Abstract
Background
Numerous clinical and experimental studies support the hypothesis that the intrauterine environment is an important determinant of cardiovascular disease and hypertension.
Objective
This review examines the mechanisms linking an adverse fetal environment and increased risk for chronic adult disease, with an emphasis on gender differences and the role of sex hormones in mediating sexual dimorphism in response to a sub-optimal fetal environment.
Methods
This is a selective review that focuses on current findings regarding sex differences in fetal programming and the mechanisms involved in the fetal programming of cardiovascular disease and hypertension.
Results
The mechanisms involved in the fetal programming of adult disease are multifactorial and involve alterations in the regulatory systems involved in the long-term control of arterial pressure. Sex differences are observed in animal models of fetal programming and recent studies suggests sex hormones modulate activity of regulatory systems leading to a lower incidence of hypertension and vascular dysfunction in females compared to males.
Conclusions
Animal models of fetal programming demonstrate that female offspring are protected from the adverse effects of fetal insult, and are providing insight into the mechanisms by which sex hormones contribute to sexual dimorphism in adult disease.
Keywords: fetal programming, sex differences, testosterone, estrogen, blood pressure
INTRODUCTION TO FETAL PROGRAMMING
Hypertension is a prevalent disorder estimated to affect about 25% of the world's adult population (1, 2). The cause of elevated blood pressure in the majority of the population is unknown; however, common risk factors include: sex, diet, ethnicity and obesity (1, 2). Cardiovascular disease (CVD) continues to be the major cause of human death worldwide and the incidence and prevalence of chronic kidney disease (CKD) is also increasing (3). Hypertension and type II diabetes mellitus (DM) are the two major causes of CKD worldwide and also contribute to death from CVD (3). Despite intense research in the field, the etiology of these common diseases remains unknown. Environmental and genetic factors have been considered the underlying cause of these diseases. However, hypertension frequently coexists with type 2 DM, as well as dyslipidemia, insulin resistance, and CKD, which may reflect a common underlying mechanism (3). One such mechanism may be related to intrauterine environment.
As hypothesized by Barker, “the fetal origins of adult disease” proposes that cardiovascular and related disorders derive from fetal adaptations to maternal undernutrition (4). This hypothesis, referred to as “fetal programming,” suggests that an adverse environmental stimulus experienced during a critical period of fetal development induces long-term structural and functional effects in the developing organism predisposing it to increased risk for development of hypertension and CVD (4). Programming is a consequence of developmental plasticity in the fetus whereby a variety of different phenotypes result from a background of a single genotype in response to a sub-optimal environment during intrauterine life (5). These phenomena are intimately linked and have far reaching implications if one considers that their effects can be perpetuated across generations (6).
Barker postulated his theory based on the correlation between birth weight and increased risk for coronary heart disease (5). Numerous epidemiological studies focused on diverse populations from various parts of the world have substantiated these initial findings and expanded them to include increased susceptibility to a number of other pathophysiological conditions such as impaired glucose tolerance, type II DM, obesity, stroke, high blood pressure and non-insulin-dependent DM (7). By far, low birth weight (LBW) which occurs when a fetus fails to achieve its genetically determined growth potential (8), and subsequent hypertension has been the most studied. Although the mechanistic pathways underlying this association remain unclear, investigators using animal models to induce a sub-optimal fetal environment are providing critical support for the fetal programming hypothesis by demonstrating that exposure to adverse conditions in utero results in offspring with marked adaptive responses and adverse adult outcomes.
METHODS
This selective review focuses on the current findings regarding mechanisms that mediate an adverse adult outcome in response to a suboptimal fetal environment, with an emphasis on the role of sex hormones and sexual dimorphism in the response to fetal programming of adult disease.
SEX DIFFERENCES IN FETAL PROGRAMMING: ANIMAL STUDIES
Numerous investigators use different animal models to induce adverse fetal conditions in order to mimic the pathophysiological conditions linked to slow fetal growth and the subsequent increased risk for adult disease in humans. Different animal models include dietary undernutrition induced by global food restriction (9, 10) or protein restriction during gestation (11, 12, 13, 14, 15), maternal hypoxia during gestation (16, 17), placental insufficiency (18, 19, 20, 21, 22), or prenatal glucocorticoid treatment (23, 24).
Numerous animal models of fetal programming exhibit sex differences in the response to a prenatal insult, with the adaptive response dependent on the timing and severity of the insult. Maternal undernutrition during the preimplantation period in the rat leads to increased blood pressure in male offspring only (25). These authors suggest that during preimplantation, male embryos have a greater capacity to respond to the maternal environment and may, as a consequence, exhibit enhanced sensitivity to specific programming influences. Female offspring appear to be protected from or exhibit reduced sensitivity to a moderate insult during development. Moderate global dietary restriction during gestation in the rat leads to gender-related hypertension with young male offspring developing increased blood pressure that is more pronounced and develops earlier than female offspring (26). Male offspring in this model also demonstrate abnormalities in vascular function in isolated peripheral arteries (26). Blood pressure is also significantly higher in males than females in response to pharmacological levels of dexamethasone administered during early gestation in sheep (27, 28). However, moderate protein restriction during gestation in the rat leads to marked increases in blood pressure in male offspring associated with a reduced nephron number (29). Only a more severe nutritional insult leads to hypertension and changes in renal structure in both male and female offspring (29). A similar pattern of response is observed in models induced by variations in sodium intake during pregnancy and lactation (30). Moderate sodium intake leads to hypertension in male, but not female offspring; whereas high sodium intake during the same window of development programs male and female offspring (30). Hypoxia during gestation in the rat results in altered vascular function in male, but not female offspring (16). Thus, animal models of fetal programming indicate that male offspring are more sensitive to insults during development; and that female offspring are less sensitive, or protected against development of adult disease in response to fetal insult. Interestingly, females appear to be protected against deficiencies in nutrients, oxygen, or both. However exposure to an excess intake of dietary fat, such as lard, is associated with endothelial dysfunction in both male and female offspring, but hypertension in female offspring, (31). Recent studies from our laboratory suggest that sex hormones may contribute to sex differences that occur in response to fetal insult (32, 33). Utilizing a model of fetal programming induced by placental insufficiency during late gestation in the rat, we observe marked increases in blood pressure in both pre-pubertal male and female growth restricted offspring (18). However, after puberty, only male growth restricted offspring remain hypertensive whereas female growth restricted offspring normalize their blood pressure (18, 32, 33). Castration abolishes hypertension in adult male growth restricted offspring in this model (32); ovariectomy induces hypertension in adult female growth restricted offspring (33). Thus, sex hormones may contribute to sexual dimorphism in this model of fetal programming of adult disease by modulating regulatory pathways important in the long-term control of blood pressure.
MECHANISMS OF FETAL PROGRAMMING
Birth weight, nephron number and glomerular size
The kidney is a central organ involved in hypertension, by mechanisms such as sodium handling, intravascular fluid volume homeostasis, as well as intrinsic renal disease (34). In many animal models, LBW is associated with a deficit in nephron number which is suggested to predispose the development of hypertension by decreasing sodium excretion (35). Total nephron number is a biological variable that is defined in humans prior to birth. Approximately 60% of the nephrons develop during the third trimester of pregnancy (36) with no new nephrons formed after birth (36). Additionally, a reduction in the number of nephrons at birth may also be associated with a diminished resistance to mechanisms of renal damage in adult life.
In humans, Brenner (35) proposes that intrauterine growth restriction (IUGR) may be associated with impaired nephrogenesis, resulting in reduced nephron number, leading to a subsequent increase in blood pressure and progressive deterioration of renal function. Many human studies demonstrate that IUGR is associated with a significant reduction in nephron number (37, 38, 39). Human studies show there are about 1 million nephrons on average in each normal kidney, with large variations depending on the counting methodology used (40, 41, 42). Although numerous studies suggest an association between birth weight and nephron number, there are also studies that suggest that reduced nephron number may not always be associated with IUGR and impaired nephrogenesis (40). The median number of glomeruli in patients with hypertension is about 700,000 per kidney compared with about 1.4 million for matched controls. Although the number of glomeruli in hypertensive patients is about half that seen in controls, it is within the normal variable observed for a full-term human fetuses (41, 42). Thus, considerable variability is observed in the total nephron number within the human population. However, despite the large variation in nephron numbers observed in these studies, a consistent observation had emerged; glomerular volume varies inversely with glomerular number. These findings suggest that larger glomeruli may be a sign of compensatory hyperfiltration and hypertrophy in subjects with fewer nephrons (43, 44). Total glomerular volume, which represents total filtration surface area, is not different among groups with different nephron numbers and birth weights (45). This observation suggests that total filtration surface area may initially be maintained, but with compensatory glomerular hypertrophy, may lead to progressive deterioration of renal function in time (46).
A marked reduction in nephron number is a common adaptive outcome observed in response to fetal insult in animal models of fetal programming. Nephron number is reduced in models induced by nutrimental insult in the rat (13, 14, 15), sheep (12), or microswine (47), and in models induced by placental insufficiency in the rat (21) and rabbit (19). Placental insufficiency in the rat leads to marked reductions in nephron number associated with significant increases in renal apoptosis and expression of key apoptosis genes and in the IUGR offspring (48). Thus, fetal insult may lead to renal apoptosis contributing to reduced nephron number.
Animal models of fetal programming demonstrate that timing and severity of the fetal insult is critical to nephron complement. Nutritional insults initiated prior to nephrogenesis in the rat do not result in changes in nephron number or hypertension (13, 49). Reductions in nephron number occur only when the nutritional insult occurs during the nephrogenic period suggesting that timing of the insult is critical to programming of adult disease. Severity of the nutritional insult also alters the adaptive response. Moderate nutrimental insult leads to reduced nephron number in male offspring only (29). Only a more severe nutritional insult leads to reduced nephron number in male and female offspring of protein restricted dams (29). To summarize, a reduction in nephron number is observed in LBW humans and in many animal models of fetal programming suggesting that an adverse fetal environment during a critical period of fetal development results in permanent structural alterations in renal structure.
Vascular reactivity
Vascular endothelial dysfunction plays an important role in the development of hypertension and CVD (50). LBW in human is associated with vascular dysfunction (51), an observation supported by animal models of fetal programming (16, 26, 52, 53). Thus, fetal programming contributes to both structural and physiological changes in the vasculature which may contribute to increased risk for development of CVD associated with LBW.
Numerous models of fetal programming exhibit sex differences in the vascular response to fetal insult. Although decreased acetylcholine-induced relaxation is observed in both male and female offspring from nutrient restricted dams, vascular abnormalities are more pronounced in males than in females (26, 52, 53). Endothelial nitric oxide synthase activity, but not gene expression of eNOS, is reduced in female offspring from undernourished dams (52). Estrogen levels are reduced in these female offspring suggesting that the vasoprotective role of the estrogen on the vascular responses is lost in females subjected to fetal undernutrition. Thus, reduced nitric oxide bioavailability may contribute to altered vascular function and sex hormones may contribute to sex differences in the programming response of vascular function.
The renin angiotensin system
The renin angiotensin system (RAS) plays a major role in the control of blood pressure and body fluid volume through both systemic and intrarenal actions (54, 55). Components of the RAS are highly expressed in the developing kidney (56). A critical role for the RAS in mediating proper nephrogenesis is suggested in studies whereby angiotensin type I receptor (AT1R) blockade during the nephrogenic period after birth in the rat leads to a decrease in nephron number associated with a reduction in renal function and an increase in arterial pressure (57). Numerous investigators have examined the role of the RAS in the etiology of reduced nephron number and hypertension in models of fetal programming (12, 15, 32, 33, 58, 59). Reduced nephron number that occurs in response to gestational protein restriction is associated with marked reductions in renal renin mRNA and tissue ANG II levels in the offspring at birth (15). Thus, suppression of the RAS during fetal development may play a key role in mediating the structural and physiological changes observed in models of fetal programming. Marked reductions in renal renin mRNA are also observed at birth in models of fetal programming induced by placental insufficiency in both the rat (60) and sheep (12) suggesting that different fetal insults lead to common alterations in the RAS.
Sex specific alterations in the renal RAS may contribute to sex differences in the fetal programming of nephron number (15, 60). Suppression of the RAS is observed at birth in male, but not female offspring from moderately protein restricted dams (29). Importanlty, only male offspring demonstrate reduced nephron number and hypertension in this model (29). Therefore, moderate gestational undernutrition may lead to sex specific programming effects on the renal RAS which contributes to sex differences in nephron number and the development of programmed hypertension.
Temporal alterations in the RAS are also observed in models of fetal programming (58, 60). Plasma renin activity (PRA) remains significantly reduced in young rats from protein restricted dams prior to the development of hypertension (58). However, PRA becomes significantly increased after the establishment of hypertension (58). ACE inhibition abolishes hypertension in young (59) and adult (58) offspring of protein restricted dams suggesting that RAS played a critical role in the etiology of hypertension programmed by undernutrition in utero. ACE inhibition also abolishes hypertension in young (60) and adult male offspring (31) in a model of placental insufficiency in the rat suggesting similar mechanisms mediate hypertension programmed by in utero insult. Therefore, evidence from these studies suggests that a reduction in nephron number induced by fetal insult is due to suppression of the fetal RAS. Additionally, later inappropriate activation of the RAS, or increased sensitivity to angiotensin II, contributes to the etiology of fetal programmed hypertension.
SEX HORMONES IN FETAL PROGRAMMING
Role of testosterone
In men, testosterone has an inverse relationship to systolic blood pressure (61), an observation strongly supported by numerous studies in animal models of hypertension. Castration of male spontaneously hypertensive rats (SHR) or Dahl salt-sensitive rats at a young age (3 to 5 weeks) delays the development of hypertension (62). Treatment of castrated male and ovariectomized female SHR with testosterone exacerbates their hypertension (62). Furthermore, chronic blockade of the androgen receptor with the antagonist flutamide attenuates blood pressure in male SHR to lower levels than those found in female SHR (63). Thus, testosterone is suggested to play an important mechanistic role in blood pressure control and may contribute to sex differences in human essential hypertension and also in animal models of hypertension.
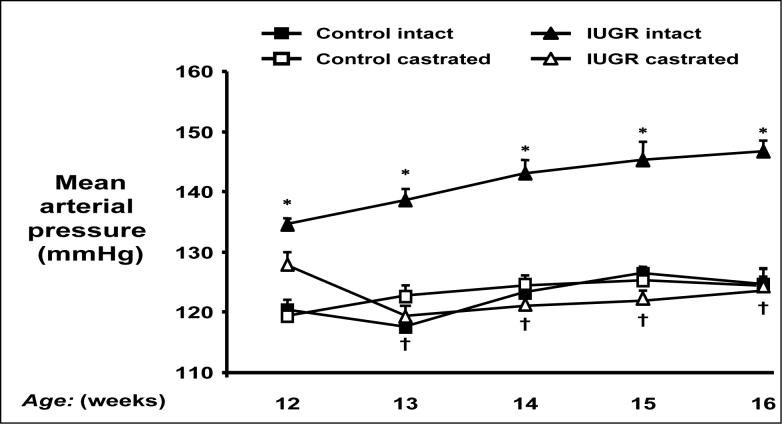
Testosterone appears to contribute to sex differences in models of hypertension programmed by in utero insult. Our laboratory reports that testosterone levels are significantly elevated in hypertensive adult male growth restricted offspring in a model of fetal programming induced by placental insufficiency in the rat (32). Castration abolishes hypertension in adult male growth restricted offspring suggesting that testosterone contributes to elevations in blood pressure in the adult animal (Figure 1) (32). The mechanism by which testosterone contributes to hypertension in adult male growth restricted offspring may involve the RAS, a regulatory systems important in sodium and water homeostasis and arterial pressure control (54, 55). We report that renal angiotensinogen mRNA is elevated in adult, but not pre-pubertal male growth restricted offspring (60). Other investigators demonstrate that angiotensinogen mRNA expression in kidney and liver is testosterone dependent (64, 65). Since hypertension in adult male growth restricted offspring can be abolished by chronic ACE inhibition (60), testosterone may serve as a stimulus to enhance intrarenal angiotensinogen in adult male growth restricted offspring, thus exacerbating the increase in blood pressure in adulthood.
Figure 1.
Castration abolishes hypertension in male intrauterine growth restricted (IUGR) offspring. Mean arterial pressure was measured by radio telemetry from 12 to 16 weeks of age in conscious, free moving animals that underwent either sham (intact) or castration (CTX) at 10 weeks of age. * P<0.05 vs. Control intact, † P<0.05 vs. IUGR intact. Used with permission from reference 32; Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R758−63.
Role of estrogen
The increased incidence of hypertension in women after the age of 50 suggests that endocrine changes associated with a decline in ovarian function play a role in the pathogenesis and clinical manifestation of hypertension (66). Consequently, the value of estrogen replacement as prophylactic to hypertension is plausible. In animal studies, ovariectomy leads to hypertension in the aging female Dahl salt sensitive and female mRen(2).Lewis rats, models whereby the female rat is normotensive to their male counterparts (67, 68). Thus, a protective role for estrogens against increases in blood pressure is suggested.
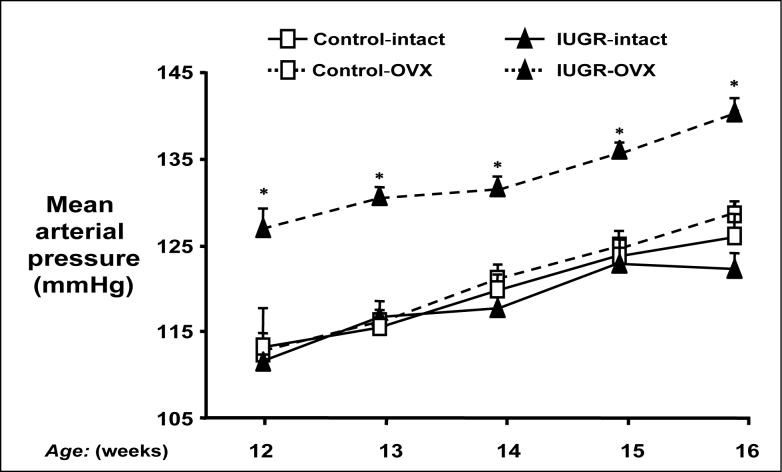
Estrogens may also contribute to sex differences in animal models of fetal programming. Our laboratory reports that placental insufficiency in the rat leads to female growth restricted offspring that normalize their blood pressure after puberty (18), or the age at which female rats reach their maximum levels of estrogen in the strain (69). Blood pressure is significantly increased following ovariectomy in adult female growth restricted offspring in this model with no effect on blood pressure in adult female control offspring (Figure 2) (33). The importance of estrogens is indicated since estrogen replacement normalizes blood pressure in ovariectomized adult female growth restricted (33). Thus, estrogens appear to provide a protective status in adult female growth restricted offspring in this model of placental insufficiency; however, the protective mechanism is not clear.
Figure 2.
Ovariectomy induces significant increases in mean arterial pressure in adult female intrauterine growth restricted (IUGR) offspring. Mean arterial pressure was measured by radio telemetry from 12 to 16 weeks of age in animals that underwent either sham (intact) or ovariectomy (OVX) at 10 weeks of age. *P<0.01 vs. IUGR intact. Used with permission from reference 33; Hypertension 2007; 50:679−685.
Presently, there is a paucity of data regarding the effects of estrogens on the natural history of hypertension. Estradiol is associated with protective cardio-renal effects in many animal models of hypertension (67, 68, 70) and the deleterious effects of ovariectomy such as induced hypertension, renal injury, or endothelial dysfunction are reversed with estradiol therapy (70, 71). Therefore, the cardio-renal protective effect of estradiol appears to be complex and includes a wide range of regulatory systems. One potential target for modulation by estrogen is the RAS. Estrogens may activate the RAS by augmenting levels of renin and angiotensinogen (72, 73). Estrogens may also act downstream of these two proteins by reducing angiotensin converting enzyme (ACE) and increasing angiotensin converting enzyme 2 (ACE2) (74). The ACE-dependent pathway of the RAS generates the potent vasoconstrictor peptide, angiotensin II (ANG II) which is critical for blood pressure regulation (75). However, the ACE2-dependent pathway generates the peptide ANG (1-7) which acts as a negative regulator of the vasoconstrictor effects of ANG II providing a counter-regulatory balance to ACE (75). A role for modulation of ACE and ACE2 is demonstrated in the DBA/Ren-2d model. In this animal model, males become severely hypertensive, but females are less hypertensive compared to age-matched males (74). Ovariectomy induces hypertension in the transgenic females; however, estrogen replacement reverses this effect suggesting estrogens shift the balance between the ACE and ACE2 pathways and their circulating peptides, ANG II and ANG 1−7, respectively (74).
Our laboratory observes similar effects by estrogen on the ACE2 pathway in a model of placental insufficiency in the rat. Significant elevations in renal ACE2 mRNA expression are observed in normotensive adult female growth restricted offspring (33). However, hypertension induced by ovariectomy in adult female growth restricted offspring is associated with a significant decrease in renal ACE2 mRNA expression (33). Ovariectomy has no effect on blood pressure or renal ACE2 mRNA expression in adult control female offspring suggesting that only in adult female growth restricted offspring, is the RAS and subsequent regulation of blood pressure sensitive to the presence of estrogens. Therefore, the abnormal response to loss of ovarian function on blood pressure regulation in adult female growth restricted offspring may reflect permanent alterations in the regulatory systems important in the long-term control of arterial pressure regulation, and the RAS, as a consequence of fetal programming, may be one system sensitive to the effects of estrogen.
SEX DIFFERENCES IN FETAL PROGRAMMING: HUMAN STUDIES
Numerous epidemiological studies examine the association between birth weight and blood pressure with significant controversy as to whether an inverse relationship is observed (4). Original population studies linking birth weight and blood pressure did not separate findings based on gender of the cohort (4). Recent population studies report that the inverse relationship between birth weight and blood pressure is observed in both men and women (76, 77, 78, 79, 80). Another study performed in children between 8 and 11 years old indicates that this inverse relationship is observed only in girls (80). Other studies suggest that the association between birth weight and coronary heart disease may be associated with sex differences in early growth patterns reflecting sex differences in the rate of fetal growth at a similar level of maternal nutrition (77). Although coronary heart disease in women is associated with LBW, it is more strongly linked to short body length at birth (77). Among men, coronary heart disease is also associated with LBW, but is more strongly linked to thinness at birth (77). Thus, whether sex differences are observed in LBW individuals in not clear and contributions from confounding variables including current BMI, catch-up growth and socioeconomic factors may limit these investigations. Sex differences are observed in human essential hypertension (81). An increase in blood pressure is more common and severe in men as compared to age-matched, pre-menopausal women. However, after menopause the risk of hypertension increases with age (82) suggesting that while the ovaries are functional, women have a lower risk for hypertension and cardiovascular disease than men. Thus, sex differences in the response to an adverse fetal environment may lead to sexual dimorphism in the severity or the age-dependent development of chronic adult disease in humans, an observation already demonstrated by animal models of fetal programming.
CONCLUSIONS
Animal models of fetal programming provide critical support for the inverse relationship between birth weight and blood pressure. Despite the model of insult, animal models of fetal programming exhibit sex differences in the pathophysiological response to an adverse fetal environment. A role for sex hormone involvement is strongly suggested with the response of regulatory systems critical to the long-term regulation of arterial pressure exhibiting increased sensitivity to sex hormones within the adult fetal programmed animal. As humans also exhibit sexual dimorphism in blood pressure in adulthood and later life, animal studies investigating sex differences in fetal programming may provide insight critical to the mechanisms linking sex hormones and factors crucial to the long-term control of blood pressure.
ACKNOWLEDGEMENTS
B.T.A. is supported by NIH grants, HL074927 and HL51971.
REFERENCES
- 1.Staessen JA, Wang J, Bianchi G, Birkenhager WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 2.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: The role of fetal programming. Hypertension. 2006;47(part 2):502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 3.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP. Mothers, babies, and disease in later life. BMJ Publishing Group; London: 1994. [Google Scholar]
- 5.Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 7.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:584–594. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 8.Cetin I, Foidart JM, Miozzo M, et al. Fetal growth restriction: a workshop report. Placenta. 2005;25:753–757. doi: 10.1016/j.placenta.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Bertram C, Trowern AR, Copin N, et al. The maternal diet during preganancy programs altered expression of the glucocorticoid receptor and type 2 11 b-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;91:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 15.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- 17.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol. 2005;288:R16–R24. doi: 10.1152/ajpregu.00462.2004. [DOI] [PubMed] [Google Scholar]
- 18.Alexander BT. Placental insufficiency leads to development of hypertension in growth restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 19.Bassan H, Trejo LL, Kariv N, et al. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol. 2000;15:192–195. doi: 10.1007/s004670000457. [DOI] [PubMed] [Google Scholar]
- 20.Hiraoka T, Kudo T, Kishimoto Y. Catecholamines in experimentally growth-retarded rat fetus. Asia Oceania J Obstet Gynaecol. 1991;17:341–348. doi: 10.1111/j.1447-0756.1991.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 21.Merlet-Benichou C, Gilbert T, Muffat-Joly M, et al. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DY, Lumbers ER, Simonetta G, et al. Effects of placental insufficiency on the ovine fetal renin-angiotensin system. Exp Physiol. 2000;85:79–84. [PubMed] [Google Scholar]
- 23.Ortiz LA, Quan A, Zarzar F, et al. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wintour EM, Moritz KM, Johnson K, et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong WY, Wild AE, Roberts P, et al. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 28.Dodic M, Abouantoun T, O'Connor A, et al. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40:729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 29.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 30.Vehaskari VM, Woods LL. Prenatal programming of hypertension: Lessons from experimental models. J Am Soc Nephrol. 2005;16:2545–2556. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 31.Khan IY, Taylor PD, Dekou V, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 32.Ojeda NB, Grigore D, Yanes LL, et al. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292:R758–763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 33.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyton AC, Coleman TG, Cowley AV, Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 35.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 36.Haycock GB. Relationship between birth weight, glomerular number, and glomerular size. Kidney Int. 2001;59:387–387. doi: 10.1046/j.1523-1755.2001.00509.x. [DOI] [PubMed] [Google Scholar]
- 37.Hinchcliffe SA, Lynch MR, Sargent PH, et al. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 38.Mañalich R, Reyes L, Herrera M, et al. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 39.Spencer J, Wang Z, Hoy W. Low birth weight and reduced renal volume in aboriginal children. Am J Kidney Dis. 2001;37:915–920. doi: 10.1016/s0272-6386(05)80006-x. [DOI] [PubMed] [Google Scholar]
- 40.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 41.Hinchcliffe SA, Sargent PH, Howard CV, et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–784. [PubMed] [Google Scholar]
- 42.Kett MM, Bertram JF. Nephron endowment and blood pressure: what do we really know? Curr Hypertens Rep. 2004;6:133–139. doi: 10.1007/s11906-004-0089-2. [DOI] [PubMed] [Google Scholar]
- 43.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 44.Znadi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 45.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003;(83):S31–37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 46.Douglas-Denton RN, McNamara BJ, Hoy WE, et al. Does nephron number matter in the development of kidney disease? Ethn Dis. 2006;16(2 Suppl 2):S2, 40–45. [PubMed] [Google Scholar]
- 47.Bagby SP, Ogden B, LeBard L, et al. Maternal protein restriction during nephrogenesis in microswine causes asymmetric intrauterine growth retardation in neonates and hypertension with body weight excess in adults. J Am Soc Nephrol. 2001;12:461A. [Google Scholar]
- 48.Pham TD, MacLennan NK, Chiu CT, et al. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285:R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 49.Langley-evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 50.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 2003;42:768–774. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 51.Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- 52.Franco Mdo C, Arruda RM, Dantas AP, et al. Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res. 2002;56:145–153. doi: 10.1016/s0008-6363(02)00508-4. [DOI] [PubMed] [Google Scholar]
- 53.do Carmo Pinho Franco M, Nigro D, Fortes ZB, et al. Intrauterine undernutrition-renal and vascular origin of hypertension. Cardiovasc Res. 2003;60:228–243. doi: 10.1016/s0008-6363(03)00541-8. [DOI] [PubMed] [Google Scholar]
- 54.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 55.Hall JE, Guyton AC, Mizelle HL. Role of renin-angiotensin system in control of sodium excretion and arterial pressure. Acte Physiol Scand Suppl. 1990;591:48–62. [PubMed] [Google Scholar]
- 56.Guron G, Friberg P. An intact renin angiotesin system is a prerequisite for normal renal development. J Hypertens. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 57.Woods LL, Rasch R. Periantal ANG II programs adult blood pressure, glomerular number and renal function in rats. Am J Physiol. 1998;275:R1593–R1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]
- 58.Manning J, Vehaskari VM. Low birth weight associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:471–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 59.Langley-Evans SC, Jackson AA. Captopril normalizes systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 60.Grigore D, Ojeda NB, Robertson EB, et al. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R804–811. doi: 10.1152/ajpregu.00725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapiro J, Christiana J, Frishman WH. Testosterone and other anabolic steroids as cardiovascular drugs. Am J Therapeut. 1999;3:167–174. doi: 10.1097/00045391-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 63.Reckelhoff JF, Zhang H, Granger JP. Androgen receptor antagonism attenuates the progression of hypertension in male SHR. Hypertension. 1998;32:601–607. [Google Scholar]
- 64.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 65.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shulman IH, Aranda P, Raij L, et al. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47:1168–1174. doi: 10.1161/01.HYP.0000218857.67880.75. [DOI] [PubMed] [Google Scholar]
- 67.Hinojosa-Laborde C, Craig T, Zheng W, et al. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 68.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 69.Sokol RZ, Okuda H, Stanczyk FZ, et al. Normative reproductive indices for male and female adult Sprague-Dawley rats. Contraception. 1999;59:203–207. doi: 10.1016/s0010-7824(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 70.Roberts CK, Vaziri ND, Barnard RJ. Protective effects of estrogen on gender-specific development of diet-induced hypertension. J Appl Physiol. 2001;91:2005–2009. doi: 10.1152/jappl.2001.91.5.2005. [DOI] [PubMed] [Google Scholar]
- 71.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol. 2004;15:1546–1556. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- 72.Nasjletti A, Masson GM. Studies on angiotensinogen formation in a liver perfusion system. Cir Res. 1972;30:187–202. [PubMed] [Google Scholar]
- 73.Tewksbury DA. Angiotensinogen- biochemistry and molecular biology. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. Raven; New York: 1990. pp. p1197–1216. [Google Scholar]
- 74.Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am Journal Physiol-Regul Integr Com Physiol. 1997:R1908–R1915. doi: 10.1152/ajpregu.1997.273.6.R1908. [DOI] [PubMed] [Google Scholar]
- 75.Ferrario CM, Chappell MC. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61:2720–2727. doi: 10.1007/s00018-004-4243-4. [DOI] [PubMed] [Google Scholar]
- 76.Forsén T, Osmond C, Eriksson JG, Barker DJ. Growth of girls who later develop coronary heart disease. Heart. 2004;90:20–24. doi: 10.1136/heart.90.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forsén T, Eriksson JG, Tuomilehto J, et al. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curhan GC, Chertow CM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 79.Rich-Edwards JW, Kleinman K, Michels KB, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330:1115–1120. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor SJ, Whincup PH, Cook DG, et al. Size at birth and blood pressure: cross sectional study in 8−11 year old children. BMJ. 1997;314:475–480. doi: 10.1136/bmj.314.7079.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 82.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]