Abstract

Treatment of γ,δ-unsaturated ketones with hydrogen peroxide and acid provides a rapid entry into the medicinally important 1,2,4-trioxane structure. Alkene substitution that stabilizes carbocationic intermediates proved to be important for the success of this transformation.
The 1,2,4-trioxane moiety of the sesquiterpene artemisinin (1, Figure 1) is considered to be an important component of the potent anti-malarial activity of this natural product.1 Artemisinin and its semi-synthetic derivatives are some of the most successful drugs for the treatment of malaria.2 Despite the effectiveness of artemisinin, malaria is still a worldwide epidemic responsible for millions of deaths annually, and strains of the Plasmodium falciparum parasite are growing increasingly resistant to older drug therapies.3 Development of new syntheses of organic peroxides could address many challenges associated with malaria treatment, such as drug resistance and availability.4 In addition to anti-malarial properties, organic peroxides, such as artemisinin, have notable activity against tumor cells5 and viruses like HIV6 and hepatitus B.7
Figure 1.
1,2,4-trioxanes.
In this Letter, we describe the efficient synthesis of 1,2,4-trioxanes 3 from simple γ,δ-enones 2 in one synthetic operation, without isolation of intermediates (Figure 2). This procedure complements the multi-step methods reported by Wu8 and Griesbeck9 because it enables access to 1,2,4-trioxanes with different substitution patterns.
Figure 2.
Synthesis of 1,2,4-trioxanes from γ,δ-unsaturated ketones
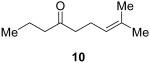
The synthesis of 1,2,4-trioxanes 3 was discovered when we attempted to form geminal-dihydroperoxides from unsaturated ketones 2 (Figure 2). Treatment of γ,δ-unsaturated ketones with acidic hydrogen peroxide10,11,12,13 solutions gave the trioxane 3 and two identifiable decomposition products: peroxide oligomers14 and Baeyer-Villiger oxidation products.15,16 Oligomerization was decreased by slow addition of the γ,δ-unsaturated ketone 2 into the reaction mixture. Lowering the reaction temperature reduced the amount of Baeyer-Villiger oxidation observed for a number of substrates.17 Finally, the addition of excess acid made a marked improvement in the efficiency of the reaction. For example, the yield of 1,2,4-trioxane 5 from γ,δ-unsaturated ketone 4 could be improved from 8% to 53% by making slight adjustments to the reaction conditions (Table 1).
Table 1.
Optimization of rearrangement.
 | ||
|---|---|---|
| entry | conditions | Yield of 5a |
| 1 | CF3CO2H (12 equiv), H2O2 (8 equiv), CH2Cl2, 23 °C | 8% |
| 2 | CF3CO2H (2 equiv), H2SO4 (2 equiv), 50% H2O2 (10 equiv), CH2Cl2, 0 °C | 53% |
Yield based on purified reaction mixtures.
The formation of 1,2,4-trioxanes occured most efficiently with acylic aliphatic γ,δ-enones with trisubstituted alkenes. Ketoalkenes with short alkyl side-chains underwent the transformation most effectively, as shown by the formation of trioxanes 7, 9, and 11 (Table 2, entries 1–3). The synthesis of trioxanes 13 and 15 demonstrated that increasing the chain length and introduction of functional groups resulted in longer reaction times and decreased yields (Table 2, entries 4 and 5). If two different alkenes were present, only the more nucleophilic alkene18 was oxidized (Table 2, entry 4). Functionalized alkenes were also tolerated (Table 2, entry 6).
Table 2.
Synthesis of 1,2,4-trioxanes from γ, δ-enones: substrate scope.
Based on purified reaction mixtures.
As determined by 1H NMR spectroscopic analysis of the product relative to CH2C12.
90:10 mixture of E/Z alkenes.
3:1 mixture of diastereomers.
Attempts to extend the scope of the rearrangement to to form trioxabicyclo[3.3.1]nonane 19 failed, instead leading to dioxabicyclo[3.2.1]octane 20 (eq 1). Others have encountered difficulties in forming trioxabicyclo[3.3.1]nonanes.8c
 |
(1) |
Structural assignment of the products from these reactions required careful analysis (Scheme 1). Without authentic samples of structures 7 and 21, both structures might be considered to be consistent with the 1H NMR spectra. Two-dimensional NMR spectroscopy, however, could differentiate between these structures. A cross-peak between the bridgehead proton and acetal carbon was observed when the HMBC experiment was optimized for 1H-13C coupling constants of 10 Hz. This result indicated that the structure of the rearrangement was the trioxabicyclo[3.2.1]octane 7, and not trioxabicyclo[2.2.2]octane 21. The proposed structure was later confirmed by X-ray crystallography.19
Scheme 1.
Two potential products.
For the formation of 1,2,4-trioxane 3, we propose the mechanism outlined in Scheme 2. Addition of hydrogen peroxide to the carbonyl group,13,20 followed by in situ epoxidation by trifluoroacetic peracid,21 would afford a mixture of epoxides 22 and 23. Cyclization of the hydroxyl group of hemi-peroxyketal 23 would provide intermediate tetrahydrofuran 24.22 Cyclization to give tetrahydrofuran 24 could also occur by hydrolysis of epoxide 22 or 23 to form a diol (not shown) followed by hemi-peroxyketalization.23 The highly acidic conditions in the reaction mixture could promote the formation of cis and trans tertiary carbocations 25 and 26. Ring closure to provide the 1,2,4-trioxane 3 occurs when the tertiary carbocation is generated and is in a cis relationship (intermediate 25) with the anomeric hydroperoxide. Ring closure to afford the bicyclic 1,2,4-trioxane would not be expected to occur if the hydroperoxide and carbocation reside in a trans relationship, as seen in intermediate 26.8c
Scheme 2.
Proposed mechanism for 1,2,4-trioxane formation.
To test the viability of the suggested mechanism, intermediates were synthesized independently and subjected to the reaction conditions. Epoxy ketal 28 was obtained by treatment of ketoalkene 6 with aqueous hydrogen peroxide and cerium ammonium nitrate (CAN)24 followed by epoxidation with dimethyldioxirane (Scheme 3).25 Exposure of epoxy ketal 28 to acid with or without hydrogen peroxide produced trioxane 7.19 These results show that geminal-dihydroperoxides like 22 (Scheme 2) are competent intermediates in the formation of trioxanes 3.
Scheme 3.
Synthesis of proposed intermediates.
The hydroperoxy ketal portion of the intermediates was necessary for the synthesis of 1,2,4-trioxanes. When the epoxide 2926 was subjected to the reaction conditions, none of the desired trioxane 7 was observed (Scheme 3). This result indicates that formation of the peroxy ketal or hemi-ketal (e.g., 22 or 23, Scheme 2) before epoxidation is necessary for production of 1,2,4-trioxanes 3.
In addition to synthesizing hypothesized intermediates and transforming them to 1,2,4-trioxanes, some products have been isolated from reaction mixtures that are consistent with the proposed mechanism. Treatment of epoxide 30 to the reaction conditions provided primarily decomposition. Protonation with a weaker Brønsted acid yielded the diastereomerically pure tetrahydrofuran 31 (eq 2), a product that is structurally related to intermediate 24 (Scheme 2).19 The isolation of the spirocyclic product 31 with an anomeric hydroperoxide suggests the formation of the tetrahydrofuran ring occurs prior to carbocation formation. The weaker acid is not likely to generate a tertiary carbocation (e.g., 25 or 26, Scheme 2), so the reaction stops at the tetrahydrofuran stage.
 |
(2) |
The presence of a carbocationic intermediate was probed with a substrate bearing a 1,2-disubstituted styryl group.27 The product ratio of the resulting trioxanes 34a/b was independent of the diastereomeric ratio of the starting alkene 32 (Table 3). The observation that the stereochemical integrity of the alkene was not maintained in the product is consistent with carbocationic intermediates.28
Table 3.
Synthesis of 1,2,4-trioxanes 34a/b from a 1,2-disubstituted alkene.a
 | ||||
|---|---|---|---|---|
| entry | substrate | E/Z ratio | overall yield of 34 | ratio 34a/34b |
| 1 | 32 | 64:36 | 21% | 86:14b |
| 2 | 32 | 98:2 | 46% | 90:10c |
Reagents and conditions: (a) H2O2 (50%), CF3CO2H, H2SO4, 0 °C, 24 h.
As determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture.
Ratio as determined by isolated yield.
In conclusion, the reaction of trisubstituted γ,δ-unsaturated ketones with acidic hydrogen peroxide solutions afforded 1,2,4-trioxanes efficiently. This reaction is believed to occur by an in situ epoxidation, hemi-peroxyketalization, and ring closure onto a carbocationic intermediate. The stabilization of this carbocation (25, Scheme 2) is a critical feature for the success of this transformation.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (GM61006) and the National Science Foundation (CHE-0135572). K. A. W. thanks Amgen and Eli Lilly for awards to support research. We thank Dr. Joseph Ziller (UCI) for X-ray crystallographic data, Dr. Phil Dennison (UCI) for assistance with NMR spectrometry, and Dr. John Greaves and Ms. Shirin Sorooshian (UCI) for mass spectrometry.
Footnotes
Supporting Information Available Complete experimental procedures and product characterization (PDF and CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Haynes RK, Vonwiller SC. Acc Chem Res. 1997;30:73–79. [Google Scholar]; b Jefford CW. Curr Med Chem. 2001;8:1803–1826. doi: 10.2174/0929867013371608. [DOI] [PubMed] [Google Scholar]; c Wu Y. Acc Chem Res. 2002;35:255–259. doi: 10.1021/ar000080b. [DOI] [PubMed] [Google Scholar]; d Dembitsky VM. Eur J Med Chem. 2008;43:223–251. doi: 10.1016/j.ejmech.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 2.a Klayman DL. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]; b Hien TT, White NJ. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]; c Bhattacharya AK, Sharma RP. Heterocycles. 1999;51:1681–1745. [Google Scholar]; d Dhingra V, Rao KV, Narasu ML. Life Sci. 2000;66:279–300. doi: 10.1016/s0024-3205(99)00356-2. [DOI] [PubMed] [Google Scholar]; e Woodrow CJ, Haynes RK, Krishna S. Postgrad Med J. 2005;81:71–78. doi: 10.1136/pgmj.2004.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Sachs J, Palaney P. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]; b Whitty CJM, Rowland M, Sanderson F, Mutabingwa TK. Brit Med J. 2002;325:1221–1224. doi: 10.1136/bmj.325.7374.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Barnes KI, White NJ. Acta Trop. 2005;94:230–240. doi: 10.1016/j.actatropica.2005.04.014. [DOI] [PubMed] [Google Scholar]; d Woodrow CJ, Krishna S. Cell Mol Life Sci. 2006;63:1586–1596. doi: 10.1007/s00018-006-6071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a McCullough KJ, Nojima M. Curr Org Chem. 2001;5:601–636. [Google Scholar]; b Jefford CW. Curr Opin Invest Drugs. 2004;5:866–872. [PubMed] [Google Scholar]; c Tang Y, Dong Y, Vennerstrom JL. Med Res Rev. 2004;24:425–448. doi: 10.1002/med.10066. [DOI] [PubMed] [Google Scholar]
- 5.a Efferth T. Drug Resist Updates. 2005;8:85–97. doi: 10.1016/j.drup.2005.04.003. [DOI] [PubMed] [Google Scholar]; b Jansen FH, Soomro SA. Curr Med Chem. 2007;12:3243–3259. doi: 10.2174/092986707782793844. [DOI] [PubMed] [Google Scholar]
- 6.Jung M, Schinazi RF. Bioorg Med Chem Lett. 1994;4:931–934. [Google Scholar]
- 7.Romero MR, Efferth T, Serrano MA, Castaño B, Macias RIR, Briz O, Marin JJG. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.a Jin HX, Liu HH, Wu Y. J Org Chem. 2005;70:4240–4247. doi: 10.1021/jo050139y. [DOI] [PubMed] [Google Scholar]; b Zhang Q, Jin HX, Wu Y. Tetrahedron. 2006;62:11627–11634. [Google Scholar]; c Zhang Q, Wu Y. Tetrahedron. 2007;63:10189–10201. [Google Scholar]
- 9.Griesbeck AG, Blunk D, El-Idressey TT, Raabe A. Angew Chem Int Ed. 2007;46:8883–8886. doi: 10.1002/anie.200701397. [DOI] [PubMed] [Google Scholar]
- 10.Kharasch MS, Sosnovsky G. J Org Chem. 1958;23:1322–1326. and references cited therein. [Google Scholar]
- 11.a Milas NA, Golubaović A. J Am Chem Soc. 1959;81:6461–6462. [Google Scholar]; b Milas NA, Golubaović A. J Org Chem. 1962;27:4119–4323. [Google Scholar]
- 12.Tsuchiya K, Hamada Y, Masuyama A, Nojima M, McCullough KJ, Kim HS, Shibata Y, Wataya Y. Tetrahedron Lett. 1999;40:4077–4080. [Google Scholar]
- 13.Ramirez A, Woerpel KA. Org Lett. 2005;7:4617–4620. doi: 10.1021/ol051703u. [DOI] [PubMed] [Google Scholar]
- 14.Peroxide oligomers are potentially hazardous and explosive: Zabicky J. In: The Chemistry of Peroxides. Rappoport Z, editor. Vol. 2. Wiley; Chchester, UK: 2006. pp. 597–774. Chapter 7.No difficulties were encountered in the handling or purification of these compounds on small scale. Details are provided in the Supporting Information.
- 15.Treatment of carbonyl compounds with acidic hydrogen peroxide or its derivatives yeilds peroxide oligomers and Baeyer–Villiger oxidation products: Renz M, Meunier B. Eur J Org Chem. 1999:737–750.and references cited therein.
- 16.Krow GR. Org React. 1993;43:251–798. [Google Scholar]
- 17.a Ruzicka L, Stoll M. Helv Chim Acta. 1928;11:1159–1173. doi: 10.1002/hlca.19480310426. [DOI] [PubMed] [Google Scholar]; b Robinson R, Smith LH. J Chem Soc. 1937:371–374. [Google Scholar]
- 18.Mayr H, Kempf B, Ofial AR. Acc Chem Res. 2003;36:66–77. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]
- 19.Details are provided in the Supporting Information.
- 20.Žmitek K, Zupan M, Iskra J. Org Biomol Chem. 2007;5:3895–3908. doi: 10.1039/b711647k. and references therein. [DOI] [PubMed] [Google Scholar]
- 21.a Emmons WD, Ferris AF. J Am Chem Soc. 1953;75:4623–2624. [Google Scholar]; b Emmons WD, Pagano AS. J Am Chem Soc. 1955;77:89–92. [Google Scholar]
- 22.a Cyclization of the 1,2-dibromo alkane in a related system has been reported: Holum JR, Jorenby D, Mattison P. J Org Chem. 1964;29:769–776.Wasserman HH, Barber EH. J Am Chem Soc. 1969;91:3674–3675.Vanderwel D, Oehlschlager AC. J Am Chem Soc. 1992;114:5081–5086.
- 23.a Gaoni Y. J Chem Soc C. 1968:2925–2934. [Google Scholar]; b Ishibashi H, Uehara C, Komatsu H, Ikeda M. Chem Pharm Bull. 1987;35:2750–2754. [Google Scholar]; c Brimble MA, Rowan DD, Spicer JA. Synthesis. 1995:1263–1266. [Google Scholar]
- 24.Das B, Krishnaiah M, Veeranjaneyulu B, Ravikanth B. Tetrahedron Lett. 2007;48:6286–6289. [Google Scholar]
- 25.Reaction conditions have not been optimized.
- 26.Ceruti M, Balliano G, Viola F, Grosa G, Rocco F, Cattel L. J Med Chem. 1992;35:3050–3058. doi: 10.1021/jm00094a020. [DOI] [PubMed] [Google Scholar]
- 27.In other experiments, 1,2-disubstituted alkenes without the phenyl group did not form the desired 1,2,4-trioxanes. It appears that the additional stability of the proposed carbocationic intermediate 33 (Table 3) conferred by the phenyl substituent allowed the product to form from a disubstituted alkene.
- 28.Loss of stereochemistry was also observed in trisubstituted alkene substrates (Table 2, entry 6).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.