates were cleared at 14,000 rpm for 10 min, and diluted 1:100 in TBS/1% skim milk for PIP Strip binding assay. Rabbit anti-GFP (ABCAM) was used at 1:5000 dilution.
Immobilised sulfatide binding assays
Lipids were pipetted, in 20 μL methanol/well, into a white 96-well plate and dried onto the wells. The wells were blocked overnight at 4 °C with 200 μL/well TBST/3% fatty acid free BSA, then emptied and incubated with recombinant proteins (100 μL/well in TBST/3% BSA) for 90 min at 37 °C. The wells were washed four times with TBST/BSA, incubated for 90 min at 37 °C with rabbit anti-MBP (1:2000), washed four times more, incubated for 45 min at room temperature with Europium labelled anti-rabbit IgG, and again washed four times. Signal was developed with 100 μL/well DELFIA reagent and read in a fluorescent plate reader.
Immunofluorescence
HeLa cells were transfected as described for HEK293 cells, and seeded onto glass coverslips 8 h after transfection. At 24 h after transfection, cells were washed twice with PBS, fixed in PBS containing 4% paraformaldehyde for 10 min, washed three times with PBS, and mounted in Biomeda mounting medium. Images were captured with an Olympus FV500 confocal microscope.
Ceramide kinase assay
C6-NBD ceramide was used to measure Cerk activity, as described previously [16]. The 100 μL reactions were started with the addition of 0.5 μg MBP-Cerk or 1 μg HEK293 cell lysate overexpressing Cerk, and incubated at 35 °C for 20 min in the dark. Sulfatides were delivered in 2 μL methanol per reaction.
For the mixed micelle assay [17], micelles containing 1.25 mM (0.125%) TritonX-100, 0.2 mM cardiolipin, and 50 μM C18:0 ceramide (solubilised in ethanol) were prepared by sonicating in reaction buffer (20 mM Hepes pH 7.4, 10 mM KCl, 15 mM MgCl2, 1 mM DTT). The micelles were diluted 5-fold with reaction buffer, and 1 mM ATP was added, together with 5 μCi per reaction of [32P]-ATP. The 100 μL reactions were started with the addition of 1 μg MBP-Cerk, and run for 20 min at 35 °C. Reactions were stopped with addition of 400 μL chloroform/methanol (1:1), then extracted four times with 180 μL 1M KCl/20 mM Hepes pH 7.4, discarding the upper aqueous phase each time. Silica Gel 60 TLC plates (Fisher) were spotted with 2 μL of the final organic phase, and resolved in butanol/acetic acid/water (3:1:1). Radioactivity was detected with a Typhoon scanner (GE Healthcare).
Sphingosine kinase assay
MBP-Sphk1 (0.5 μg/reaction) or MBP-Sphk2 (1 μg/reaction) was pre-incubated for 5 min with test lipid in reaction buffer (50 mM Hepes, pH 7.4, 10 mM KCl, 15 mM MgCl2, 10% glycerol), and reactions were started with the addition of 1 mM ATP and 10 μM NBD-sphingosine. Reactions (50 μL) were run for 20 min at 35 °C, in the dark, and product was detected as described previously [15].
Results and Discussion
Sulfatide binds the N-terminus of Sphk2 and Cerk
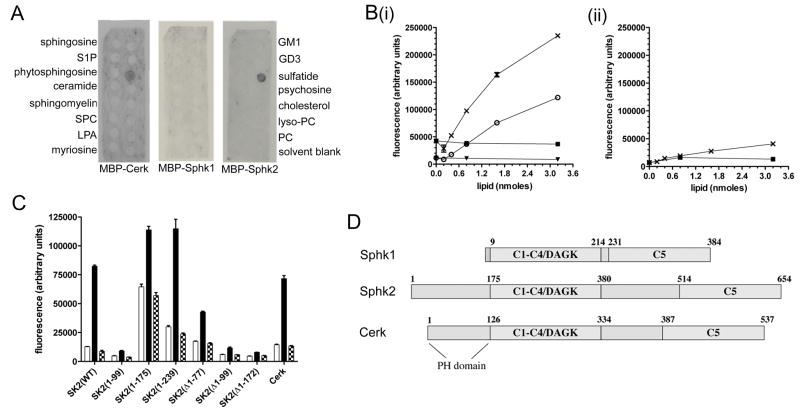
Using Sphingostrip membranes, we identified a specific interaction between 3-O-galactosylceramide (sulfatide) and recombinant Sphk2, purified as a C-terminal fusion to maltose binding protein (MBP). This interaction was also seen with MBP-Cerk, but not with MBP-Sphk1 (Figure 1A). To further explore this interaction, we developed a 96-well plate assay for binding of recombinant proteins to immobilised sulfatide. MBP-Sphk2 bound efficiently to immobilised sulfatide, but not to galactosylceramide (cerebroside) (Figure 1Bi). MBP-Sphk1 (Figure 1Bii) and MBP alone (data not shown) did not bind either sulfatide or cerebroside.
Figure 1. Sphk2 and Cerk bind sulfatide.
(A) Interaction of MBP-Cerk, MBP-Sphk1, and MBP-Sphk2 with lipids immobilised on Sphingostrip membranes. Identity of the lipid in each 100 nmole spot is indicated alongside the blots: S1P, sphingosine 1-phosphate; SPC, sphingosylphosphorylcholine; LPA, lysophosphatidic acid; GM1, monosialoganglioside type GM1; GD3, disialoganglioside type GD3; PC, phosphatidylcholine. No signal was observed with MBP control protein. (B) Binding of MBP-Sphk2 (i) or MBP-Sphk1 (ii), at 0.25 (○ and ▼) or 1 mg/mL (X and ■), to immobilised sulfatide (○ and X) or cerebroside (▼ and ■). (C) Binding of MBP fusion proteins (0.5 μg/mL) to wells coated with BSA only (open bars), 2 nmole sulfatide (closed bars), or 2 nmole cerebroside (hatched bars). Sphk2(1-99), Sphk2(1-175), and Sphk2(1-239) refer to the first 99, 175, and 239 residues of Sphk2, respectively, whilst Δ1-77, Δ1-99, and Δ1-172 indicate deletion of those residues. Results shown are mean and standard error of triplicate wells. (D) Domain structures of human Sphk1, Sphk2 and Cerk; numbers indicate primary amino acid sequence. C1–C5: sphingolipid kinase conserved domains; DAGK: diacylglycerol kinase active site.
To define the sulfatide binding region of Sphk2, we prepared a series of truncations from both the N- and C-termini of Sphk2. Deleting the first 99 residues eliminated binding to sulfatide (Figure 1C). The first 175 residues of Sphk2 [MBP-Sphk2(1-175)] bound strongly to sulfatide, but displayed non-specific binding to BSA-coated wells. Increasing the Sphk2 fragment to the first 239 residues restored specificity for sulfatide, indicating that the sulfatide binding domain localises to the N-terminus of Sphk2, upstream of the conserved diacylglycerol kinase active site (Figure 1C and D).
Sphk2 N-terminus binds phosphatidylinositol phosphates
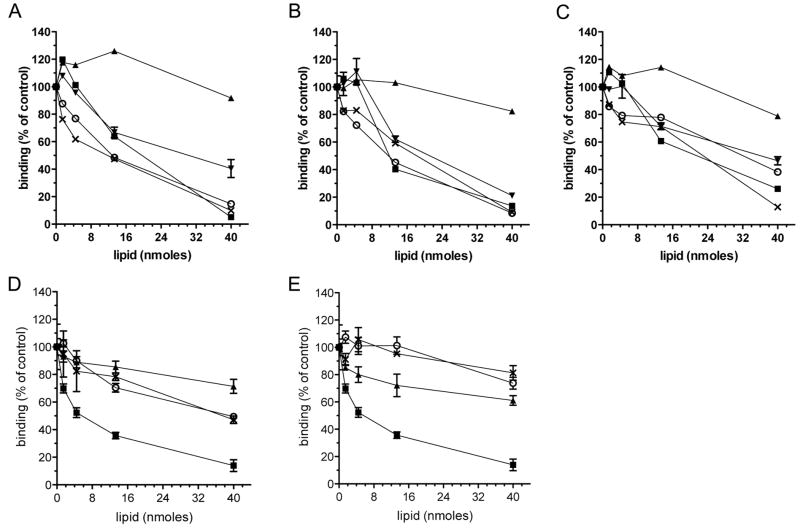
The N-terminal region of Cerk has been identified as a pleckstrin homology (PH) domain [18; 19] (Figure 1D). The PH domain is essential for Cerk activity, binding to phosphatidylinositol phosphates in vitro, and localisation to the golgi and plasma membranes. To determine whether the sulfatide binding region of Cerk and Sphk2 overlaps with a phosphatidylinositol phosphate binding site, we tested the capacity for soluble lipids to compete with immobilised sulfatide for binding to recombinant Sphk2 and Cerk. Soluble phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 4-phosphate, and phosphatidic acid were able to compete with sulfatide for binding to MBP-Sphk2 (Figure 2A) and MBP-Cerk (Figure 2B), as well as MBP-Sphk2(1-175) (Figure 2C), indicating that these lipids are competing for a common binding site within Cerk and the Sphk2 N-terminus. Abundant anionic cellular phospholipids - phosphatidylinositol, phosphatidylserine, and phosphatidylethanolamine - did not compete effectively with sulfatide for binding to Sphk2 (Figure 2D), nor did the anionic lipids cholesterol sulfate and ganglioside GM3 (Figure 2E).
Figure 2. Phosphoinositides and sulfatide compete for a common binding site in Sphk2 and Cerk.
Binding of (A) MBP-Sphk2, (B) MBP-Cerk, and (C) MBP-Sphk2(1-175), to immobilised sulfatide (1 nmole/well), with sulfatide (■), cerebroside (▲), phosphatidylinositol 4,5-bisphosphate (○), phosphatidylinositol 4-phosphate (▼), or phosphatidic acid (X) present during the enzyme binding step. Recombinant proteins were at 0.5 μg/mL. (D) Binding of MBP-Sphk2 to immobilised sulfatide, with competing sulfatide (■), phosphatidylserine (○), phosphatidylethanolamine (▲), or phosphatidylinositol (X) present during the enzyme binding step. (E) Binding of MBP-Sphk2 to immobilised sulfatide, with competing sulfatide (■), cholesterol sulfate (○), cholesterol (▲), or ganglioside GM3 (X) present during the enzyme binding step. Results are mean and error of triplicate assays, and represent two independent experiments.
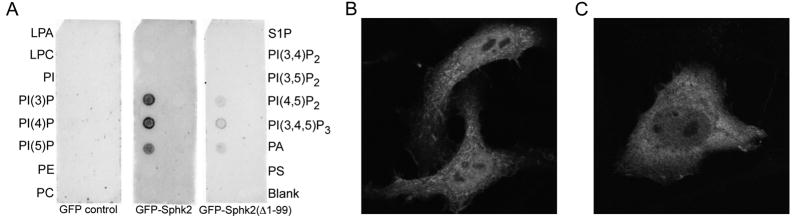
The specificity of the Sphk2 binding interaction with phosphatidylinositols was further investigated using PIP Strip membranes (Figure 3A). Cerk has been shown to bind multiple different phosphatidylinositols on PIP Strips [18; 19]. As MBP bound to several lipids on these membranes, we used lysates of HEK293 cells expressing GFP-tagged Sphk2. GFP alone did not bind to any lipids on the membrane, whilst GFP-Sphk2 bound specifically to the three phosphatidylinositol monophosphates. In agreement with our immobilised sulfatide binding assay, deleting the first 99 residues of Sphk2 greatly reduced binding to phosphatidylinositol phosphates. GFP-Sphk2(Δ1-172) was poorly expressed in HEK293 cells, and therefore unsuitable for this assay.
Figure 3. Sphk2 N-terminus binds to phosphoinositides.
(A) PIP Strip membranes were incubated overnight with lysates of HEK293 cells expressing GFP alone, GFP-Sphk2(WT), or GFP-Sphk2(Δ1-99), then blotted with rabbit anti-GFP. Lipid abbreviations not defined under Figure 1 are as follows: LPC, lysophosphatidylcholine; PI, phosphatidylinositol; PI(3)P, phosphatidylinositol 3-phosphate; PI(3,4)P, phosphatidylinositol 3,4-bisphosphate; PE, phosphatidylethanolamine; PA, phosphatidic acid; PS, phosphatidylserine. (B and C) Fluorescent confocal images, at 600X magnification, of HeLa cells transfected with (B) GFP-Sphk2(WT) or (C) GFP-Sphk2(Δ1-172).
Sphk2 N-terminus is required for membrane localisation
The lipid binding properties of the Sphk2 N-terminus suggest that this region may be important for the enzyme’s constitutive membrane localisation. In HeLa cells, GFP-Sphk2 was expressed in a punctate, reticular pattern, primarily on intracellular membranes, but also present in the nucleus (Figure 3B). Untagged Sphk2, detected with a polyclonal antibody to Sphk2, showed an identical localisation (not shown). GFP-Sphk2(Δ1-172) was distributed throughout the cytosol, with a much less punctate and less reticular pattern (Figure 3C), indicating that deletion of the N-terminal lipid binding domain adversely affects the association of Sphk2 with intracellular membranes.
Sulfatide inhibits Sphk2 and Cerk
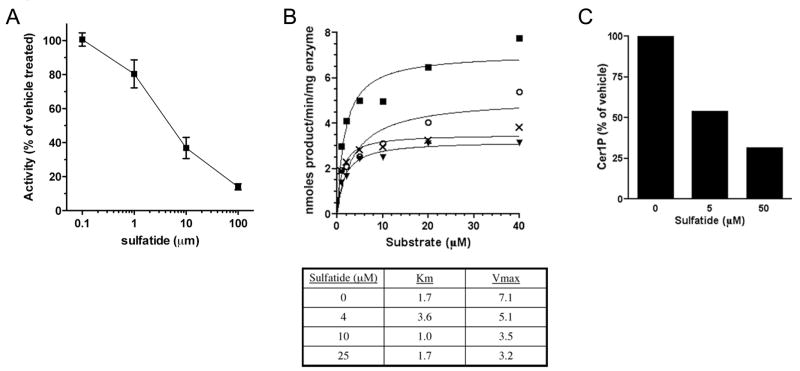
The strong interaction between sulfatide and Sphk2/Cerk suggested that sulfatide might inhibit these enzymes. Sulfatide inhibited Sphk2 with an IC50 of 10–13 μM (Figure 4A) and Cerk with an IC50 of 2.5–3.5 μM (based on 2 independent titrations for each enzyme; graph not shown for Cerk). Sulfatide did not inhibit Sphk1 at concentrations up to and including 100 μM. The MBP group on the recombinant proteins did not influence the interaction, since untagged Cerk and Sphk2 expressed in HEK293 cells were both inhibited by sulfatide. The sulfate group and long N-acyl chain of sulfatide were both required for inhibition, as galactosylceramide and C2-sulfatide did not inhibit either enzyme (Table 1). The inhibition was non-competitive with substrate (Figure 4B; data not shown for Cerk), providing further evidence that sulfatide binds these enzymes at a site that is remote from the active site. For these experiments Cerk activity was measured using the substrate C6-NBD ceramide [16]. We also tested for inhibition of Cerk using the natural substrate C18:0 ceramide, in a mixed micelle assay (Figure 6C). Phosphorylation of C18:0 ceramide was inhibited by sulfatide with an IC50 of approximately 5 μM (1.6% molar fraction of the micelles).
Figure 4. Sulfatide inhibits Sphk2 and Cerk.
(A) Recombinant Sphk2 activity as a function of brain sulfatide concentration. Activity was measured as described in experimental procedures. (B) Reaction rate as a function of substrate concentration for MBP-Sphk2, in the presence of 0 (■), 4 (○), 10 (X), or 25 (▼) μM sulfatide. Data points are the mean of two assays. Accompanying table shows Km and Vmax values calculated from the curves, using GraphPad PRISM software. Sulfatide produced a decrease in the enzyme turnover rate (Vmax), without a significant increase in substrate affinity (Km), indicative of non-competitive enzyme inhibition. (C) Inhibition of Cerk activity on C18:0 ceramide, by brain sulfatide incorporated into TritonX-100/cardiolipin/ceramide micelles at 5 or 50 μM (1.6% or 14.3% of micelle lipids). Results are the mean of two assays, and representative of two independent experiments.
Table 1. Activity of sphingosine and ceramide kinases in the presence of lipids.
Values are percentage activity, relative to vehicle control, for sphingosine or ceramide kinases in the presence of 10 μM test lipid. Vehicle was methanol for all compounds except cerebroside (DMSO vehicle).
| Lipid | Sphk2 | Sphk1 | Cerk |
|---|---|---|---|
| galactosylceramide 3-sulfate (sulfatide) | 37 | 156 | 36 |
| galactosylceramide (cerebroside) | 84 | 109 | 101 |
| C16 sulfatide | 47 | ND | 22 |
| C2 sulfatide | 160 | ND | 99 |
| cholesterol | 107 | ND | 109 |
| phosphatidylserine | 203 | ND | 65 |
| phosphatidic acid | 102 | 265 | 102 |
| phosphatidylinositol | 187 | ND | 62 |
| phosphatidylinositol 4-phosphate | 89 | 175 | 102 |
| phosphatidylinositol 4,5-bisphosphate | 60 | 99 | 94 |
ND: not determined. Results are the mean of triplicate assays.
Given their distribution throughout intracellular membranes, phosphoinositides are likely to be physiologically relevant binding partners for Sphk2. Accordingly, they did not inhibit Sphk2 or Cerk (Table 1). Nor were Sphk2 and Cerk inhibited by the common cellular phospholipids phosphatidylserine, phosphatidylinositol, cholesterol and phosphatidic acid. The inhibitory nature of sulfatide probably results from the greater electronegativity of the sulfate group, compared to the phosphate group of phosphatidylinositol phosphates. Binding of sulfatide to the N-terminus of Sphk2 or Cerk presumably locks these enzymes into a conformation that does not permit catalysis.
The inhibitory binding interaction between sulfatide and Sphk2 served as a useful tool for defining a lipid binding domain. At this stage we can not assign any physiological significance to the interaction between Sphk2 or Cerk and sulfatide. Sulfatide is formed by sulfation of galactosylceramide in the lumen of the golgi, then exported to the outer leaflet of the plasma membrane, where it plays an important role in cell adhesion and migration [20; 21]. It may also be secreted, and is a major lipid component of myelin [22]. The spatial separation of sulfatides from Cerk and Sphk2 would probably limit any inhibitory effects under normal circumstances. It would, however, be of great interest to the cell biology community to determine whether sulfatide also binds the PH domain of other enzymes, and whether this interaction disrupts enzyme activity, as sulfatide is found in the golgi and plasma membrane of many cell types. Defective lysosomal catabolism of sulfatide, resulting from a deficiency in arylsulfatase A, gives rise to the lysosomal storage disease, Metachromatic Leukodystrophy [23]. In this and other situations involving the excessive accumulation of sulfatide, there may be inhibition and altered sub-cellular localisation of Sphk2, Cerk, and related enzymes.
In summary, this is the first study to identify a lipid binding domain in Sphk2 (independent of the active site), and to describe the lipid binding properties of this enzyme that influence its membrane association in cells. Specific interactions with membrane lipids are likely to be critical for Sphk2 localisation, as the enzyme is not thought to contain any transmembrane helices, and localises to both membranes and cytosol. Studies are ongoing to define elements of predicted secondary structure and specific amino acids that create the N-terminal lipid binding domain in Sphk2, and to determine the role for this domain in the control of S1P synthesis.
Acknowledgments
This work was supported by the National Institutes of Health (AI055509 and NIMH-074404 for H.R.), a grant from Kyorin Pharmaceutical Company, and a CJ Martin fellowship from the National Health and Medical Research Council of Australia (A.S.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH., Jr Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–84. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–9. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 3.Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev. 2008;223:221–35. doi: 10.1111/j.1600-065X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 4.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–21. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–9. [PubMed] [Google Scholar]
- 7.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–29. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–25. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–6. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–9. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 11.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–9. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 12.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–25. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 13.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, Dent P, Milstien S, Spiegel S. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–74. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 15.Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem. 2007;282:15833–42. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- 16.Don AS, Rosen H. A fluorescent plate reader assay for ceramide kinase. Anal Biochem. 2008;375:265–71. doi: 10.1016/j.ab.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesinghe DS, Massiello A, Subramanian P, Szulc Z, Bielawska A, Chalfant CE. Substrate specificity of human ceramide kinase. J Lipid Res. 2005;46:2706–16. doi: 10.1194/jlr.M500313-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Rovina P, Jaritz M, Hofinger S, Graf C, Devay P, Billich A, Baumruker T, Bornancin F. A critical beta6-beta7 loop in the pleckstrin homology domain of ceramide kinase. Biochem J. 2006;400:255–65. doi: 10.1042/BJ20060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TJ, Mitsutake S, Igarashi Y. The interaction between the pleckstrin homology domain of ceramide kinase and phosphatidylinositol 4,5-bisphosphate regulates the plasma membrane targeting and ceramide 1-phosphate levels. Biochem Biophys Res Commun. 2006;342:611–7. doi: 10.1016/j.bbrc.2006.01.170. [DOI] [PubMed] [Google Scholar]
- 20.Merten M, Beythien C, Gutensohn K, Kuhnl P, Meinertz T, Thiagarajan P. Sulfatides activate platelets through P-selectin and enhance platelet and platelet-leukocyte aggregation. Arterioscler Thromb Vasc Biol. 2005;25:258–63. doi: 10.1161/01.ATV.0000149675.83552.83. [DOI] [PubMed] [Google Scholar]
- 21.Aruffo A, Kolanus W, Walz G, Fredman P, Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991;67:35–44. doi: 10.1016/0092-8674(91)90570-o. [DOI] [PubMed] [Google Scholar]
- 22.Svennerholm L, Bostrom K, Fredman P, Jungbjer B, Mansson JE, Rynmark BM. Membrane lipids of human peripheral nerve and spinal cord. Biochim Biophys Acta. 1992;1128:1–7. doi: 10.1016/0005-2760(92)90250-y. [DOI] [PubMed] [Google Scholar]
- 23.von Figura K, Gieselmann V, Jaeken J. Metachromatic leukodystrophy: Lysosomal disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3695–3724. [Google Scholar]