Abstract
Wnt/β-catenin signaling plays an important role in liver development and regeneration. Its aberrant activation, however, is observed in a subset of primary hepatocellular cancers (HCCs). In the present study we compare and contrast the tumor characteristics of HCC in the presence or absence of mutations in the β-catenin gene (CTNNB1). Frozen HCC (n=32) including five fibrolamellar (FL) variants, and control livers (n=3) from Health Sciences Tissue Bank and Department of Surgery at the University of Pittsburgh Medical Center, were examined for mutations in CTNNB1, protein levels of β-catenin, tyrosine-654-phosphorylated-β-catenin (Y654-β-catenin) and glutamine synthetase (GS). Missense mutations in the exon-3 of CTNNB1 were identified in 9/32 HCCs. Total β-catenin levels were higher than controls in most tumors, however GS was exclusively increased in HCC with mutations. Phenotypically, greater percentages of mutated HCCs showed macro- and micro-vascular invasion. Also the tumor size was greater than double in mutated HCCs. High levels of total β-catenin protein were observed in multinodular tumors independent of β-catenin mutations. In addition, significant cases with mutations showed absence of cirrhosis. Finally, highest levels of Y654-β-catenin were exclusively observed in FL-HCC cases.
Conclusion
Thus, HCCs that harbor missense mutations in exon-3 of CTNNB1 exhibit histologically, a more aggressive phenotype. Also, CTNNB1 mutations might lead to HCC, in absence of cirrhosis. Finally, FL-HCC cases display a unique upregulation of tyrosine-phosphorylated-β-catenin suggesting robust receptor tyrosine kinase signaling in this tumor type.
INTRODUCTION
Hepatocellular cancer (HCC) is the most common primary tumor of the liver accounting for 85% of all primary malignant tumors. It is the fifth most common malignancy worldwide and third most frequent cause of death related to cancers (1, 2). Common risk factors of HCC include hepatitis, chronic alcohol abuse, toxins such as aflatoxins and non-alcoholic fatty liver disease (NAFLD). While it used to be a common malignancy in underdeveloped or developing countries, it is now on the rise in developed countries (3, 4). This shifting trend is attributable to the increasing incidence of hepatitis C and B as well as NAFLD. Also, while there is an overall increase in incidence of HCC with age, especially in western world, these trends are also shifting. In fact peak incidence of HCC in a recent study was between the ages of 45−60 years (1). Thus, it is imperative to identify the molecular basis of this malignancy.
Wnt/β-catenin signaling plays a multitude of functions in liver biology (reviewed in (5)). Its role has been identified in normal liver development in regulating hepatoblast proliferation as well as differentiation (6). It has also been shown to play a role in liver stem cell or oval cell activation (7-9). β-Catenin is also important in normal liver regeneration (10, 11). In addition, this pathway plays a critical role in normal liver zonation and is also thought to regulate key metabolic processes in the liver (11-13). These varying functions of this pathway might be due to specific target genes that are under the co-transcriptional control of β-catenin and T-cell factor family. Other than being the chief downstream effector of the canonical Wnt pathway where it is regulated by phosphorylation at specific serine/threonine residues, β-catenin has also been shown to be activated via tyrosine phosphorylation, especially at its C-terminal by growth factors such as EGF and HGF, enabling nuclear translocation and activation (14-16). Several target genes of β-catenin are known to play a role in proliferation (cyclin-D1), differentiation (c-myc), survival (survivin), protein metabolism (glutamine synthetase or GS), xenobiotic metabolism (P450s such as cyp2e1, cyp1a2) and regulating oxidative stress (glutathione s-transferases) in liver or in other tissues (6, 11, 13, 17-22).
The current study was aimed at identifying the frequency of activating β-catenin gene mutations in HCC samples from the University of Pittsburgh Medical Center, and to compare and contrast the tumor characteristics attributable to presence or absence of such mutations. Herein we report an aggressive HCC phenotype based on coincident increase in tumor size and presence of vascular invasion, in the presence of CTNNB1 mutations. Also, increased total β-catenin (irrespective of mutations) was associated with a multinodular HCC. Finally, we report an increased tyrosine-phosphorylated-form of β-catenin in FL-HCC cases, which in our dataset, did not exhibit any mutations in the CTNNB1 or increase in GS levels, but did show elevated levels of cyclin-D1. These findings support an alternate pathogenesis of FL-HCC, suggestive of pronounced receptor tyrosine kinase activation.
MATERIALS AND METHODS
Generation and testing of the custom Y654-phospho-β-catenin antibody
A hen antibody against a KLH-conjugated phosphorylated peptide sequence CZ SRN EGV AT (pY) AAA VLF RMS EDK, corresponding to mouse, human and rat β-catenin amino acids 646−666, was generated at the Aves Labs, Inc. (Tigard, OR). The antibody was affinity purified against the phosphorylated sequence. The eluted fraction was further affinity purified against the non-phosphorylated peptide sequence. The final double affinity purified antibody recognized only the phosphorylated form of β-catenin at tyrosine-654 residue as tested in primary hepatocyte culture, which is shown in results. Primary rat hepatocytes were isolated by two-step collagenase perfusion as described elsewhere (23). Following attachment on wet collagen, the hepatocytes were cultured in the chemically defined HGM medium with additional HGF (25 ng/ml), EGF (10ng/ml) or in the presence or absence of 10% serum for 30 minutes (15, 24). Total cell lysates from these cells was used for western blot analysis. The pre-immune egg fraction collected prior to the first injection was used to verify the specificity of the Y654-phospho-β-catenin antibody. Whole liver lysates from β-catenin-conditional-null livers described by our laboratory, were also utilized to verify the presence of Y654-β-catenin only in the wild type littermates (11).
Patient Tissues
All tissues and materials used in this study were obtained under an approved Institutional Review Board (exempt) protocol. Frozen tissue samples from 32 HCC cases and 3 control-tumor-free livers were obtained from the Department of Pathology Tissue Bank and the Department of Surgery at the University of Pittsburgh, School of Medicine. The corresponding anonymized pathology reports were obtained and pertinent patient information of all cases in this study is detailed in Table 1.
Table 1. Patients and Controls in the study.
HCC (non-fibrolamellar) patient demographics and additional tumor attributes.
| Case | Age | Sex | Cirrh. | Vascular Invasion | Hepatitis | Numbers of Nodules | Nodule Size (cm) | TNM (Classical) | |
|---|---|---|---|---|---|---|---|---|---|
| Macro | Micro | ||||||||
| M-1 | 60−69 | F | Y | N | Y | C | 4 | 2.5 | T2NxMx |
| M-2 | 70−79 | M | N | N | Y | 1 | 12.5 | T3NxMx | |
| M-3 | 80−89 | F | N | N | Y | 1 | 13 | T2NxMx | |
| M-4 | 50−59 | M | Y | N | N | C | 3 | 4.2 | T2NxMx |
| M-5 | 50−59 | M | Y | N | N | 2 | 1.8 | T2NoMx | |
| M-6 | 80−89 | M | N | Y | Y | 1 | 5 | T2NxMx | |
| M-7 | 70−79 | M | N | Y | Y | C | 1 | 15 | T2N0Mx |
| M-8 | 70−79 | M | N | N | Y | 1 | 14 | T3NxMx | |
| M-9 | 50−59 | M | N | N | Y | 1 | 5 | T3NxMx | |
| NM-1 | 40−49 | M | N | N | N | 1 | 7 | T2NxMx | |
| NM-2 | 50−59 | F | Y | N | Y | 1 | 2 | NA | |
| NM-3 | 50−59 | M | Y | N | N | 1 | 2 | T1NxMx | |
| NM-4 | 60−69 | M | Y | N | Y | 2 | 3 | T3N0Mx | |
| NM-5 | 50−59 | M | Y | N | N | 2 | 0.7 | T2NxMx | |
| NM-6 | 60−69 | M | Y | N | N | 2 | 1.5 | T2NxMx | |
| NM-7 | 60−69 | F | Y | N | N | 2 | 4.5 | T4NxMx | |
| NM-8 | 50−59 | F | Y | N | N | 1 | 8 | T2N0Mx | |
| NM-9 | 60−69 | M | N | N | Y | 4 | 4 | T2NxMx | |
| NM-10 | 50−59 | M | Y | N | N | C | 1 | 1.5 | T1NxMx |
| NM-11 | 50−59 | M | Y | N | N | 1 | 1 | T1NxMx | |
| NM-12 | 50−59 | M | Y | N | N | C | 1 | 2.5 | T1NxMx |
| NM-13 | 50−59 | M | Y | Y | Y | 1 | 4.5 | T3NxMx | |
| NM-14 | 50−59 | M | N | N | Y | C | 1 | 4 | T2NxMx |
| NM-15 | 60−69 | F | N | N | Y | 1 | 2 | NA | |
| NM-16 | 70−79 | M | N | N | N | 3 | 8 | T3NxMx | |
| NM-17 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NM-18 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Fibrolamellar HCC patient demographics and additional tumor attributes. | |||||
|---|---|---|---|---|---|
| Case | Age | Sex | Numbers of Nodules | Nodule Size (cm) | TNM |
| FL-1 | 20−29 | M | 1 | 14 | T3N1M1 |
| FL-2 | 20−29 | F | 1 | 15.3 | T4N1M1 |
| FL-3 | 20−29 | F | 1 | 20 | T3N0Mx |
| FL-4 | 20−29 | F | 1 | 17 | T3N1Mx |
| FL-5 | 20−29 | M | 1 | 3 | NA |
| Controls livers used in the current study: | ||||
|---|---|---|---|---|
| Case | Age | Sex | Cirrhosis | Diagnosis* |
| C1 | 50−55 | M | N | Metastatic papillary renal cell carcinoma in liver |
| C2 | 70−79 | M | N | Unremarkable Liver |
| C3 | 70−79 | M | N | Nodular regenerative hyperplasia, liver |
Abbreviations: M-HCC cases with mutations in exon-3 of CTNNB1; NM-HCC cases without mutations in exon-3 of CTNNB1; FL-fibrolamellar HCC; C*-Control livers; NA-not available; Cirrh-Cirrhosis; (*For controls, livers utilized were from disease-free areas)
Genomic DNA Isolation and Mutational Analysis
Genomic DNA (gDNA) was extracted using 5M potassium acetate and 100% ethanol. Precipitated DNA was spooled and dissolved in 50μL of TE buffer. Exon 3 of the CTNNB1 was amplified by the polymerase chain reaction (PCR) and the product was resolved on agarose gel followed by gel purification as described previously (25). Purified PCR products were sent for sequencing using the primer hCat-E3-S1 (5'-GCT GAT TTG ATG GAG TTG GAC-3’), to the University of Pittsburgh, DNA sequencing facility. All mutations were verified by sequencing the sense and antisense strands.
Protein Extraction and Western Blot Analysis
Frozen tumors and control livers were utilized for whole cell lysates in RIPA buffer (26). Isolated protein was assayed by bicinchoninic acid assay and 25μg or 100μg of protein was resolved by SDS-PAGE analysis and transferred to Immobilon-PVDF membrane (Millipore, Bedford, MA) or to nitrocellulose membrane (Bio-Rad Labs, Hercules, CA) for the Hen antibody only. Western blots for the following primary antibodies: β-Catenin (BD Biosciences, San Jose, CA), cyclin-D1, glutamine synthetase (Santa Cruz Biotechnology, Santa Cruz, CA) and actin (Millipore, Bedford, MA), were performed using standard protocol (26). GS antibody detected at least 2 major species at around 45 and 49kDa. Based on literature, the 45kDa size was labeled as the true GS band as it was also clearly the predominant species in β-catenin-mutated HCCs as published previously (17, 27-29). A modified protocol was used for the Y654-phospho-β-catenin chick antibody. The nitrocellulose membranes were blocked for 30 minutes at room temperature with 10% Block Hen (Aves Labs, Inc, Tigard, OR) and then for 30 minutes with 5% milk in blotto. Primary antibody was prepared in 5% milk and probed overnight at 4°C with standard protocol followed from hereon. The signal was detected using the SuperSignal West Pico Chemiluminiscent substrate (Perkin Elmer, Boston, MA) for 5 minutes. The blots were visualized by autoradiography. The membranes were stripped using IgG Elution Buffer (Pierce) and stained with Ponceau S Solution for verifying equal loading (Sigma-Aldrich, St. Louis, MO).
Autoradiograph film and Ponceau S stained membranes were scanned and quantitatively analyzed using Image J (NIH, Bethesda, MD). The three non-HCC control liver samples were loaded with each gel for meaningful interpretation and excluding technical variations such as exposure times. The average densitometry values of the controls were used to normalize the densitometry for each HCC case. Student's T Test was used to calculate the p values, which are listed on each graph along with the standard error bars. Statistically insignificant values were depicted as NS. Microsoft Excel was used to perform the statistical calculations and generate the graphs. Also, separately, to reaffirm quantitative analysis, all western blots were arbitrarily scored for band intensity relative to controls and absolute values presented in table 3. The total β-catenin was scored + to +++++, Y654-β-catenin was scored + to +++++ and GS was scored + to ++++++. Control livers for the three proteins were usually +.
TABLE 3.
Arbitrary western blot scores in all HCC samples and controls
| Case | Total β-Catenin | Y654-β-catenin | GS |
|---|---|---|---|
| M-1 | +++ | + | ++++++ |
| M-2 | +++ | +++ | ++++++ |
| M-3 | +++ | ++ | ++++++ |
| M-4 | ++ | ++ | ++++ |
| M-5 | ++ | ++ | + |
| M-6 | +++ | + | ++++++ |
| M-7 | +++ | +++ | ++++++ |
| M-8 | +++ | +++ | +++ |
| M-9 | NA | NA | NA |
| NM-1 | ++ | +++ | + |
| NM-2 | + | + | + |
| NM-3 | ++ | ++ | ++++++ |
| NM-4 | ++ | + | ++ |
| NM-5 | +++ | + | ++ |
| NM-6 | +++++ | ++ | + |
| NM-7 | +++++ | + | ++ |
| NM-8 | ++++ | +++ | + |
| NM-9 | +++++ | +++ | + |
| NM-10 | + | + | + |
| NM-11 | ++ | + | + |
| NM-12 | +++ | ++ | + |
| NM-13 | ++ | + | ++ |
| NM-14 | ++ | ++ | + |
| NM-15 | + | ++ | ++++++ |
| NM-16 | +++++ | ++ | ++ |
| NM-17 | +++ | ++ | ++ |
| NM-18 | ++++ | +++ | + |
| FL-1 | +++ | +++++ | ++ |
| FL-2 | +++ | +++ | + |
| FL-3 | ++++ | ++++ | ++ |
| FL-4 | ++ | +++ | ++ |
| FL-5 | + | +++ | + |
| C-1 | + | + | + |
| C-2 | + | ++ | + |
| C-3 | + | + | + |
RESULTS
Specificity of the custom beta-catenin-Y654-phospho-β-catenin (Y654-β-catenin) antibody
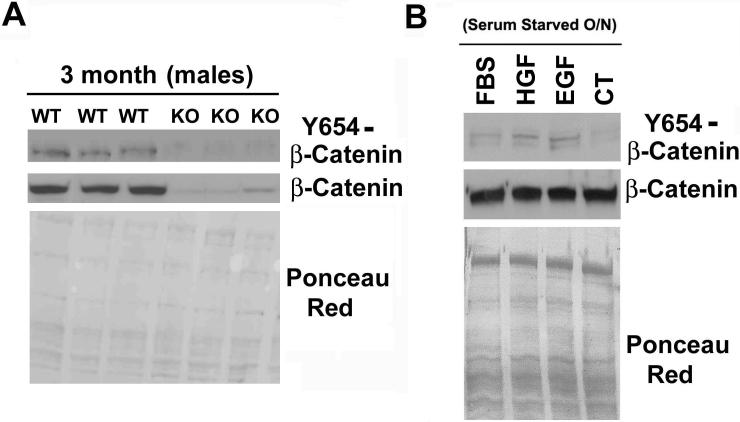
Two separate experiments were done to verify the specificity of the Y654-β-catenin antibody. First, extracted protein from the livers of wild type (WT) and β-catenin-conditional-null (KO) livers were used to verify specificity of the antibody (11). WT livers showed moderate levels of Y654-β-catenin whereas the KO showed no Y654-β-catenin (Figure 1A). Next, western blot analysis was done using the protein isolated from primary rat hepatocytes that were serum starved and treated with either 10% FBS, HGF, EGF, or no serum as described in the methods. As shown in Figure 1B, while all four groups contained equal levels of β-catenin, the groups treated with FBS, EGF and HGF display a clear increase in Y654-β-catenin. Thus, the Y654-phospho-β-catenin antibody specifically identified the tyrosine-phosphorylated form of β-catenin in cells and tissues.
Figure 1. Validation of custom phospho-Y654-β-catenin antibody.
(A) Total protein from wild type and β-catenin knock out mice were analyzed by western blot with the Y654-β-catenin antibody. (B) Total protein from primary rat hepatocytes treated with FBS, HGF, EGF or no serum was analyzed by western blot for Y654-β-catenin and total β-catenin. Ponceau Red staining verified equal loading of protein in both instances.
Significant subset of HCCs display missense mutations in exon-3 of CTNNB1
Thirty-two HCC samples were analyzed for mutations in exon-3 of the β-catenin gene as described in the methods and previously (25). As shown in table 2, we identified mutations in nine HCC cases. These mutations were all point mutations affecting the GSK3β or casein kinase-phosphorylation sites in the exon-3 of β-catenin rendering it stable and active (30). None of the five fibrolamellar HCCs showed any mutation in CTNNB1. Thus, in our data set around 28% of all HCCs displayed missense mutations in the exon-3 of β-catenin gene.
TABLE 2.
Missense mutations in exon-3 of CTNNB1 in 9/32 cases of HCC.
| Case No. | Mutation | Sequence |
|---|---|---|
| M-1 | S33C | TCT-TGT |
| M-2 | D32V | GAC-GTC |
| M-3 | L30Q, S37F | CTG-GAC, TCT-TTT |
| M-4 | T41F | ACC-GCC |
| M-5 | S37F | TCT-TTT |
| M-6 | S45P | TCT-CCT |
| M-7 | S33C | TCT-TGT |
| M-8 | T41P | ACC-CCC |
| M-9 | D32G | GAC-GGC |
Protein levels in non-fibrolamellar HCC with and without β-catenin gene mutations
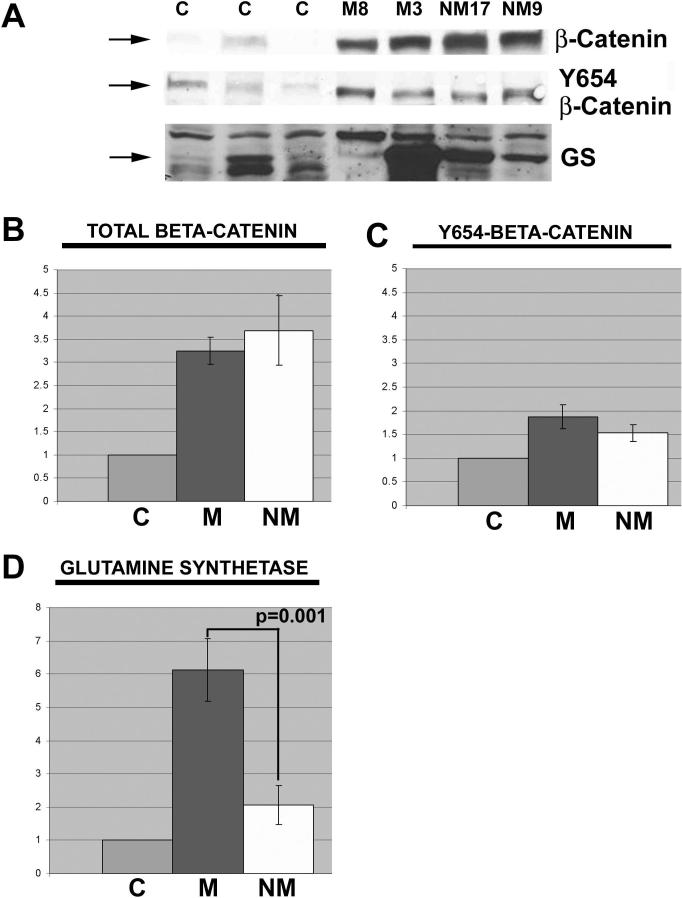
Next, we examined all non-fibrolamellar HCCs and the three control human livers for total protein levels of total β-catenin, Y654-β-catenin, and GS (liver-specific downstream target of β-catenin) as shown in two representative mutated and non-mutated HCCs along with the controls (Figure 2). Missense mutations in exon-3 of CTNNB1 render the β-catenin protein non-degradable and thus available to translocate to the nucleus to transactivate target genes. Comparison of the average densitometry values between the two HCC groups revealed no changes in total β-catenin levels, although both were significantly higher than controls (p<0.05) (Figure 2B). No significant differences were observed in the levels of Y654-β-catenin between both HCC groups and control livers (Table 3). The samples shown in Figure 2C for Y654-β-catenin were in the minority displaying higher levels of Y654-β-catenin. GS levels were overall around 3-fold and significantly greater in HCCs displaying CTNNB1 mutations, signifying β-catenin activation (Figure 2D). A case-by-case analysis of absolute protein levels of β-catenin, Y654-β-catenin and GS are detailed in Table 3.
Figure 2. Protein quantities relative to control in tumors with and without β-catenin gene mutations.
(A) Representative western blots for total β-catenin, Y654-β-catenin, and GS in HCCs with or without mutations in exon-3 of CTNNB1 as compared to three normal livers. (B) Quantitative analysis of western blots shows a significant increase (p<0.001) in total β-catenin in HCC cases with CTNNB1 mutations (M, n=9), and HCC cases without mutations (NM, n=16) over controls (C, n=3). Data for the bar graph is normalized to control. The difference of total β-catenin between CTNNB1 mutation and no mutation HCCs was insignificant (C) Quantitative analysis of Y654-β-catenin in the same data set shows insignificant differences. (D) Similar quantitative analysis displays a significant increase in GS only in HCC cases with CTNNB1 mutations. Each graph includes standard error bars.
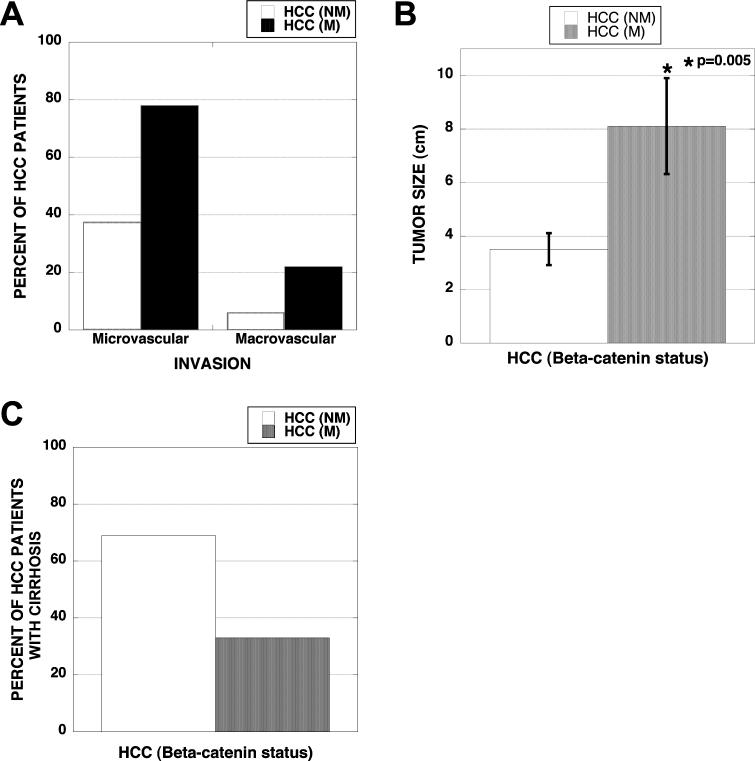
Vascular invasion and greater tumor size observed in non-fibrolamellar HCC with missense CTNNB1mutations
Next, the CTNNB1 mutated and non-mutated HCCs were compared for histological attributes such as vascular invasion and tumor size that are known to influence tumor behavior and patient survival (Table 1). Out of the nine tumors with CTNNB1 mutations, seven showed evidence of microvascular invasion including two cases that showed large vessel involvement. This was notably different in the non-mutated group of sixteen HCC samples, where only six showed microvascular invasion including one, which also displayed macrovascular invasion (Table 1). Thus microvascular invasion was observed in 78% of HCC cases with CTNNB1 mutations versus 37% in the non-mutated group (Figure 3A). Similarly, incidence of macrovascular invasion was 22% in the mutated group versus 6% in the non-mutated group (Figure 3A). Next, we analyzed tumor size as a function of β-catenin gene mutations. The average tumor sizes in HCC cases with missense CTNNB1 mutations were significantly greater (>2-fold) than the tumors without mutation (Figure 3B). In fact four of the nine tumors in mutated group showed >10 cm tumors and two additional tumors measured 5 cm in their greatest dimensions (Table 1). Only three tumors in the non-mutated group were ≥5 cm. These findings suggest higher incidence of vascular invasion and greater tumor size in the β-catenin gene-mutated group of HCC cases.
Figure 3. Vascular invasion, tumor size and cirrhosis in HCC with and without CTNNB1 mutations.
(A) Around 80% of HCC cases with β-catenin-gene mutations (n=9) show microvascular and 20% display macrovascular invasion. Less than 40% of non-mutated HCCs (n=16) show microvascular whereas around 6% displayed macrovascular involvement. (B) Average diameter (cm) of the tumor (largest tumor in case with multiple nodules) was significantly greater in HCC cases with β-catenin gene mutations as compared to HCCs with wild type β-catenin gene (p=0.005). (C) Only 30% of HCC cases with missense CTNNB1 mutations exhibited concomitant cirrhosis whereas more than 70% HCC cases without β-catenin mutations displayed full-blown cirrhosis.
Lower incidence of cirrhosis is evident in HCC with missense mutations in CTNNB1
Next, we analyzed any association of CTNNB1 mutations with ongoing cirrhosis in HCC patients. Only 33% (3/9) of HCC cases with mutations in CTNNB1 showed any histopathological evidence of cirrhosis. On the other hand, 75% (12/16) of HCC cases without mutations, showed clear evidence of cirrhosis (Figure 3C). Thus, it appears that β-catenin gene mutations are an important risk factor for HCC, independent of cirrhosis.
Increased total β-catenin protein in HCC with multiple nodules compared to solitary tumors
A separate analysis was then conducted to observe any differences in protein levels between cases with multiple HCC nodules and solitary HCC. All cases were included initially and the analysis was first done irrespective of CTNNB1 mutations. Average densitometry relative to control was compared between the two groups and on average a 2-fold increase in total β-catenin levels was observed in the HCC with multiple nodules (Figure 4A). No differences in Y654-β-catenin or GS were observed (Figure 4A). The results were the same when CTNNB1 mutated cases were excluded. (Figure 4B.) The apparent trend towards higher GS levels in solitary HCC was due to only 2 cases, which inexplicably had very high GS levels. Table 3 shows results for individual cases.
Figure 4. Protein quantities relative to control in tumors with HCC with multiple nodules compared to solitary HCC tumors.
(A) β-Catenin, Y654-β-catenin and GS densitometry in all cases with either multiple HCC nodules (n=9) or solitary tumors (n=15), normalized to controls. (B) β-Catenin, Y654-β-catenin, and GS densitometry in non-β-catenin-mutated cases with either multiple HCC nodules (n=6) or solitary HCC (n=9). All graphs include standard error bars and p values wherever significant differences were observed. NS-not significant.
Missense mutations in exon-3 of CTNNB1 lead to aggressive histological phenotype in HCC
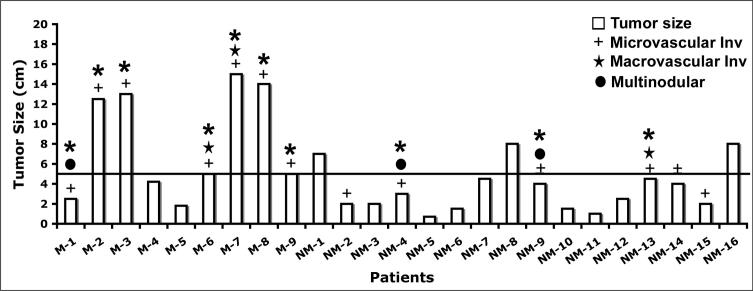
Next, the histopathological surrogates identified above, including vascular invasion, tumor size and tumor multinodularity were assessed on a case-by-case basis to address the overall tumor behavior in the presence or absence of CTNNB1 mutations as well as to exclude any random associations (Table 1). Greater tumor size along with the evidence of vascular invasion (micro and macro) is associated with an advanced tumor stage and poor prognosis (31), Six of the nine tumors with mutations in CTNNB1 that measured ≥5 cm in their maximum dimension including four that were greater than 10 cm, showed evidence of concomitant microvascular invasion (Figure 5, Table 1). In fact two patients in this group (M-6 and M-7) also showed macrovascular invasion (Figure 5). In another relevant subanalysis it was noted that the only additional patient in the mutated group with evidence of microvascular invasion (M-1) but tumor size of 2.5 cm, also showed a multifocal disease with four nodules (Figure 5). Based on the same study (31), the presence of microvascular invasion and multiple nodules stratifies tumor to a higher stage, irrespective of tumor size (Figure 5, Table 1). In the group of CTNNB1-non-mutated HCCs, none of the three tumors with size ≥5 cm, showed any evidence of micro- or macro-vascular invasion (Figure 5, Table 1). In fact, all HCC cases in this group with evidence of microvascular invasion, had tumor size of less than 5 cm (Figure 5, Table 1). One patient in this group (NM-13) did show portal vein invasion (Table 1). Finally, in a subanalysis in this non-mutated group, only two of the six tumors that showed microvascular invasion, also displayed multiple nodules, implying an advanced tumor stage (Figure 5, Table 1). Thus, taken together, these data clearly reflect evidence an aggressive histological phenotype in HCC with missense mutations in the β-catenin gene as compared to the non-mutated HCC.
Figure 5. Mutations in β-catenin gene lead to histologically more aggressive tumor phenotype than non-mutated group.
All six tumors in β-catenin-gene mutated group of HCCs with tumor size greater than 5 cm (horizontal line) showed microvascular (+) and/or macrovascular (Η) invasion whereas none of the three tumors in non-mutated group that were greater than 5 cm showed any evidence of micro- or macro-vascular invasion. M-1 in the mutated group showed coexisting microvascular invasion and multiple tumor nodules (λ). Two patients in non-mutated group (NM-4 and NM-9) also showed multinodular HCC along with evidence of microvascular invasion. Finally, only 1 patient in the non-mutated group had macrovascular involvement. Thus overall, a greater number of HCC with CTNNB1 mutations stratified into histologically aggressive phenotype (Θ) than the non-mutated HCC group.
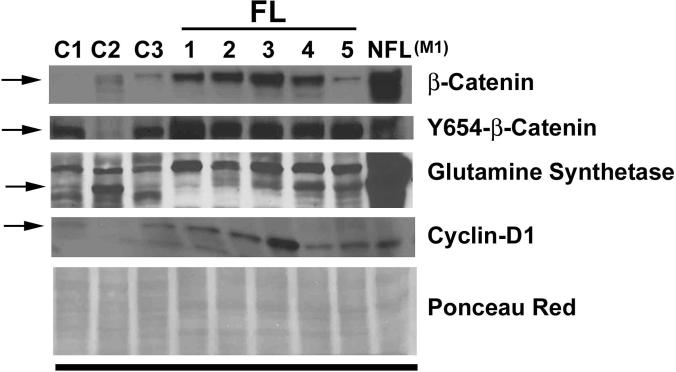
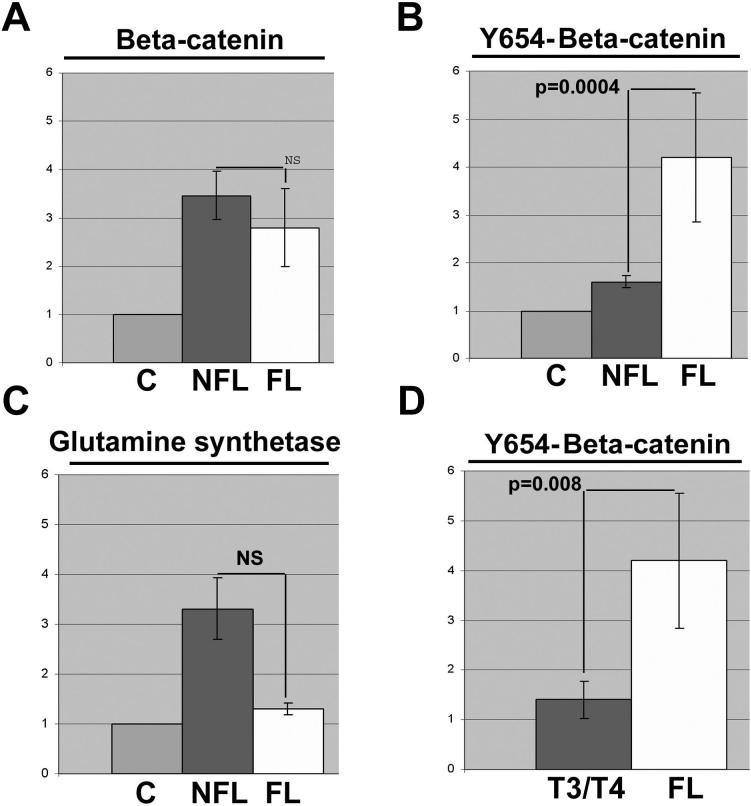
Fibrolamellar HCC displays significantly higher levels of Y654-β-catenin
Next, we examined FL-HCC cases (n=5) as a group with the remainder of HCC samples (n=27) and controls (n=3) for Wnt/β-catenin signaling (Figure 6). As mentioned previously, none of the FL-HCC displayed any mutations in the exon 3 of CTNNB1. Total β-catenin protein in FL-HCC was higher than controls, but insignificantly different from other HCCs (Figure 7A). However, Y654-β-catenin levels were consistently and significantly higher in FL-HCCs than both the controls and conventional HCCs by around 4-fold and 3-fold, respectively (Figure 7B). Interestingly, GS levels were comparable to the control livers, lower than HCC with β-catenin gene mutation (Figure 5) and non-FL-HCC cases (Figure 7C). However, cyclin-D1, another established target of β-catenin, displayed higher levels in FL-HCC as compared to the controls (Figure 6).
Figure 6. Protein analysis of FL-HCC cases for β-catenin signaling.
Western blot analysis done utilizing 25μg protein was examined for total β-catenin and cyclin-D1, and 100μg protein for Y654-β-catenin, and GS. Ponceau red staining was performed to verify equal loading. C (1-3) are three normal control livers; FL (1-5) are FL-HCC cases; and NFL (M1) is a non-fibrolamellar HCC case M-1 from Table 1, which harbored exon-3 mutation in CTNNB1.
Figure 7. Enhanced tyrosine-phosphorylated-β-catenin levels in FL-HCC cases.
(A) Total β-catenin, (B) Y654-β-catenin, and (C) GS densitometry of fibrolamellar HCCs (FL, n=5) and all other non-fibrolamellar HCC for which tissue was available for protein analysis (NFL, n=24), relative to three control livers, identifies a unique and dramatic increase in Y654-β-catenin in FL-HCC livers. (D) Densitometry of Y654-β-catenin western blots comparing non-fibrolamellar advanced stage tumors (T3/T4) to FL-HCCs. Graphs include standard error bars and significant p values; NS indicates not statistically significant difference.
Since all FL-HCCs in our data set were classified as T3 or T4 (Table 1), we wanted to exclude selection bias as a basis of observed differences in Y654-β-catenin levels. As shown in figure 6D, non-FL-HCCs that were classified as T3 and T4 tumors showed significantly lower levels of Y654-β-catenin than the FL-HCC, clearly demonstrating elevated tyrosine-phosphorylated β-catenin as a unique characteristic of the FL-HCCs.
DISCUSSION
Several reports have implicated aberrant activation of the Wnt/β-catenin signaling in a significant subset of HCCs (32). This has been shown to be secondary to different mechanisms including mutations in the CTNNB1 in around 10−40% cases, AXIN-1 and AXIN-2 in around 5% of cases; changes in absolute expression levels of several components of the Wnt pathway such as Wnts, Frizzled receptors; and finally epigenetic inactivation in the expression of certain negative regulators of the pathway such as sFRPs (33-38). However, irrespective of the mechanism, the canonical signaling converges at β-catenin, which is the chief downstream effector and transcriptional coactivator regulating expression of many genes playing key roles in tumor cell survival, proliferation and plays other roles in tumorigenesis, such as in cancer stem cell biology (5, 39). Indeed others and we have demonstrated a critical role of Wnt/β-catenin signaling in oval cell activation, which is a candidate cancer stem cell in the liver and also subset of HCCs have been identified with stem cell signatures that have shown to have aggressive disease (7-9, 40-42). It is important to also point out that the mode of activation of β-catenin might ultimately dictate extent of activation or specificity of target gene expression, which might impact the overall hepatic tumor biology and behavior (29, 43). Finally targeting β-catenin in HCC has been shown to have important therapeutic implications in several preclinical studies, where β-catenin inhibition has been associated with decreased tumor cell proliferation and growth, increased apoptosis and decreased cell migration and invasion (28, 44, 45).
We were interested in identifying the dysregulation of the canonical Wnt signaling in HCC patients at the University of Pittsburgh Medical Center. We identified missense mutations affecting the exon-3 in around 28% of HCC patients, which is in agreement with the previous reports. This correlated with the overexpression of GS in these tissues as reported previously (27, 29, 46). Interestingly one of the eight HCC cases (for one HCC no tissue was available for protein studies, Table 3), showed low GS levels despite β-catenin gene mutation. Au contraire, 2/18 non-β-catenin mutated HCCs showed very high levels of GS. Thus GS is an overall reliable marker of β-catenin activation secondary to CTNNB1 mutations. However, there are some exceptions to this rule and might be due to β-catenin-independent regulation of GS expression also reported elsewhere, recently (47).
Our additional analysis was aimed at extrapolating tumor characteristics in the presence or absence of mutations in β-catenin to elucidate role of β-catenin in HCC biology. A major correlate that was identified in the CTNNB1-mutated HCC cases was the presence of microvascular and macrovascular invasion. 78% of HCCs with mutations displayed invasion, whereas only 38% of non-mutated group revealed any evidence of invasion. Interestingly, Y654-β-catenin, which has been shown to negatively regulate cell-cell adhesion, was only modestly upregulated in subset of β-catenin mutated and non-mutated HCCs (48). Thus it seems unlikely that Y654-β-catenin itself may be responsible for invasive characteristics of the HCC, which appears to be more closely related to the activation of β-catenin and downstream target gene regulation. Several known targets of β-catenin that are known to play a role in invasion and cell-matrix regulation and include uPAR, MMP-2, MMP-7, MMP-9, MMP-26, Nr-CAM, and others (49). However, which targets are specifically playing a role in HCC would need to be addressed in future studies.
Tumor size was another significant attribute identified as a function of β-catenin mutation. The tumors in β-catenin-mutated HCC group were greater than twice the size in non-mutated group. Indeed similar observation have been associated with activating β-catenin mutations, previously (32). β-Catenin was also upregulated significantly in HCC presenting as multiple nodules as compared to single nodules. This was independent of mutations in CTNNB1 and did not correspond to an increase in GS. Since multinodular disease often, but not always, reflects a more invasive phenotype, it was again interesting to see a positive correlation between total β-catenin levels and multinodular disease.
To derive a functional outcome of the histopathological differences observed between the CTNNB1-mutated and non-mutated HC cases, we turned to revised AJCC staging that follows new stratification criteria based on vascular invasion, tumor number (multiple tumor nodules) and tumor size (31). This study emphasized tumor size alone being a good predictor of disease prognosis. However, this study, which has been recently validated by a follow up analysis in 2007, stratifies patients to higher stages based on the presence of microvascular and macrovascular invasion in a tumor size greater than 5 cm (50). Based on these recommendations, our studies identified in the predominant group of β-catenin gene-mutated HCC cases, a consistently larger tumor size, along with evidence of predominantly microvascular invasion and in a smaller subset-macrovascular invasion. This clearly reflects a more aggressive phenotype in this group of HCC patients, which is attributable to aberrant β-catenin activation due to missense mutations in CTNNB1. Thus, despite a relatively smaller sample size, our observations are important, since the verdict on the HCCs with β-catenin mutations has been mixed so far, with a few studies demonstrating better and others a poor survival in such HCC cases (29, 41, 51-53). Our studies classify CTNNB1-mutated HCCs to be histologically aggressive, and thus β-catenin activation might have a prognostic significance, although future prospective studies with greater patient population will be necessary to further strengthen these observations.
Another relevant observation in β-catenin mutated-HCC was the lack of cirrhosis in a significant number of tumors. Only 30% of β-catenin-mutated HCC had ongoing cirrhosis while more than 70% of non-β-catenin-mutated HCCs had coexisting cirrhosis. This suggests β-catenin gene mutations to be a risk factor for HCC, independent of cirrhosis. Indeed, it has been shown that hepatic adenomas that typically occur in absence of any ongoing cirrhosis, progress to HCC if CTNNB1 mutations were identified in these benign tumors (27, 46). What is the mechanism of occurrence of such random mutations remains unknown at this stage, although recent studies have uncovered Met-β-catenin dysregulation to work synergistically to promote tumorigenesis in the liver (54).
Our final analysis was aimed at identifying the status of canonical Wnt signaling in FL-HCC, a variant of HCC whose molecular basis remains obscure. While originally thought to have better prognosis, it is now thought to be due to the absence of cirrhosis in these tumors (55). None of the FL-HCCs displayed any β-catenin mutations. However, total β-catenin levels and more importantly Y654-β-catenin levels were dramatically higher in all FL-HCC. While GS levels remained unequivocal, cyclin-D1 levels were higher in FL-HCC. Indeed the consistency in overexpression of target genes during β-catenin/TCF activation and how it is determined remains a dilemma. To address this issue, studies have now shown the role of cofactors such as histone acetlyltransferases to dictate stage- and tissue-specific target gene specificity of β-catenin (56). While the expression and activity of such cofactors remains unknown in the context of various tumors, our observation of elevated Y654-β-catenin and higher cyclin-D1, which is an established target in liver (11) and other tissues (21), does raise an important question of whether such phosphorylation of β-catenin might also have any role in regulating target gene specificity. Currently, our observations in FL-HCC supports excessive receptor tyrosine kinase signaling in this variant of HCC as β-catenin is known to be tyrosine-phosphorylated by many such factors as EGF, HGF and others (14, 15, 57). Indeed, EGFR overexpression has been reported in FL-HCC recently (58). Also, EGFR is known to impact β-catenin phosphorylation at Y654 (14). Thus, the finding of high levels of Y654-β-catenin in FL-HCC makes this tumor specifically susceptible to RTK targeting. Perhaps specific inhibitors of EGFR, which have been shown to be upstream of β-catenin or non-specific RTK inhibitors such as Sorafenib, might have strong therapeutic implications in the fibrolamellar variant of HCC (59, 60).
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support of Dr. Tong Wu for his critical discussions and Dr. Rajiv Dhir for his tissue bank leadership and organization. We would like to also thank Ms. Amanda Micsenyi and Ms. Emily Boyd for their technical support. Ms. Boyd was the recipient of the Summer Undergraduate Research Program fellowship from the University of Pittsburgh Cancer Institute.
Grant Support: This study was funded by NIH, grant numbers 1R01DK62277 and 1R01CA124414 to SPSM and by Rango's Fund for Enhancement of Pathology Research.
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bergsland EK, Venook AP. Hepatocellular carcinoma. Curr Opin Oncol. 2000;12:357–361. doi: 10.1097/00001622-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 4.McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136:125–135. doi: 10.1016/j.jss.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 8.Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287–4295. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 10.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 11.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, et al. Apc tumor suppressor gene is the ”zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 14.Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A, Dunach M, et al. Geldanamycin abrogates ErbB2 association with proteasome-resistant beta-catenin in melanoma cells, increases beta-catenin-E-cadherin association, and decreases beta-catenin-sensitive transcription. Cancer Res. 2001;61:1671–1677. [PubMed] [Google Scholar]
- 15.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 16.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 18.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 19.Loeppen S, Koehle C, Buchmann A, Schwarz M. A beta-catenin-dependent pathway regulates expression of cytochrome P450 isoforms in mouse liver tumors. Carcinogenesis. 2005;26:239–248. doi: 10.1093/carcin/bgh298. [DOI] [PubMed] [Google Scholar]
- 20.Loeppen S, Schneider D, Gaunitz F, Gebhardt R, Kurek R, Buchmann A, Schwarz M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002;62:5685–5688. [PubMed] [Google Scholar]
- 21.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]
- 23.Monga SP, Micsenyi A, Germinaro M, Apte U, Bell A. beta-Catenin regulation during matrigel-induced rat hepatocyte differentiation. Cell Tissue Res. 2006;323:71–79. doi: 10.1007/s00441-005-0045-8. [DOI] [PubMed] [Google Scholar]
- 24.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, Malhotra V, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 28.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 30.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 31.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 32.Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, et al. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 33.Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, Kim M, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 37.Takagi H, Sasaki S, Suzuki H, Toyota M, Maruyama R, Nojima M, Yamamoto H, et al. Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J Gastroenterol. 2008;43:378–389. doi: 10.1007/s00535-008-2170-0. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 39.Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 42.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 43.Kim YD, Park CH, Kim HS, Choi SK, Rew JS, Kim DY, Koh YS, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:110–118. doi: 10.1111/j.1440-1746.2007.05250.x. [DOI] [PubMed] [Google Scholar]
- 44.Behari J, Zeng G, Otruba W, Thompson MD, Muller P, Micsenyi A, Sekhon SS, et al. R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells. J Hepatol. 2007;46:849–857. doi: 10.1016/j.jhep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, Herault A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 46.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 47.Austinat M, Dunsch R, Wittekind C, Tannapfel A, Gebhardt R, Gaunitz F. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol Cancer. 2008;7:21. doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nusse R. In; 1997-2008.
- 50.Vauthey JN, Ribero D, Abdalla EK, Jonas S, Bharat A, Schumacher G, Lerut J, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg. 2007;204:1016–1027. doi: 10.1016/j.jamcollsurg.2006.12.043. discussion 1027−1018. [DOI] [PubMed] [Google Scholar]
- 51.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 52.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–710. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 54.Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol. 2005;18:1417–1423. doi: 10.1038/modpathol.3800449. [DOI] [PubMed] [Google Scholar]
- 56.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 57.Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buckley AF, Burgart LJ, Kakar S. Epidermal growth factor receptor expression and gene copy number in fibrolamellar hepatocellular carcinoma. Hum Pathol. 2006;37:410–414. doi: 10.1016/j.humpath.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Furuse J. Growth factors as therapeutic targets in HCC. Crit Rev Oncol Hematol. 2008;67:8–15. doi: 10.1016/j.critrevonc.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]