Abstract
Arc, c-fos and zif268 are immediate early genes (IEGs) important for adult brain plasticity. The current study examines developmental expression of these IEGs and the effect of neonatal noradrenergic lesion on their expression in developing and mature brain. N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), a specific noradrenergic neurotoxin, was administered to rats on postnatal day (PND) 3 and in situ hybridization was used to assay Arc, c-fos and zif268 mRNA on PND 13, 25 and 60. In contrast to decreases in Arc, c-fos and zif268 expression produced by noradrenergic lesions of mature brain, lesions on PND3 yield a strikingly different effect. Neonatal lesions produce increases in c-fos and zif268 expression in specific frontal cortical layers on PND13, while Arc shows no change. These lesions lead to increases in zif268 expression in frontal cortical layers on PND 25, with no changes in c-fos or Arc expression, and on PND60 they produce a significant increase in c-fos expression in hippocampus with no significant changes in Arc or zif268 expression. RX821002, an A2AR antagonist, administered to control PND 60 animals produces elevations of Arc, zif268 and c-fos mRNAs. This response was eliminated in animals lesioned with DSP-4 on PND 3. These data indicate that norepinephrine regulation of IEG expression differs in developing and mature brain and that the loss of developmental norepinephrine leads to abnormally high postnatal IEG expression. Several studies have shown an important role for norepinephrine in brain development, including the regulation of synaptic densities and neuronal morphology. Our data support the idea that norepinephrine plays an important role during CNS development and that changes in noradrenergic signaling during development may have long lasting effects, potentially on learning and memory.
Keywords: Arc, c-fos, zif268, DSP-4, Cerebral cortex, Alpha-2 adrenergic receptors
Activation of neurotransmitter receptors initiates an intracellular signaling cascade that frequently leads to changes in the transcription of genes. The immediate early genes (IEGs) are critically poised signaling intermediates within this cascade. These transcription factors are rapidly activated by neuronal depolarization and regulate a diverse array of promoters, allowing the neuron to translate its activity into cellular modifications subserving plasticity (Tischmeyer and Grimm, 1999).
Many studies have demonstrated the importance of IEG expression for brain functions. Including learning and memory. Induction of long-term potentiation (LTP), proposed to be the physiological basis of memory, is associated with rapid and robust transcription of the IEG, zif268 (egr-1, Krox-24, NGFI-A, TIS8), in the hippocampus (Davis et al., 2000). Mice with a targeted disruption of zif268 exhibit an impaired ability to form LTP and long term memory (Jones et al., 2001). It has also been shown that activation of zif268 is necessary for the reconsolidation and persistence of memories (Lee et al., 2004;). Expression of zif268 in the anterior cingulate cortex is proposed to favor the retrieval of remote fear memory in mice (Frankland et al., 2004). Induction of zif268 within the medial prefrontal cortex and amygdala has been implicated in the extinction of conditioned fear (Herry and Mons, 2004). Zif268 may be important across diverse neuronal populations, including cortex and hippocampus which are the focus of this study, since there is evidence that zif268 regulates the neuronal proteasome (James et al., 2006). This transcription factor also may play an important role in brain development since it is expressed within developing dendrites (Crino et al., 1998).
Activity regulated cytoskeletal protein (Arc) is another IEG involved in regulating brain plasticity. This IEG is unique in that neural activity causes it to be rapidly enriched in rat neuronal dendrites in hippocampus and cortex (Steward et al., 1998; Steward and Worley, 2001, 2002; Kelly et al., 2008). Arc also has attracted attention as a protein expressed in the postsynaptic density/NMDA receptor complex in mice (Husi et al., 2000; Steward and Worley, 2001). These studies suggest that Arc allows selective strengthening of postsynaptic junctions following their activation. Similar to zif268, Arc expression is increased during LTP in mice, and disruptions in learning and memory occur when Arc induction is blocked (Guzowski et al., 2000).
Studies have supported the idea that c-fos also plays a role in learning and memory in many species (Dragunow, 1996). Expression of c-fos in rat amygdala is important for encoding taste aversion memory (Lamprecht and Dudai, 1996). Expression of c-fos in the rat medial frontal cortex is important for the acquisition of conditioned fear (Morrow et al., 1999). Several studies have shown that while c-fos expression may not be necessary for short-term memory, it does play an important role in many forms of long term memory in rat and chicken (Mileusnic et al., 1996; Countryman et al., 2005a; Countryman et al., 2005b; Yasoshima et al., 2006a; Yasoshima et al., 2006b). Similarly, c-fos involvement in synaptic plasticity has been demonstrated in Drosophila (Sanyal et al., 2002).
In light of the roles played by Arc, c-fos and zif268 in brain plasticity, understanding what signals regulate their expression in the postnatal period and guide them to mature patterns of expression is important. In the current study we examined the role of norepinephrine in this process. Norepinephrine is an important candidate for multiple reasons. In mature animals norepinephrine has a documented role in regulating IEG expression. Lesioning noradrenergic fibers with DSP-4 results in a reduction in brain expression of zif268, Arc and c-fos in postsynaptic cells (Pompeiano et al., 1994; Cirelli et al., 1996). Further studies have pointed to a role for specific adrenergic receptors, particularly alpha-2 adrenergic receptors (A2AR), in regulating IEG expression. For example, local administration of the A2AR antagonist, yohimbine, increases c-fos within the cerebral cortex (Stone et al., 1993). RX821002, another A2AR antagonist, increases zif268 in cortex, hippocampus and amygdala (Shen et al., 1995; Shen and Gundlach, 2000).
In addition to its role in the adult brain, data indicate norepinephrine is a regulatory factor in brain development. Noradrenergic neurons in the rat differentiate by gestational day (GD) 12 and project to the cortex at GD 17 (Lauder and Bloom, 1974; Coyle and Molliver, 1977; Schlumpf et al., 1980). The timing of noradrenergic cortical innervation coincides with many important events relevant to cortical development including neurogenesis, neuronal migration, sprouting of cellular processes, and the formation of synaptic contacts. This occurs largely within the first three weeks of postnatal development, a time period in which noradrenergic innervation is established and subsequently increases to adult levels (Markus and Petit, 1987; Berger-Sweeney and Hohmann, 1997; Murrin et al., 2007). The timing of noradrenergic cortical and hippocampal innervation also coincides with the developmental expression of A2AR and IEGs within these areas. Similar to postnatal IEG expression, the norepinephrine transporter and alpha-2 adrenergic receptors exhibit low cortical and hippocampal expression at PND 5, with large increases in expression by the second to third postnatal week (Herms et al., 1994; Happe et al., 2004; Sanders et al., 2005). Also similar to IEGs, this expression decreases with further maturation (Happe et al., 2004; Sanders et al., 2005). Other markers for the noradrenergic system, including tyrosine hydroxylase, alpha-1 and beta adrenergic receptors and norepinephrine itself follow a similar pattern (see Murrin et al., 2007).
Taken together, these data suggest that norepinephrine plays an important role in regulating developmental expression of IEGs.. As a consequence, altering noradrenergic signaling during the neonatal period may alter the development of IEG expression and regulation by A2AR. This could have long term consequences for functions in which IEGs play an important role, such as learning and memory. In the current study we characterize the postnatal expression of zif268, Arc and c-fos in rat cortex and hippocampus. We also examine the role of norepinephrine in regulating the expression of these IEGs during this developmental period using neonatal noradrenergic lesions and we show differences compared to IEG regulation in mature brain.
EXPERIMENTAL PROCEDURES
MATERIALS
[3H]Nisoxetine (85 Ci/mmol) was obtained from Perkin-Elmer (Boston, MA). N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4) and 2-[2-(2-methoxy-1,4-benzodioxanyl)]imidazoline hydrochloride (RX821002 HCl) were obtained from Sigma-Aldrich (St. Louis, MO). For in situ hybridization studies, all reagents were molecular biology grade and were RNAase free and were from Sigma-Aldrich. All other chemicals were reagent grade and were from Sigma-Aldrich.
ANIMALS AND DSP-4 LESIONS
Sprague-Dawley rats (Sasco, Kingston, NY) were bred in our colony. On PND 3 animals of either sex were treated with 50 mg/kg of DSP-4 in sterile saline, i.p. (n=4).. Control animals received saline (n=4). Brains from control animals were collected on PNDs 5, 13, 14, 25 and 60. Brains from DSP-4 lesioned animals were collected on PND 13, 25 and 60. Animals were allowed to remain quietly in their home cage for one hour and were then taken to a separate room where they were decapitated under isoflurane anesthesia. Brains were removed, divided in half, and one hemisphere was immediately homogenized and used for [3H]nisoxetine binding, a specific marker for noradrenergic terminals, to determine lesion efficacy. The opposite hemisphere was frozen on dry ice, stored at −80 °C until used for in situ hybridization studies. All animal use procedures were in strict accordance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications 80–23) revised 1996 and were approved by the University of Nebraska Medical Center Animal Care and Use Committee.
DRUG TREATMENTS
At PND 60 rats that were neonatally treated with DSP-4 (n=8) or with saline (n=8) received injections of either RX821002 (α2AR-antagonist, 5 mg/kg, i.p.) dissolved in sterile saline (n=4) or vehicle alone (n=4). Animals were allowed to remain quietly in their home cage for one hour and were then taken to a separate room where they were decapitated under isoflurane anesthesia. Brains were removed, divided in half, and one hemisphere was immediately homogenized and used for [3H]nisoxetine binding. The opposite hemisphere was frozen on dry ice, stored at −80 °C until used for in situ hybridization studies.
[3H]NISOXETINE BINDING
[3H]Nisoxetine binding to brain homogenates used a modification of the methods of Gehlert and colleagues (Gehlert et al., 1995). Freshly dissected cortex was homogenized in 30 volumes of 50 mM Tris, pH 7.4, for 30s on a low Tissumizer setting (10,000 rpm, Tekmar Co., Cincinnati, OH). This homogenate was then centrifuged at 1000g for 10 min at 2 °C and the supernatant (S1) transferred to a clean, pre-weighed tube. Following centrifugation of S1 at 20,000g for 20 min, the resulting supernatant was discarded and the pellet was resuspended in 15 vol of 50 mM Tris using a low Tissumizer setting for 10 sec. This homogenate was then centrifuged at 20,000g, the supernatant was discarded and the tube was weighed again and its empty weight was subtracted to obtain the pellet weight. The pellet was suspended in 10 volumes of assay buffer: 50 mM Tris, 300 mM NaCl, and 5 mM KCl, pH 7.4. A 250 μL aliquot of this suspension was mixed with 50 μL of 20 nM [3H]nisoxetine in the assay buffer (final concentration: 2 nM) and 200 μL of assay buffer. Nonspecific binding was defined by 100 μM desmethylimipramine. Samples were incubated at 4 °C for 4 hr, followed by addition of 5 mL of ice cold buffer. Membranes were then collected by vacuum filtration with a Cell Harvester (Brandel, Gaithersburg, MD) on GF-B filters (Whatman) that had been presoaked in 0.3% polyethyleneimine (Sigma-Aldrich, St. Louis, MO). Samples were washed twice with 5 mL of ice-cold buffer. Bound radioligand was determined by liquid scintillation spectrophotometry.
IN SITU HYBRIDIZATION
Sixteen micron tissue sections were cut in a cryostat and thaw-mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were fixed in ice cold 4% paraformaldehyde in phosphate buffered saline (PBS) pH 7.4, for 5 min, washed in PBS for 1 min and in 75% ethanol for 2 min, and stored in 100% ethanol at 4 °C. Slides were allowed to dry at room temperature prior to in situ hybridization. Oligonucleotide probe sequences were as follows: Arc: 5′-CTT-GGT-TGC-CCA-TCC-TCA-CCT-GGC-ACC-CAA-GAC-TGG-TAT-TGC-TGA-3′ (complementary to bases 789–833, NM_019361.1)’; zif268: 5′-CCG-TTG-CTC-AGC-AGC-ATC-ATC-TCC-TCC-AGT-TTG-GGG-TAG-TTG-TCC-3′ (complementary to bases 355–399, NM_012551.1); c-fos: 5′-CAG-GGC-TAG-CAG-TGT-GGG-GGA-GCT-CAG-TGA-GTC-AGA-GGA-3′ (complementary to bases 1465–1503, XM_234422.2). A Blast search (Altschul et al., 1990) of Genbank found that these sequences do not have significant homology with any other sequences. Probes were 3′ end labeled with [35S]-dATP (1200 Ci/mmol, Perkin Elmer, Boston, MA) using terminal deoxyribonucleotidyl transferase (3′ End Labelling System, Perkin Elmer). Labeling reactions were purified of unincorporated radionucleotide with Biospin-6 chromatography columns (BioRad, Hercules, CA) by centrifuging at 2500 rpm for 5 min. Hybridization buffer (150 μl of 50% formamide, 4XSSC, 8% dextran sulfate, 1XDenhardt’s, 500 μg/mL salmon sperm DNA, 270 μg/mL yeast tRNA, 0.1 mM DTT) containing 1×106 cpm of labeled probe was applied to each slide. Non-specific labeling was determined by inclusion of 10X unlabeled probe. Slides were coverslipped, sealed with D.P.X. (Aldrich Chemical Co., Milwaukee, WI) and placed overnight in a 1XSSC humidified sealed Tupperware container at 42 °C. The next day coverslips were removed in 55°C 1XSSC and slides were washed 4×15min in 1XSSC at 55 °C. After washing, slides were briefly dipped in room temperature water to remove salts and in 70% ethanol to facilitate drying. Slides were apposed to Biomax film (Kodak, Rochester, NY) for 2–3 weeks. Films were developed using standard techniques and analyzed using the MCID-M7 image analysis system (Interfocus Imaging, Ltd., Linton, England). For figure 1 images were normalized to the same color calibration scale for presentation of comparative data. This does not change the underlying density data for each image.
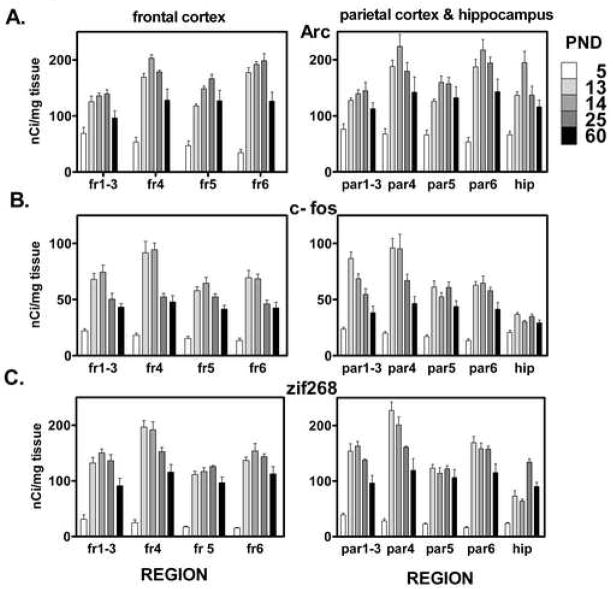
Figure 1.
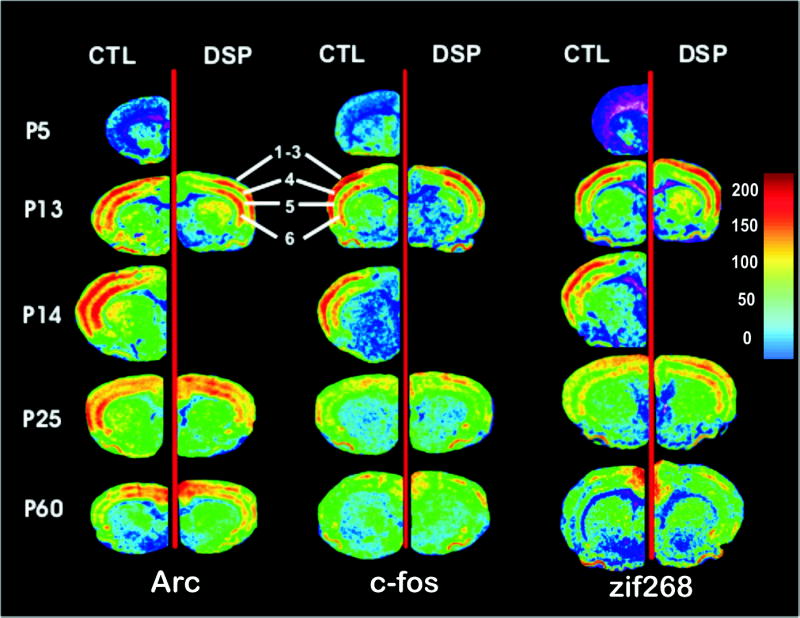
In situ hybridization autoradiographs for Arc, c-fos and zif 268 at PND 5, 13, 14, 25 and 60. These images correspond to 0.7 mm anterior to bregma in adult animals (plate 15, Paxinos and Watson, 1998) and present frontal cortex. The greatest enrichment of Arc, c-fos and zif268 mRNA is seen in layers 4 and 6 at PND 13 and 14. This decreases through P25 to adulthood. IEG expression in control brains (CTL) is compared to expression in rats that were treated with DSP-4 on PND 3. Images are normalized to one another in terms of color. Data for DSP4 treated animals were not collected on PND 14. PND 14 animals were used only for comparison with PDN 13 animals during a period of robust synaptogenesis, as indicated in the text.
The calibration bar indicates the density of mRNA and is calibrated in nCi/mg tissue. All images are at the same magnification. Differences in size between images are due to age, variations in shrinkage during processing or to differences in the sex of the animals.
Autoradiographic densities were quantified using commercial tritium standards (American Radiochemicals, St. Louis, MO) that were previously calibrated to 35S (Miller and Zahniser, 1987). Cortical expression was measured at two coronal levels in order to view IEG development in rostral and caudal cortex. These levels corresponded to 0.7 mm anterior to the bregma and 3.3 mm posterior to the bregma (plates 15 and 33; Paxinos and Watson, 1998), and are referred to as frontal and parietal cortex, respectively Determinations were taken from one to two sections per coronal level. Neuroanatomy was assessed by comparing sections to the rat brain atlas of Paxinos and Watson. Assays involving different ages of animals were carried out on the same day using the same hybridization probe preparation.
STATISTICS
Development of IEG expression was compared across ages using a two-way ANOVA with the Bonferroni post hoc test. Differences in control and DSP-4-treated brain areas were analyzed with Student’s two-tailed T test. Data from studies examining the effects of neonatal DSP-4 on adult IEG expression and their response to RX821002 were analyzed by a one-way ANOVA with Tukey multiple comparison post hoc test. There were no significant differences between sexes at the ages examined and so data were collapsed across sexes. Data from the RX821002 treatment studies are presented as the percentage change compared to the average basal expression level. [3H]Nisoxetine binding data were analyzed with Student’s two-tailed t-test. Tables of detailed values and statistical comparisons are presented as supplementary material.
RESULTS
BRAIN EXPRESSION OF IMMEDIATE EARLY GENES DURING DEVELOPMENT
Expression of IEGs was assayed at PND 5, 13, 14, 25 and 60. These ages were chosen since they represent important periods of postnatal brain development. By PND 5 most neuronal division and migration is complete, but differentiation and synaptogenesis have not been well initiated. PND 13 and 14 were assayed since a robust proliferation of synapses has been documented between ~PND 10 and ~PND 25–30 in many brain regions, including the cortex (Markus and Petit, 1987). We measured differences in expression over 24 hrs to determine whether differences could be detected over a short period during intense synaptogenesis. By PND 25 synaptogenesis reaches its peak (Markus and Petit, 1987). Finally, PND 60 was analyzed to view the IEG expression profile in the mature brain.
There is evidence that Arc, c-fos and zif268 are highly expressed in neurons but are also found in astrocytes and glia (Worley et al., 1991; Lyford et al., 1995; Hung et al., 2000; Rodriguez et al., 2008; Nakazawa et al., 2008; Reznikov et al., 2008). Therefore the expression detected in this study reflects contributions from both of these neuronal populations.
Cortical expression was measured at two coronal levels in order to view IEG development in rostral and caudal cortex. These levels corresponded to 0.7 mm anterior to the bregma and 3.3 mm posterior to the bregma (plates 15 and 33; Paxinos and Watson, 1998), and are referred to as frontal and parietal cortex, respectively. Tables of detailed values and statistical comparisons are presented as supplementary material.
Arc
Cortical Arc levels are low at PND 5 with higher expression in superficial cortical layers compared to deep cortical layers (Figs. 1, 2A). By PND 13 Arc mRNA has increased greatly, with 2 to 5 fold increases in cortical expression compared to PND 5. Arc mRNA at PND 13 is rich in layers 4 and 6 of the frontal cortex, especially in the motor and somatosensory cortices, while the increase in cingulate cortex is less (Fig. 1). In the parietal cortex pronounced expression is apparent in layers 4 and 6 as well. Statistical analysis shows an effect of both age (p < 0.0001, df = 4 for both levels) and cortical layer (p < 0.0001, df = 3 for frontal, df = 4 for parietal) and an interaction between layer and age in frontal cortex (p < 0.0002, df = 12). Statistical analysis also shows that, for most regions, Arc expression from PND 13 onward is significantly greater (+39 to +470%) than the expression at PND 5. At PND 14 layers 4 and 6 of the frontal and parietal cortices exhibit Arc mRNA levels that are statistically greater (+51 to +58%) than those found in the PND 60 cortex. The high expression levels at PND 14 begin to diminish by PND 25, with only layer 6 of the frontal cortex still significantly greater than PND 60. Within the PND 60 cortex, Arc is generally decreased compared to its expression at the end of the second postnatal week (Figs. 1, 2A), although expression in cingulate and medial cortex is at its highest level (Fig. 1).
Figure 2.
Development of (A) Arc (B) c-fos and (C) zif 268 expression in cortical layers and hippocampus (CA1–3). Ages represented are PND 5, 13, 14, 25 and 60. Peak expression for the majority of regions occurs at PND 14. Data are expressed as nCi/mg tissue and are mean ± SEM, n=3–4. Cortical expression was measured at two coronal levels corresponding to 0.7 mm anterior to the bregma and 3.3 mm posterior to the bregma in adult rats (plates 15 and 33 in Paxinos and Watson, 1998) and are referred to as frontal and parietal cortex, respectively. For the sake of clarity, statistical differences are not presented in this figure. A complete statistical analysis comparing brain regions by age is presented in supplementary tables.
Abbreviations: fr=frontal cortex, par=parietal cortex, hip=hippocampus; numbers refer to cortical layer.
Hippocampal measurements were taken by combining densities of CA1, CA2 and CA3 regions, all of which were similar. In general, Arc development in hippocampus mirrors that in the cortex. Low Arc mRNA expression levels are seen at PND 5, they peak at PND 14 and then decrease to lower levels of expression with adulthood. Interestingly hippocampal Arc exhibits a strong and statistically significant increase (+42%) in expression from PND 13 to PND 14 (p < 0.05; Fig. 2A). This increase over 24 h of development illustrates the dynamic and rapid changes in Arc expression that can occur during periods of early synaptogenesis and suggests Arc plays an important role in this process.
c-fos
Developmental expression of c-fos generally parallels the pattern found with Arc. Low c-fos mRNA levels are found at PND 5, followed by a pronounced increase at PND 13. c-fos expression is significantly higher (+58 to +422%) in most regions at all later ages compared to PND 5. At the end of the second postnatal week c-fos expression is especially high in layer 4 of the frontal and parietal cortex (Figs. 1, 2B), especially in the somatosensory cortex (Fig. 1). Statistical analysis shows an effect of both age (p < 0.0001, df = 4 for both levels) and cortical layer (p < 0.0001, df = 3 for frontal, df = 4 for parietal) and an interaction between layer and age for frontal (p < 0.032, df = 12) and parietal cortex (p < 0.0001, df = 16). At PND 14 c-fos transcript levels are statistically greater (+55 to +102%) in all regions examined compared to adult, with the exception of parietal cortex layer 5 and the hippocampus. In contrast to Arc, c-fos expression is most enriched developmentally in superficial cortical layers, with less expression in layer 6. This is especially evident in the parietal cortex. c-fos also differs from Arc, as well as zif268, at PND 25. At this age Arc and zif268 are highly expressed in cortical layers 4 and 6, while c-fos is more homogenous in its expression across cortical layers (Fig. 2B). Similar to Arc and zif268, c-fos expression decreases with adulthood particularly in superficial cortical layers (Fig 1B, Fig 2), a pattern also seen with zif268.
Within the hippocampus developmental c-fos expression is less dynamic than within cortex. Expression increases to PND 13, and from this age on is elevated relative to PND 5 values, although none of these differences reached statistical significance with two-way ANOVA. Unlike cortex, hippocampal c-fos does not exhibit a peak in expression at PND 13, but remains at a similar level at all ages after PND 5. (Figs. 1, 2B).
zif268
zif268 has a very low level of expression at PND 5 across frontal and parietal cortical layers. At this age expression is characterized by higher densities in the superficial layers of the cerebral cortex compared to deeper cortical layers. Statistical analysis shows an effect of both age (p < 0.0001, df = 4 for both levels) and cortical layer (p < 0.0001, df = 3 for frontal, df = 4 for parietal) and an interaction between layer and age for frontal (p < 0.01, df = 12) and parietal cortex (p < 0.0001, df = 16). By PND 13 a striking 4- to 10-fold increase in zif268 expression is evident (Figs. 1, 2C), particularly in somatosensory and lateral motor cortices (Fig. 1). At this age cortical zif268 mRNA expression is highest in layers 4 and 6, with lower expression in layers 1–3 and layer 5. At PND 25 levels of zif268 transcript decrease slightly from PND 13 levels across most cortical layers. With adulthood most cortical layers contain less zif268 mRNA than is found at PND 13, 14 and 25. Statistical analysis of cortical expression across different ages demonstrates that in all cortical layers zif268 expression from PND 13 onward is significantly greater (+150 to +680%) than expression at PND 5. By adulthood, expression decreases from levels found between PND 13 and PND 25 and many layers exhibit statistically significant decreases (−27 to −41%) in expression relative to PND 13 and 25 (Figs. 1, 2C).
zif268 mRNA expression in the hippocampus is similar to the cortex in that it is low at PND 5 and dramatically increases by PND 13. However, unlike the cortex, the hippocampus does not exhibit peak zif268 expression until PND 25 as opposed to a peak at PND 13 in cortical layers (Fig 2C). Hippocampal zif268 expression decreases significantly by PND 60. The peak at PND 25 in hippocampal zif268 expression is unique to zif268, compared to the other two IEGs examined.
EFFECT OF NEONATAL DSP-4 NORADRENERGIC LESION ON POSTNATAL BRAIN IEG EXPRESSION
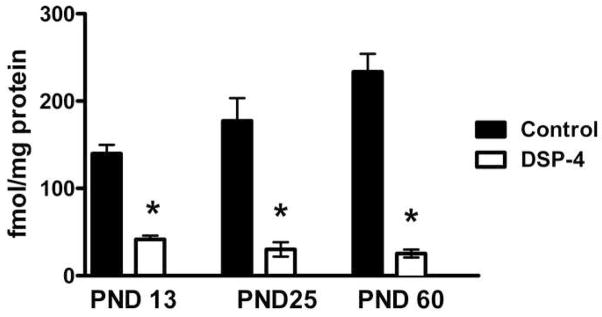
Rat pups were administered 50 mg/kg DSP-4 i.p. on PND 3. This protocol has been shown to produce a ~90% depletion of endogenous norepinephrine (Jonsson et al., 1982). Our own studies have verified that neonatal administration of DSP-4 leads to a comparable loss of norepinephrine (data not shown) and that this decrease correlates well with decreases in [3H]nisoxetine binding. Nisoxetine binds with high selectivity to the norepinephrine transporter (NET), which is located on noradrenergic terminals (Tejani-Butt, 1992), reflecting noradrenergic innervation. Brains from rats lesioned with DSP-4 on PND 3 were collected on PND 13, 25 and 60. One hemisphere of the brain was dedicated to [3H]nisoxetine binding to verify the lesion. The opposite hemisphere was used for in situ hybridization analysis of Arc, c-fos and zif268 mRNA. We found that injecting DSP-4 on PND 3 produced major decreases in hemispheric NET at PND 13, 25 and 60 (−70%, −83%, −89% respectively; Fig 3). Autoradiographic studies show that [3H]nisoxetine binding is reduced to background levels in cortex and hippocampus, the regions examined in this study. Normal binding remains in ventral regions of the brain innervated by projections from the A1 and A2 cell groups (data not shown).
Figure 3.
Effects of DSP-4 lesion at PND 3 on cortical NET levels. DSP-4 lesions at PND 3 decrease [3H]nisoxetine binding in cortical synaptosomes at PND 13, 25 and 60.
* - p<0.001: significantly different from control at the same age; Students two-tailed t test.
Lesioning adult rats with DSP-4 has been shown to cause a pronounced reduction in Arc, c-fos and zif268 expression (Pompeiano et al., 1994; Cirelli et al., 1996; Cirelli and Tononi, 2000). We conducted preliminary studies on rats administered DSP-4 at 48 days of age to verify that DSP-4 would decrease IEG expression. Examining these animals on PND 62 (two weeks later) revealed an ~80% decrease in Arc expression across the frontal and parietal cortices and a ~25% decrease in zif268 expression across frontal cortical layers (data not shown), in agreement with the previous studies. In striking contrast to the adult, we found that neonatal DSP-4 either produced no change or increases in IEG expression compared to controls.
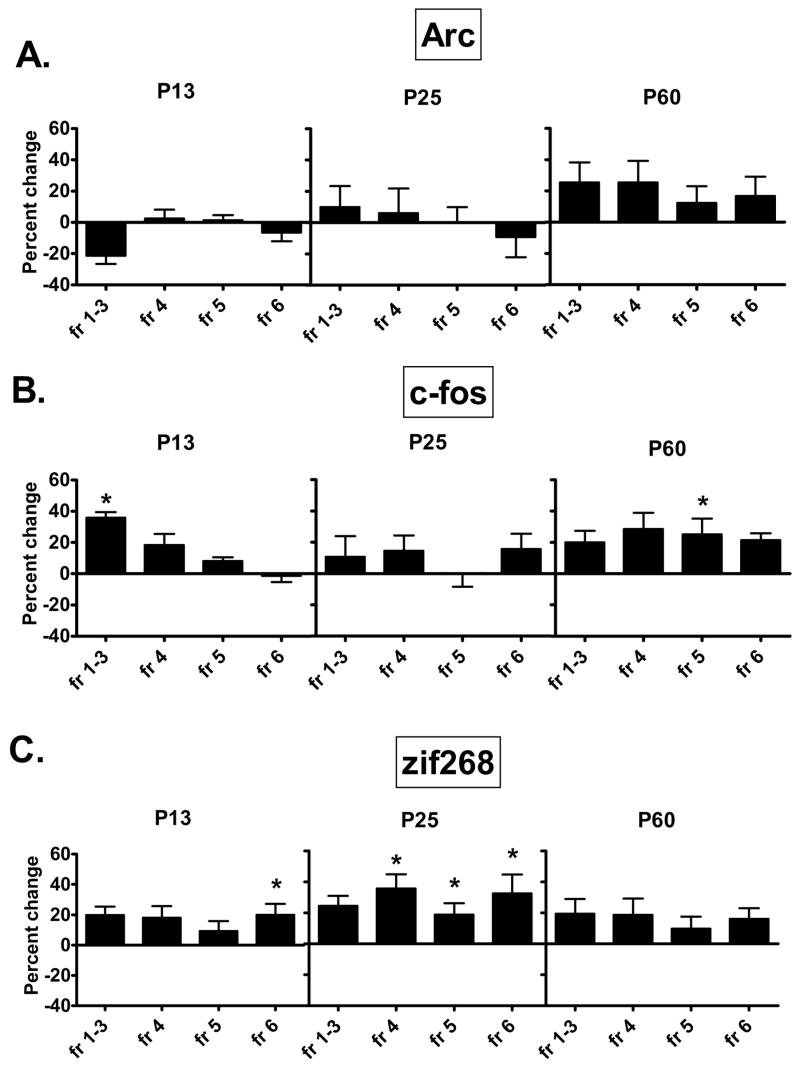
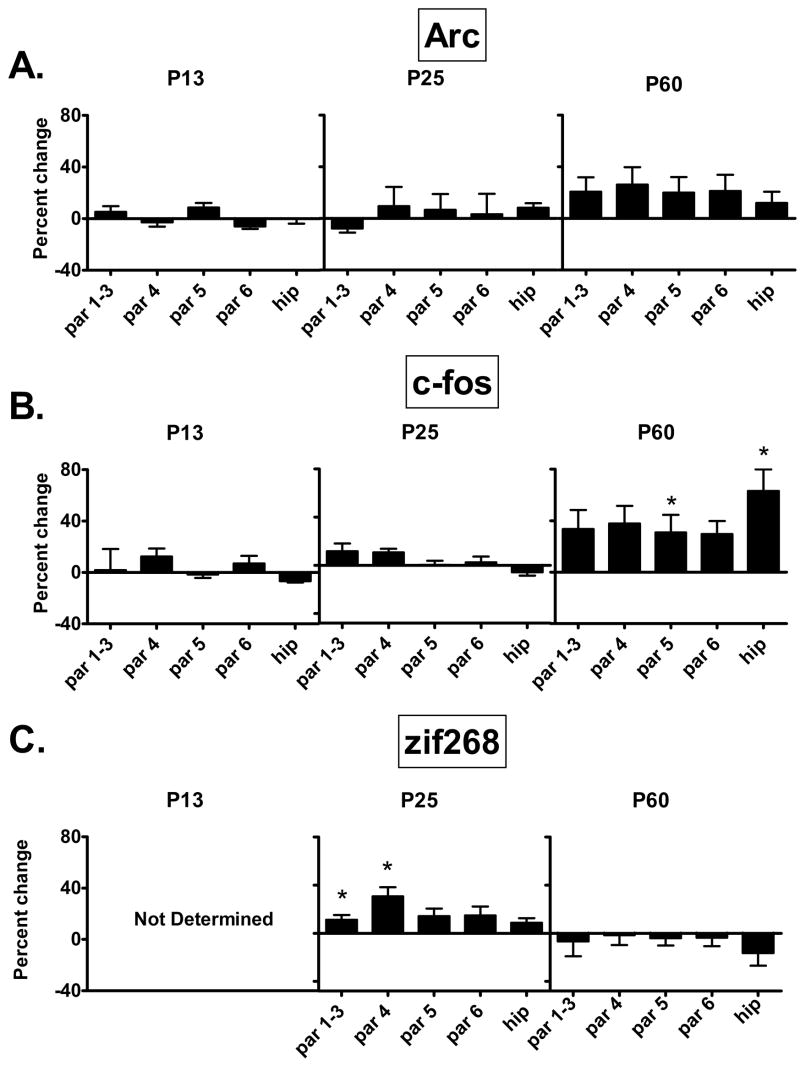
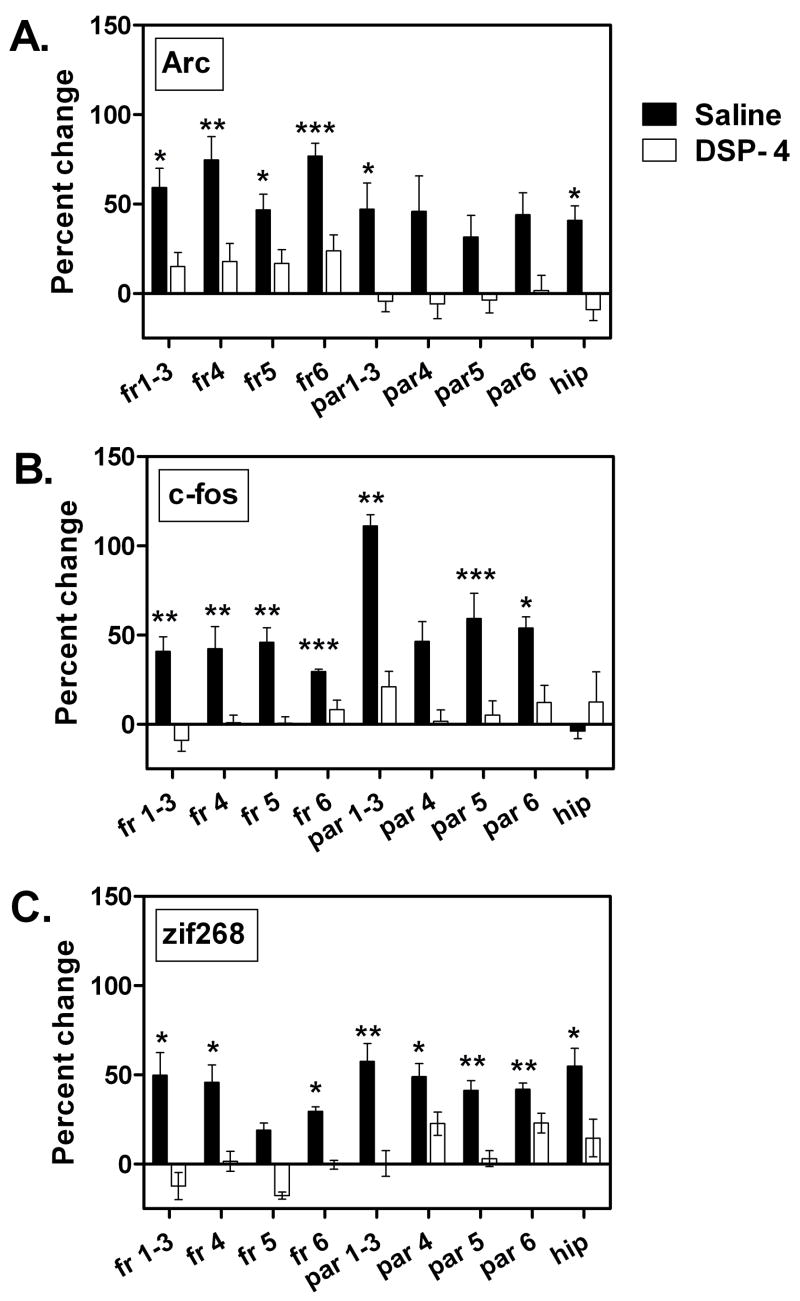
Arc mRNA was expressed at similar levels compared to controls across frontal and parietal cortical layers in animals administered DSP-4 on PND 3. This is evident at PND 13, 25 and 60 (Figs. 1,4A,5A). In general the effect of the DSP-4 lesion on c-fos was to produce a slight increase in expression and in a few regions this increase reaches statistical significance. Frontal cortical layers 1–3 display a 36% increase in c-fos mRNA at PND 13. In the mature brain of animals lesioned on PND 3, layer 5 of frontal and parietal cortices and hippocampus have statistically significant (+25%) increases in c-fos expression (Figs. 1,4B,5B). Animals examined on PND 13 show a 20% increase in zif268 mRNA within layer 6 of the frontal cortex due to the lesion By PND 25 frontal cortical increases of 36%, 19% and 32% are seen in layers 4, 5 and 6, respectively, with similar small increases seen in layers 1–3 and 4 of the parietal cortex (Figs. 1, 4C, 5C). By PND 60, differences between control and DSP-4 treated animals disappear and zif268 expression is similar across cortical layers (Figs. 1,4C,5C).
Figure 4.
The effect of neonatal noradrenergic denervation on (A) Arc, (B) c-fos, and (C) zif268 expression in layers of frontal cortex. Arc expression during development is unaltered by the neonatal lesion. In DSP-4 lesioned animals c-fos and zif268 display transient increases compared to controls at PND 13 and 25. These disappear by PND 60. Each graph shows the percent change in IEG levels of animals treated with DSP on PND 3, relative to animals injected with saline on PND 3. Data were analyzed by a one-way ANOVA with Tukey multiple comparison post hoc test.
fr=frontal cortex; numbers refer to cortical layer.
* - p<0.05: Significantly different from control at the same age.
Figure 5.
The effect of neonatal noradrenergic denervation on (A) Arc, (B) c-fos, and (C) zif268 expression in layers of parietal cortex and in the hippocampus (CA1–3). Arc expression during development is unaltered by the neonatal lesion. c-fos expression shows small increases in layer 5 and hippocampus compared to controls at PND 60. In DSP-4 lesioned animals zif268 displays transient increases in expression at PND 13 and PND 25 compared to controls, and these disappear by adulthood. Each graph shows the percent change in IEG levels of animals treated with DSP on PND 3, relative to animals injected with saline on PND 3. All data are from the same group of animals (n=4). Data were analyzed by a one-way ANOVA with Tukey multiple comparison post hoc test. Tissue for PND 13 examination of zif268 were lost during processing.
par=parietal cortex; hip=hippocampus (CA1–3); numbers refer to cortical layer.
*p<0.05: Significantly different from control at the same age.
EFFECT OF NEONATAL DSP-4 ON RX821002 INDUCED INCREASES IN Arc, c-fos AND zif268
Previous studies of the role of alpha-2 adrenergic receptors (A2AR) in IEG regulation have found increases in IEG mRNA levels (c-fos, c-jun, zif268) subsequent to the administration of RX821002, a selective A2AR antagonist (Shen and Gundlach, 2000). We assayed levels of Arc, c-fos and zif268 mRNA after administration of RX821002, and we investigated whether this response was altered in adult rats that were treated with DSP-4 as neonates.
Administration of RX821002 (5 mg/kg, i.p.) to PND 60 intact rats produces a 25–100% increase in Arc, c-fos and zif268 mRNA expression across layers of the frontal and parietal cortex (Fig. 6). This response is most robust for Arc within the frontal cortex (Fig. 6A). In frontal cortical layers 4 and 6 Arc levels increase by ~75% over control levels. Arc increases by ~50% in layers 1–3 and layer 5. In the parietal cortex and hippocampus increases in Arc are also found, but only parietal cortex layers 1–3 and hippocampus show a statistically significant elevation (Fig. 6A). c-fos increases by approximately 50% in layers of the frontal cortex, and increases by ~50–100% in the parietal cortex. The greatest elevation is in layers 1–3 of the parietal cortex (Fig. 6B). In contrast to Arc and zif268, c-fos does not increase in hippocampus after RX821002. Zif268 increases by ~25–50% across frontal and parietal cortical layers as well as in the hippocampus (Fig. 6C). These effects demonstrate that A2AR maintain a tonic inhibition of Arc, c-fos and zif268 in mature cortex and hippocampus. This inhibition may reflect contributions from presynaptic auto-inhibitory A2AR. This inhibition may further consist of a contribution from postsynaptic A2AR, which are considered to constitute the majority of the A2AR population within rat cortex and hippocampus, comprising approximately 80% of the total A2AR population in the cortex and hippocampus (Heal et al., 1993).
Figure 6.
The effect of RX821002 administration (5 mg/kg, i.p.) on (A) Arc, (B) c-fos and (C) zif268 in PND 60 animals with or without neonatal DSP-4 lesion. Black bars show changes in IEG expression 1 hr after RX821002 administration in control (saline-treated) animals. Clear bars show changes in IEG expression 1 hr after RX821002 administration in rats treated on PND 3 with DSP-4. Each graph shows the percent change in IEG levels following treatment with RX821002 relative to saline injected PND 60 animals that had the same treatment on PND 3. Data are quantified according to cortical layer. Data were analyzed by a one-way ANOVA with Tukey multiple comparison post hoc test.
fr=frontal cortex, par=parietal cortex, hip=hippocampus; numbers refer to cortical layer.
* - p<0.05, ** - p<0.01, *** - p<0.001: significantly different from control.
Treating PND 3 rats with DSP-4 results in a dramatic loss of noradrenergic innervation to the cortex at PND 60 (Fig. 3). In situ hybridization quantification of Arc, zif268 and c-fos IEG mRNA levels shows that neonatal DSP-4 treatment left basal levels of mRNA for these IEGs unaltered at PND 60 in nearly all regions (Figs. 4,5). In contrast to control rats, treatment of neonatally DSP-4-lesioned rats with RX821002 at PND 60 does not result in a significant increase in these mRNAs within the frontal and parietal cortices (Fig. 6). [125I]Para-iodoclinidine binding (Sanders et al., 2006) was used on adjacent sections to determine changes in the density of A2AR due to treatment with neonatal DSP-4. We found no significant differences between control and DSP-4 lesioned brains (J. Sanders, unpublished data). Therefore the changes we have observed do not appear to be due to a developmental alteration in A2AR density as a result of the lesion.
DISCUSSION
Immediate early genes (IEGs) are a widely conserved component of genomic signaling. These genes are critical signaling intermediates in systems from viruses to mammals. IEGs were originally uncovered as components of viral genomes (Grasso and Buchanan, 1969; Salser et al., 1970; Honess and Roizman, 1974). The synthesis of these viral transcripts within host cells occurs within minutes of infection and is independent of de novo protein synthesis, hence the name “immediate early.”
Analogous gene expression cascades, characterized by transcription that is protein synthesis-independent, accompany the growth of mammalian cells. The IEG, c-fos, increases rapidly in mammalian cells, with mRNA levels peaking at 40 to 120 minutes after growth factor stimulation (Lau and Nathans, 1987). Studies in neurons identified transcripts that exhibited similar rapid increases in expression and did not require de novo protein synthesis (Hughes and Dragunow, 1995). Zif268, for instance, was identified when nerve growth factor (NGF) stimulated PC12 cells, producing large increases in zif268 mRNA 30 to 60 minutes post-stimulation (Milbrandt, 1987). A similar profile of Arc induction is seen in PC12 cells stimulated with NGF, fibroblast growth factor (FGF) or epidermal growth factor (EGF) (Lyford et al., 1995).
Though the investigation of IEGs in neurons historically originated with in vitro studies of cellular development, in vivo studies have identified IEG activation to be a robust correlate of new memory formation. Arc, c-fos and zif268 mRNA levels increase in the rat hippocampus subsequent to spatial learning tasks (Guzowski et al., 2001). Song-learning in zebra finch is accompanied by increases in ZENK, an ortholog of zif268, within specific auditory nuclei (Mello et al., 1995). During visual discrimination learning paradigms monkeys exhibit an increase in zif268 protein within brain regions known to mediate such learning (Okuno and Miyashita, 1996).
Studies have used inhibition of Arc and zif268 protein synthesis during learning tasks to further examine the significance of their expression. Stereotactic infusions of antisense oligonucleotides to Arc within the rat dentate gyrus disrupts formation of long term memories (Guzowski et al., 2000). Mice with a targeted disruption of zif268 display a similar deficit in the ability to form long term memory (Jones et al., 2001). Infusion of zif268 antisense oligonucleotides into the amygdala interferes with learning associations between neutral stimuli and the reinforcing properties of cocaine (Lee et al., 2005). Given the important role for IEGs in brain function, it is important to understand what signals guide these genes to mature expression and regulation by brain receptors.
IEG in Developing Cortex and Hippocampus
In the current study we report the postnatal developmental expression of the IEGs, Arc, c-fos and zif268, in frontal and parietal cortex and hippocampus, regions that exhibit robust expression of these genes. All three IEGs are weakly expressed in all regions examined at PND 5, followed by a sharp increase at PND 13. By adulthood expression levels decrease from the high levels found at the end of the second postnatal week. We focused on the cerebral cortex and hippocampus since our data and the findings of others have shown Arc and zif268 to be enriched within these structures (Worley et al., 1991; Lyford et al., 1995), and these are regions well innervated by norepinephrine. Lower levels of expression are detectable in regions such as thalamus, hypothalamus and brainstem. Furthermore, DSP-4, a noradrenergic neurotoxin used in these studies, causes long lasting depletion of norepinephrine from cortex and hippocampus but is less effective in pruning noradrenergic terminals in other brain areas such as hypothalamus (Jonsson et al., 1982; J. Sanders, unpublished data).
Norepinephrine Regulation of Brain Development and IEG Expression
We hypothesized that norepinephrine was a likely candidate for regulating the development of IEG expression, including the responsiveness of the IEGs to stimulation in adult brain. The concept that norepinephrine regulates aspects of brain development has been supported by studies showing altered dendritic morphology and orientation secondary to neonatal lesions of the noradrenergic system at the time of cortical differentiation. Treating rats with 6-OHDA during the first four days of postnatal life produces a significant increase in synaptic density through PND 8 (Blue and Parnavelas, 1982). Increases in cortical layer VI dendritic length have been found in the brains of adult rats in which the locus coeruleus was electrolytically lesioned as neonates (Maeda et al., 1974). Similar lesions increase the number of dendritic branches of pyramidal neurons in cortical layers III and IV (Wendlandt et al., 1977). In mice, neonatal 6-OHDA lesions result in changes in the dendritic orientation of layer IV neurons of the somatosensory cortex (Loeb et al., 1987). Because these studies involve lesion of noradrenergic neurons during cortical differentiation, they suggest norepinephrine is important in this phase of neuronal maturation.
Within the mature brain norepinephrine plays an important role in regulating basal IEG levels. An intact noradrenergic projection from the locus coeruleus is required for high levels of expression of several IEGs during the waking state (Cirelli and Tononi, 2004). In contrast to periods of sleep, which are associated with protein synthesis and membrane trafficking and maintenance, the waking state and the activation of IEGs is associated with increased plasticity and with learning and memory (Cirelli and Tononi, 2004). Previous studies have shown >60% decreases in c-fos mRNA and ~50% decreases in zif268 and Arc mRNAs in mature brains 5 days after lesion of noradrenergic neurons with DSP-4 (Yamada et al., 1999; Cirelli and Tononi, 2000). A 30% decrease in Arc, c-fos and zif268 mRNA has been found in adult brains 21 days after lesioning (Cirelli and Tononi, 2004). Our own studies confirmed that Arc and zif268 mRNA decreased 25–80% when animals were lesioned with DSP-4 on PND 48 and brains harvested on PND 62 (J. Sanders, unpublished data).
The findings presented in this paper demonstrate an important role for norepinephrine in early postnatal IEG expression that differs from its role in the mature brain. We show that lesion with DSP-4 at PND 3 effectively denervates cortex and hippocampus of noradrenergic terminals in developing brain as revealed by dramatic decreases in [3H]nisoxetine binding, in agreement with earlier studies (Grzanna et al., 1989; Tejani-Butt et al., 1990). In contrast to the decreases in IEG expression which follow DSP-4 lesions of adult brain, neonatal lesions produce either increases or no change in IEG expression. zif268, in particular, shows 20–30% increases across most layers of the frontal and parietal cortex. This increase is quantitatively the opposite of decreases in zif268 expression seen in mature brains after a similar number of days post-lesion (Cirelli and Tononi, 2004). Arc expression remains unchanged throughout development following DSP-4 lesion. c-fos also remains similar to control with the exception of transient PND 13 increases in cortical layers 1–3 and a small increase in cortical layer 5 of PND 60 brain. These contrast dramatically with the 30% decreases in Arc and c-fos mRNA seen in adult brains after similar post-lesion duration (Cirelli and Tononi, 2004). Our studies agree with similar findings in the dopamine-β-hydroxylase knockout mouse (Szot et al., 1999), a model of developmental norepinephrine deficit. These studies point to significant changes in the regulation of IEG expression in the adult brain due to lack of noradrenergic innervation in the developing brain, they indicate the role of norepinephrine differs in developing and adult brain, and they support the idea that norepinephrine plays an important role in normal developmental processes.
Clinical Implications
Clinically, drugs acting on A2AR are used in the treatment of a variety of psychiatric disorders. Mirtazapine is an antidepressant which acts as an antagonist at A2AR as well as at 5-HT2 and 5-HT3 receptors (Puzantian, 1998). A2AR antagonistic properties are also found in clozapine, an atypical antipsychotic (Richelson and Nelson, 1984) and S18327, an experimental compound with promising potential as an antipsychotic (Millan et al., 2000). It also has been suggested that A2AR antagonists may be therapeutically useful for treatment of neurodegenerative conditions (Marien et al., 2004). Consistent with this idea is the finding that A2AR antagonists protect rats from 6-hydroxydopamine-induced Parkinsonian symptoms (Srinivasan and Schmidt, 2004).
Considered in the context of our findings, a potential explanation for the beneficial effects of A2AR antagonists is their activation of plasticity-associated IEGs. In particular, the therapeutic properties of A2AR antagonists may arise from their ability to produce a brain state with greater potential to respond to insult, a state similar to the early neonatal period. In studies comparing the effects of brain injury at PND 1, PND 10 and in adults, optimal recovery occurs from lesions produced at PND 10 (Kolb et al., 2000; Gonzalez et al., 2003). This timeline for optimal reorganization parallels the developmental pattern of IEG expression in the current study. We find peak IEG expression at the end of the second postnatal week, close to PND 10. As IEGs are important for adult brain plasticity (Guzowski et al., 2000; Jones et al., 2001), a high level of expression may be important for or contribute to optimal recovery. Accordingly, A2AR antagonists that increase the expression of these IEGs in the adult brain may recapitulate states of developmental plasticity and promote functional recovery.
Conclusion
Our current studies provide a detailed analysis of Arc, c-fos and zif268 expression during cortical development. Expression of each of these transcripts is low in the neonatal brain but increases dramatically at PND 13, coincident with the onset of synaptogenesis. This is followed by a decrease in expression of Arc and zif268, whereas basal c-fos expression remains steady. We report that the neonatal brain responds to DSP-4 with moderate increases or no change in the levels of these transcripts at PND 13, 25 and 60 and this is in sharp contrast to the effects of a similar lesion in adult brain. This indicates regulation of these IEGs by noradrenergic innervation is very different when comparing developing to adult brain. Since these IEGs are involved in developmental synaptogenesis and also play important roles in memory and learning, these results indicate that the noradrenergic system is important in both of these critical processes. Our studies also suggest that alterations of noradrenergic system function during development may have significant consequences not only on the normal development of the CNS but on memory and learning throughout life and on other functions in which norepinephrine plays an important role, such as regulation of affective state.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health NS33194 (LCM) and MH64772 (DBH).
Abbreviations
- A2AR
alpha-2 adrenergic receptor
- Arc
activity regulated cytoskeletal protein
- DSP-4
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride
- DTT
dithiothreitol
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GD
gestational day
- IEG
immediate early gene
- LTP
long term potentiation
- NET
norepinephrine transporter
- NGF
nerve growth factor
- PBS
phosphate buffered saline
- PND
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav Brain Res. 1997;86:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The effect of neonatal 6-hydroxydopamine treatment on synaptogenesis in the visual cortex of the rat. J Comp Neurol. 1982;205:199–205. doi: 10.1002/cne.902050211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem. 2005a;84:175–183. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Countryman RA, Orlowski JD, Brightwell JJ, Oskowitz AZ, Colombo PJ. CREB phosphorylation and c-Fos expression in the hippocampus of rats during acquisition and recall of a socially transmitted food preference. Hippocampus. 2005b;15:56–67. doi: 10.1002/hipo.20030. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME. Major innervation of newborn rat cortex by monoaminergic neurons. Science. 1977;196:444–447. doi: 10.1126/science.850788. [DOI] [PubMed] [Google Scholar]
- Crino P, Khodakhah K, Becker K, Ginsberg S, Hemby S, Eberwine J. Presence and phosphorylation of transcription factors in developing dendrites. Proc Natl Acad Sci U S A. 1998;95:2313–2318. doi: 10.1073/pnas.95.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M. A role for immediate-early transcription factors in learning and memory. Behav Genet. 1996;26:293–299. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Schober DA, Gackenheimer SL. Comparison of (R)-[3H]tomoxetine and (R/S)-[3H]nisoxetine binding in rat brain. J Neurochem. 1995;64:2792–2800. doi: 10.1046/j.1471-4159.1995.64062792.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Whishaw IQ, Kolb B. Complete sparing of spatial learning following posterior and posterior plus anterior cingulate cortex lesions at 10 days of age in the rat. Neuroscience. 2003;122:563–571. doi: 10.1016/s0306-4522(03)00295-1. [DOI] [PubMed] [Google Scholar]
- Grasso RJ, Buchanan JM. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969;224:882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Berger U, Fritschy JM, Geffard M. Acute action of DSP-4 on central norepinephrine axons: biochemical and immunohistochemical evidence for differential effects. J Histochem Cytochem. 1989;37:1435–1442. doi: 10.1177/37.9.2768812. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Butler SA, Prow MR, Buckett WR. Quantification of presynaptic alpha 2-adrenoceptors in rat brain after short-term DSP-4 lesioning. Eur J Pharmacol. 1993;249:37–41. doi: 10.1016/0014-2999(93)90659-6. [DOI] [PubMed] [Google Scholar]
- Herms J, Zurmohle U, Schlingensiepen R, Brysch W, Schlingensiepen KH. Developmental expression of the transcription factor zif268 in rat brain. Neurosci Lett. 1994;165:171–174. doi: 10.1016/0304-3940(94)90737-4. [DOI] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Hung AC, Huang HM, Tsay HJ, Lin TN, Kuo JS, Sun SH. ATP-stimulated c-fos and zif268 mRNA expression is inhibited by chemical hypoxia in a rat brain-derived type 2 astrocyte cell line, RBA-2. J Cell Biochem. 2000;77:323–332. doi: 10.1002/(sici)1097-4644(20000501)77:2<323::aid-jcb14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Regulation of the neuronal proteasome by Zif268 (Egr1) J Neurosci. 2006;26:1624–1634. doi: 10.1523/JNEUROSCI.4199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Sundstrom E. Effects of noradrenaline neurotoxin DSP4 on the postnatal development of central noradrenaline neurons in the rat. Neuroscience. 1982;7:2895–2907. doi: 10.1016/0306-4522(82)90112-9. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Cheung YF, Favilla C, Siegel SJ, Kanes SJ, Houslay MD, Abel T. Constitutive activation of the G-protein subunit Galphas within forebrain neurons causes PKA-dependent alterations in fear conditioning and cortical Arc mRNA expression. Learn Mem. 2008;15:75–83. doi: 10.1101/lm.723708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Cioe J, Whishaw IQ. Is there an optimal age for recovery from motor cortex lesions? I. Behavioral and anatomical sequelae of bilateral motor cortex lesions in rats on postnatal days 1, 10, and in adulthood. Brain Res. 2000;882:62–74. doi: 10.1016/s0006-8993(00)02828-6. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Lau LF, Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987;84:1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di CP, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Loeb EP, Chang FF, Greenough WT. Effects of neonatal 6-hydroxydopamine treatment upon morphological organization of the posteromedial barrel subfield in mouse somatosensory cortex. Brain Res. 1987;403:113–120. doi: 10.1016/0006-8993(87)90129-6. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maeda T, Tohyama M, Shimizu N. Modification of postnatal development of neocortex in rat brain with experimental deprivation of locus coeruleus. Brain Res. 1974;70:515–520. doi: 10.1016/0006-8993(74)90261-3. [DOI] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mileusnic R, Anokhin K, Rose SP. Antisense oligodeoxynucleotides to c-fos are amnestic for passive avoidance in the chick. NeuroReport. 1996;7:1269–1272. doi: 10.1097/00001756-199605170-00010. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Rivet JM, Audinot V, Newman-Tancredi A, Maiofiss L, Queriaux S, Despaux N, Peglion JL, Dekeyne A. S18327 (1-[2-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)piperid-1-yl]ethyl]3- phenyl imidazolin-2-one), a novel, potential antipsychotic displaying marked antagonist properties at α1- and α2-adrenergic receptors: II. Functional profile and a multiparametric comparison with haloperidol, clozapine, and 11 other antipsychotic agents. J Pharmacol Exp Ther. 2000;292:54–66. [PubMed] [Google Scholar]
- Miller JA, Zahniser NR. The use of 14C-labeled tissue paste standards for the calibration of 125I- labeled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Ryu M, Nishida K, Pages G, Pouyssegur J, Endo S. ERK1 plays a critical protective role against N-methyl-D-aspartate-induced retinal injury. J Neurosci Res. 2008;86:136–144. doi: 10.1002/jnr.21472. [DOI] [PubMed] [Google Scholar]
- Okuno H, Miyashita Y. Expression of the transcription factor Zif268 in the temporal cortex of monkeys during visual paired associate learning. Eur J Neurosci. 1996;8:2118–2128. doi: 10.1111/j.1460-9568.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Puzantian T. Mirtazapine, an antidepressant. Am J Health Syst Pharm. 1998;55:44–49. doi: 10.1093/ajhp/55.1.44. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Richelson E, Nelson A. Antagonism by neuroleptics of neurotransmitter receptors of normal human brain in vitro. Eur J Pharmacol. 1984;103:197–204. doi: 10.1016/0014-2999(84)90478-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Davies HA, Errington ML, Verkhratsky A, Bliss TV, Stewart MG. ARG3.1/ARC expression in hippocampal dentate gyrus astrocytes: ultrastructural evidence and co-localization with glial fibrillary acidic protein. J Cell Mol Med. 2008;12:671–678. doi: 10.1111/j.1582-4934.2007.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W, Bolle A, Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the “early” RNA appear at different times and that some are “turned off” at late times. J Mol Biol. 1970;49:271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res. 2006;1109:45–53. doi: 10.1016/j.brainres.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature. 2002;416:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Lichtensteiger W, Shoemaker WJ, Bloom FE. Biogenic Amines in Development. 1980. Fetal monoamine systems: early stages and cortical projections; pp. 567–590. [Google Scholar]
- Shen P, Gundlach AL. Differential modulatory effects of alpha- and beta-adrenoceptor agonists and antagonists on cortical immediate-early gene expression following focal cerebrocortical lesion-induced spreading depression. Mol Brain Res. 2000;83:133–144. doi: 10.1016/s0169-328x(00)00216-3. [DOI] [PubMed] [Google Scholar]
- Shen PJ, Burazin TCD, Gundlach AL. Noradrenergic regulation of immediate early gene expression in rat forebrain: differential effects of α1- and α2-adrenoceptor drugs. Mol Brain Res. 1995;28:222–230. doi: 10.1016/0169-328x(94)00208-v. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Treatment with alpha2-adrenoceptor antagonist, 2-methoxy idazoxan, protects 6-hydroxydopamine-induced Parkinsonian symptoms in rats: neurochemical and behavioral evidence. Behav Brain Res. 2004;154:353–363. doi: 10.1016/j.bbr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, John S, Filer JD, Bing G. Effect of locus coeruleus lesion on c-fos expression in the cerebral cortex caused by yohimbine injection or stress. Brain Res. 1993;603:181–185. doi: 10.1016/0006-8993(93)91236-l. [DOI] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, Palmiter RD. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]Nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- Tejani-Butt SM, Brunswick DJ, Frazer A. [3H]Nisoxetine: a new radioligand for norepinephrine uptake sites in brain. Eur J Pharmacol. 1990;191:239–243. doi: 10.1016/0014-2999(90)94155-q. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendlandt S, Crow TJ, Stirling RV. The involvement of the noradrenergic system arising from the locus coeruleus in the postnatal development of the cortex in rat brain. Brain Res. 1977;125:1–9. doi: 10.1016/0006-8993(77)90355-9. [DOI] [PubMed] [Google Scholar]
- Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1991;88:5106–5110. doi: 10.1073/pnas.88.12.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Hada Y, Imamura K, Mataga N, Watanabe Y, Yamamoto M. Differential expression of immediate-early genes, c-fos and zif268, in the visual cortex of young rats: effects of a noradrenergic neurotoxin on their expression. Neuroscience. 1999;92:473–484. doi: 10.1016/s0306-4522(99)00003-2. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Sako N, Senba E, Yamamoto T. Acute suppression, but not chronic genetic deficiency, of c-fos gene expression impairs long-term memory in aversive taste learning. Proc Natl Acad Sci U S A. 2006a;103:7106–7111. doi: 10.1073/pnas.0600869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Scott TR, Yamamoto T. Memory-dependent c-Fos expression in the nucleus accumbens and extended amygdala following the expression of a conditioned taste aversive in the rat. Neuroscience. 2006b;141:35–45. doi: 10.1016/j.neuroscience.2006.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.