Abstract
Red Asian ginseng (Panax ginseng C. A. Meyer, Araliaceae) is used in many Oriental countries. In this study, the saponin constituents and anticancer activities of steamed American ginseng (Panax quinquefolius L.) roots were evaluated. The contents of 12 ginsenosides in the roots were determined using high performance liquid chromatography (HPLC). After the steaming treatment (100 – 120 °C for 1 h and 120 °C for 0.5 – 4 h), the quantity of 7 ginsenosides decreased and that of 5 others increased. The content of ginsenoside Rg3, a previously recognized anticancer compound, increased significantly when the root was steamed at 120 °C for 0.5 – 3 h. The antiproliferative effects of unsteamed and steamed (120 °C for 1 h and 2 h) American ginseng root extracts were assayed by the modified trichrome stain (MTS) method using three cancer cell lines (SW-480, HT-29, NSCLC). Heat-processing increased the antiproliferative effect of American ginseng significantly, and the activity of the extract from roots steamed for 2 h was greater than that of roots steamed for 1 h. Chemical constituents and antiproliferative activities of white and red Asian ginseng have also been evaluated. Five representative ginsenosides, Rb1, Rd, Re, Rg2 and Rg3, were studied. Ginsenoside Rg3 had the most potent effect. The antiproliferative activities of red American ginseng are augmented when ginsenoside Rg3 is increased.
American ginseng (Panax quinquefolius L.) root is a herb commonly used in the USA. [1]. In oriental countries, Asian ginseng (Panax ginseng C. A. Meyer) root is air-dried into white ginseng or steamed at 100 °C to red ginseng [2]. A number of studies point to anticancer properties and other pharmacological activities of Asian ginseng [3], [4], [5], and ginsenosides Rg3 and Rh2 are recognized as active anticancer compounds [3]. Compared with Asian white ginseng, red ginseng has stronger anticancer activities [6], [7]. Recently, there was a report using a steaming process to treat American ginseng root [8]. In the study, however, the treatment temperature was 100 °C and thus, chemical constituents did not change significantly. Additionally, only 6 ginsenosides (excluding ginsenoside Rg3) were analyzed.
We treated American ginseng root at a variety of temperatures and heating times to observe changes in ginsenoside contents and anticancer activities. After heat-processing, the root of American ginseng, like Asian ginseng, changes from white to red [2]; steamed P. quinquefolius root is therefore referred to as red American ginseng.
Twelve ginsenosides (Fig.1) were determined in American ginseng using high performance liquid chromatography (HPLC). All the assayed ginsenosides were dammarane glycosides [9], [10]. The influence of steaming at 100 °C and 120 °C on the ginsenoside content of American ginseng was tested. Compared with unsteamed American ginseng, the roots treated at 100 °C for 1 h had a slightly decreased total ginsenoside content, from 7.95% to 7.32%. For the main saponin contents, ginsenoside Rb1 was changed from 4.940% to 4.463%, and Re from 1.756% to 1.630%; ginsenoside Rg3 increased from 0.003% to 0.048%. Steaming at 120 °C for 1 h decreased the total ginsenoside content to 5.85%, as follows: Rb1, 3.252%; Re, 0.968%; and Rg3, 0.271%. Ginsenoside Rg3 increased significantly at 120 °C.
Fig. 1.
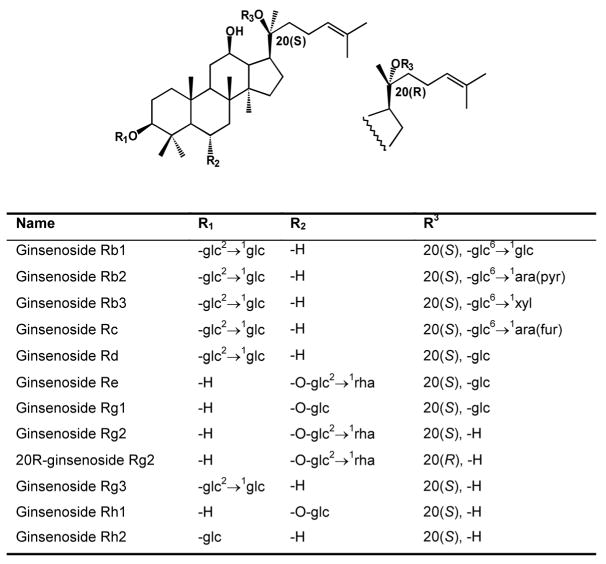
Chemical structures of the assayed ginsenosides.
Chromatograms of unsteamed and steamed American ginseng roots for 2 and 4 h at 120 °C are shown in Figs.2A–C. The peak areas of Rb1, Rd and Re decreased during the steaming process. On the other hand, ginsenoside Rg3, which is a trace saponin in unsteamed root [11], was augmented during the steaming process (Figs. 2B and C). The content of the 12 ginsenosides in steamed American ginseng roots is shown in Table 1. During the steaming process, Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd decreased while Rh1, Rg2, 20R-Rg2, Rg3 and Rh2 increased. Ginsenoside Rg3 increased significantly from 0.5 to 3 h of steaming. Ginsenoside Rh2 is also an active anticancer saponin, however, after steaming treatment, the content of Rh2 was very low (Table 1).
Fig. 2.
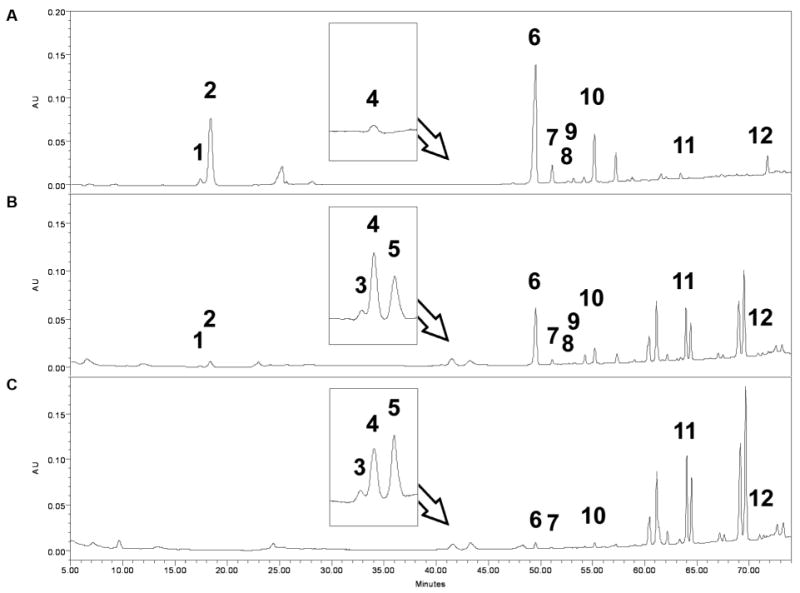
HPLC analysis of ginsenosides in unsteamed and steamed American ginseng roots. Chromatograms of unsteamed (A), or steamed at 120 °C for 2 h (B) and 4 h (C) are shown. Ginsenoside peaks: (1) Rg1, (2) Re, (3) Rh1, (4) Rg2, (5) 20R-Rg2, (6) Rb1, (7) Rc, (8) Rb2, (9) Rb3, (10) Rd, (11) Rg3, (12) Rh2. Peak numbers are not shown if the saponins were not detected.
Table 1.
Ginsenoside contents in Asian ginseng, red Asian ginseng and American ginseng which were unsteamed or steamed at 120 °C for 0.5 – 4h
| Ginsenoside | Asian Ginseng | Red Asian Ginseng | American Ginseng | Steaming Time (Red American Ginseng) | ||||
|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 2 h | 3 h | 4 h | ||||
| Rg1 | 0.279 | 0.276 | 0.133 | 0.098 | 0.075 | 0.017 | ND | ND |
|
| ||||||||
| Re | 0.234 | 0.188 | 1.750 | 1.347 | 0.968 | 0.221 | 0.032 | ND |
|
| ||||||||
| Rh1 | ND | 0.015 | ND | 0.008 | 0.017 | 0.040 | 0.042 | 0.049 |
|
| ||||||||
| Rg2 | 0.031 | 0.034 | 0.015 | 0.154 | 0.266 | 0.405 | 0.444 | 0.363 |
|
| ||||||||
| 20R-Rg2 | ND | 0.022 | ND | 0.086 | 0.155 | 0.388 | 0.425 | 0.422 |
|
| ||||||||
| Rb1 | 0.545 | 0.462 | 4.940 | 3.918 | 3.252 | 1.743 | 0.735 | 0.177 |
|
| ||||||||
| Rc | 0.523 | 0.294 | 0.387 | 0.325 | 0.278 | 0.141 | 0.087 | 0.018 |
|
| ||||||||
| Rb2 | 0.443 | 0.200 | 0.043 | 0.042 | 0.036 | 0.026 | 0.018 | ND |
|
| ||||||||
| Rb3 | 0.067 | 0.032 | 0.063 | 0.064 | 0.050 | 0.035 | 0.017 | ND |
|
| ||||||||
| Rd | 0.257 | 0.073 | 0.604 | 0.510 | 0.460 | 0.335 | 0.206 | 0.097 |
|
| ||||||||
| Rg3 | 0.004 | 0.033 | 0.003 | 0.143 | 0.271 | 0.664 | 1.053 | 1.225 |
|
| ||||||||
| Rh2 | ND | 0.009 | 0.007 | 0.007 | 0.018 | 0.047 | 0.057 | 0.066 |
|
| ||||||||
| Total | 2.38 | 1.64 | 7.95 | 6.70 | 5.85 | 4.05 | 3.11 | 2.42 |
n = 3; ND, not detected; values are expressed as percentage of dry weight; RSD (relative standard derivation) are less than 15%.
The chemical constituents of white ginseng and red ginseng (P. ginseng C. A. Meyer) were also determined. Data shown in Table 1 indicated that for the Asian ginseng, after steaming at 100 °C, the contents of the main ginsenosides (Rg1, Re, Rb1, Rb2, Rc and Rd) were decreased. Ginsenoside Rg3 increased from 0.004% to 0.033%. The changes of ginsenoside contents are similar to those of American ginseng steamed at 100 °C.
The antiproliferative effects on SW-480 and HT-29 human colorectal cancer cells and non-small cell lung cancer cells (NSCLC) of white ginseng and red ginseng extracts have been evaluated. At the concentrations of 0.1, 0.25, 0.5 and 1 mg/mL, although the antiproliferative activities of red ginseng were stronger than that of white ginseng extract, in most cases, there were no significant differences between them. The only exception was that at 0.5 mg/mL for HT-29 cells, the antiproliferative effect of red ginseng (42.9 ± 2.3%) was significantly higher than that of white ginseng (26.4 ± 3.3%) (Fig. 3).
Fig. 3.
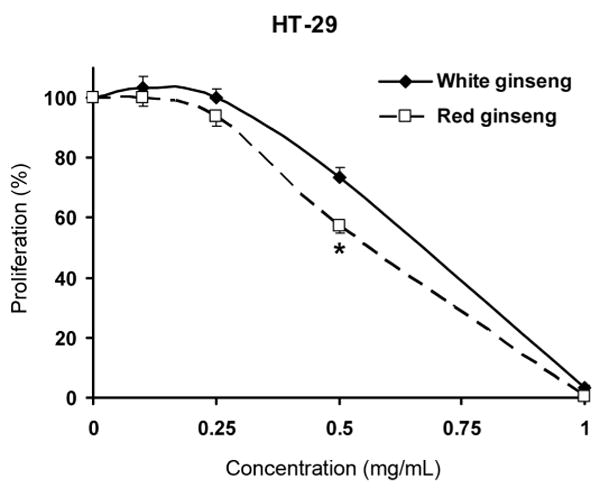
Antiproliferative effects of Asian ginseng root extract on HT-29 cells. Cancer cells were exposed to white ginseng and red ginseng extract (0.1, 0.25, 0.5, 1 mg/mL) for 72 h and cell proliferation was determined by the MTS assay. The results are expressed as the mean ± SD of three independent experiments. * p < 0.05, red ginseng vs. white ginseng.
For the American ginseng, to evaluate the trend of anticancer activities, extracts from roots steamed for 1 h and 2 h were added to SW-480, HT-29 and NSCLC cells (Fig. 4). Unsteamed American ginseng root extract had antiproliferative effects on the three cancer cell lines, but the effects were minimal at 0.1 – 0.5 mg/mL. Treated red American ginseng extracts had significant antiproliferative effects. At 0.5 mg/mL, the steamed root extracts inhibited the growth of three cancer cell lines completely. Antiproliferative effects of red American ginseng were influenced by the steaming times. The effects of extract from root steamed for 2 h were stronger than from root steamed for 1 h. Moreover, the antiproliferative effect of red American ginseng is greater than that of red Asian ginseng (see Fig. 3 and Fig.4B).
Fig. 4.
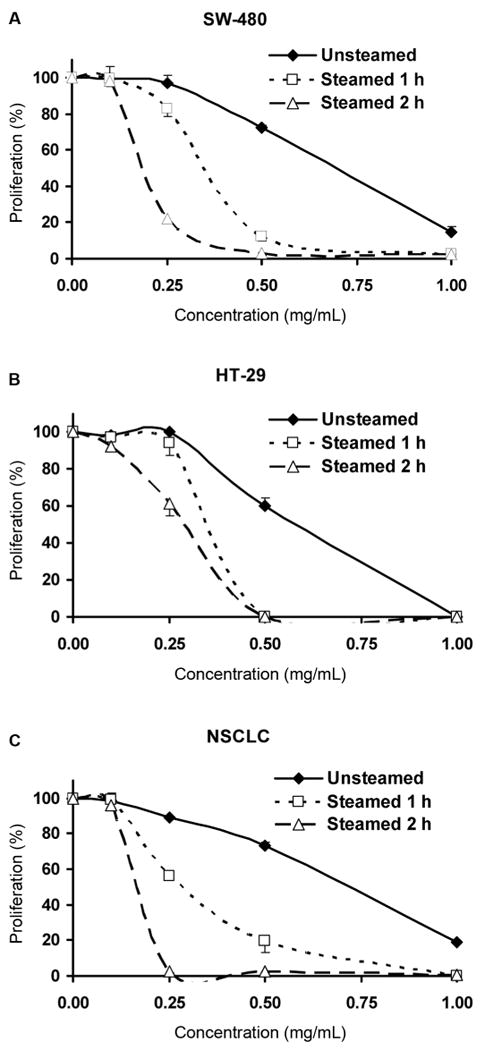
Antiproliferative effects of unsteamed and steamed (120 °C for 1 h or 2 h) American ginseng root extract on cancer cells determined by the MTS assay. Four doses (0.1, 0.25, 0.5 and 1 mg/mL) were used and the treatment time for SW-480 (A) and HT-29 (B) cells was 72 h; for NSCLC cells (C), it was 48 h. The results are expressed as the mean ± SD of three independent experiments.
The biological activity of red American ginseng extract is the result of saponins, believed to be the main active constituents in American ginseng. In this diverse group of saponins, five representative ginsenosides, three major constituents in unsteamed root (Rb1, Rd and Re) and two main constituents in steamed root (Rg2 and Rg3), were used to test for antiproliferative effects on cancer cells. From the results shown in Fig. 5, at 30 – 300 μM, ginsenosides Rb1, Rd, Re, and Rg2 did not show strong antiproliferative effects on SW-480 cells. Ginsenoside Rg3, which is the main constituent in steamed American ginseng root, showed strong antiproliferative effects on SW-480 cells at 300 μM. Similar activities were observed on HT-29 and NSCLC cells. Since several unconfirmed compounds were also detected (Fig. 2), the structure and antiproliferative activities of these compounds will be the focus of future studies.
Fig. 5.
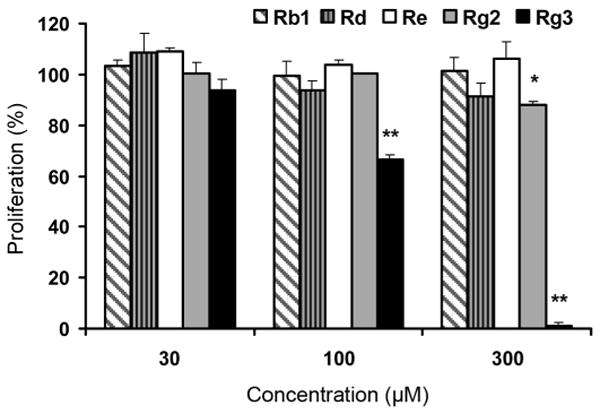
Antiproliferative effects of ginsenosides on SW-480 cells. Cancer cells were exposed to ginsenosides (30, 100, and 300 μM) for 72 h and cell proliferation was determined by the MTS assay. At the concentration of 300 μM, the antiproliferative effects of Rg3 were 99.0 ± 1.3%, respectively. The results are expressed as the mean ± SD of three independent experiments. * p < 0.05; ** p < 0.01 vs. control.
Human colorectal cancer is a significant public health problem in the Western world [12]. In the United States, this cancer is the second-leading cause of cancer-related deaths and the second most prevalent cancer worldwide [13]. The lung is one of the common sites of metastasis of colorectal cancer. About 85% of all lung cancers are of the non-small cell type [14], [15]. In this study, the anticancer activities of steamed American ginseng root extracts on two human colorectal cancer cell lines and one non-small cell lung cancer cell line were evaluated. After steaming at 120 °C, the antiproliferative effects of American ginseng root extracts increased significantly in all the three cancer cell lines. The main anticancer ginseng saponins, ginsenosides Rg3 and Rh2, can be obtained by hydrolysis from other ginsenosides such as Rb1, Rb2, Rc and Rd. Data from our study suggested that steaming at a higher temperature (120 °C) is advantageous for the conversion of Rg3 from other saponins. Red American ginseng, which is the heat-processed root of P. quinquefolius, may be a potent anticancer herbal medicine.
Materials and Methods
Materials
The root of P. quinquefolius L. (No. Pq2004003) was collected from Roland Ginseng, LLC (Wausau, WI, USA). White ginseng (P. ginseng C. A. Meyer) (No. Pg2005001) and red ginseng (No. Pg2005006) were purchased from Tongrentang Pharmacy, Beijing, P. R. China. The voucher specimens were authenticated by Dr. Chong-Zhi Wang and deposited at the Tang Center for Herbal Medicine Research at University of Chicago (Chicago, IL, USA). Ginsenoside standards were purchased from Delta Information Center for Natural Organic Compounds (Xuancheng, P. R. China). Other chemicals were obtained from Fisher Scientific (Norcross, GA, USA) and Promega (Madison, WI, USA).
Steaming treatment and extraction
For the heat-processing of American ginseng, the roots were steamed at 100 °C, and 120 °C (AMSCO 2022 Autoclave; AMSCO Scientific; Apex, NC, USA) for 1 h, or steamed at 120 °C for 0.5, 1, 2, 3, and 4 h. Fresh and steamed roots were lyophilized to obtain dried samples. For the HPLC analysis, dried root samples were ground and extracted with methanol in Soxhlet extractors, and then assayed. For the in vitro anticancer studies, root samples unsteamed or steamed at 120 °C for 1 h and 2 h were ground and extracted with 70% ethanol. The solvent of the extract solution was evaporated under vacuum. The dried extract was dissolved in water, and then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under vacuum and then lyophilized.
HPLC analysis
The HPLC system was a Waters 2960 instrument with a 996 photodiode array detector (Milford, MA, USA). The separation was carried out on an Alltech Ultrasphere C18 column (5 μ, 250×3.2 mm I.D.) (Deerfield, IL, USA) with a guard column (Alltech Ultrasphere C18, 5 μ, 7.5×3.2 mm I.D.) [16]. Acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 18% solvent A and 82% solvent B, was changed to 21% A for 20 min, to 26% A for 3 min and held for 19 min; to 36% A for 13 min; to 50% A for 9 min; to 95% A for 2 min and held for 3 min; and finally changed to 18% A for 3 min and held for 8 min. The flow rate was 1.0 mL/min and the detection wavelength was set to 202 nm. All tested solutions were filtered through Millex 0.2-μm nylon membrane syringe filters before use. The contents of saponins in each sample were calculated using standard curves of ginsenosides.
Cell culture
The human colorectal cancer cell lines SW-480 (Leibovitz's L-15), HT-29 (McCoy's 5A), and non-small cell lung cancer cells (NSCLC, DMEM) were purchased from American Type Culture Collection, ATCC (Manassas, VA, USA) and grown in the indicated media supplemented with 10% FBS and 50 IU penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C.
Cell proliferation analysis
Unsteamed, steamed American ginseng root extract, and five ginsenosides were dissolved in 50% ethanol. Cells were seeded in 96-well plates (1×104 cells/well). After 1 d, various concentrations of extracts/ginsenosides were added to the wells. The final concentration of ethanol was 0.5%. Controls were exposed to culture medium containing 0.5% ethanol without drugs. All experiments were performed in triplicate and repeated 3 times. Drug treatment time was dependent on the cell growth rate. We selected 72 h for SW-480 and HT-29 cells, and 48 h for NSCLC cells. Cell proliferation was evaluated using an MTS assay according to the manufacturer's instructions. Briefly, at the end of the drug exposure period, the medium was replaced with 100 μL of fresh medium, 20 μL of MTS reagent (CellTiter 96 Aqueous Solution) in each well, and the plate was returned to the incubator for 1 – 2 h. A 60-μL aliquot of medium from each well was transferred to an ELISA 96-well plate and its absorbance at 490 nm was recorded [17]. Since 0.5% ethanol did not influence the proliferation of three cell lines, results were expressed as percent of control (ethanol vehicle set at 100%).
Statistical analysis of the results
Data are presented as mean ± standard deviation (SD) with n = 3. A one-way ANOVA determined whether the results had statistical significance. In some cases, Student's t test was used for comparing two groups. The level of statistical significance was set at P < 0.05. For the antiproliferative effects of white and red ginseng extract on cancer cells, the significance of red ginseng extract vs. white ginseng extract was assessed by Student's t test. For the antiproliferative effects of ginsenosides on SW-480 cells, the significance of ginsenoside treatment vs. control was assessed by Student's t test.
Acknowledgments
This work was supported in part by research grants from the American Cancer Society (RSG-05-254-01DDC), the NIH/NCI (1RO1 CA106569-01), and the NIH/NCCAM (AT002176 and AT002445).
References
- 1.Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208–16. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 2.Takaku T, Kameda K, Matsuura Y, Sekiya K, Okuda H. Studies on insulin-like substances in Korean red ginseng. Planta Med. 1990;56:27–30. doi: 10.1055/s-2006-960877. [DOI] [PubMed] [Google Scholar]
- 3.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–74. [PubMed] [Google Scholar]
- 4.Xie JT, Mchendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 5.Liou CJ, Huang WC, Tseng J. Long-term oral administration of ginseng extract modulates humoral immune response and spleen cell functions. Am J Chin Med. 2005;33:651–61. doi: 10.1142/S0192415X05003247. [DOI] [PubMed] [Google Scholar]
- 6.Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16:S6–18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo HH, Yokozawa T, Satoh A, Kang KS, Kim HY. Effects of ginseng on the proliferation of human lung fibroblasts. Am J Chin Med. 2006;34:137–46. doi: 10.1142/S0192415X06003709. [DOI] [PubMed] [Google Scholar]
- 8.Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79 – 4 cells induced by oxidative stress. J Ethnopharmacol. 2007 doi: 10.1016/j.jep.2007.01.004. advance online publication. [DOI] [PubMed] [Google Scholar]
- 9.Park JD, Rhee DK, Lee YH. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem Rev. 2005;4:159–75. [Google Scholar]
- 10.Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) F. H. Chen. J Nat Med. 2006;60:97–106. [Google Scholar]
- 11.Wang CZ, Wu JA, McEntee E, Yuan CS. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem. 2006;54:2261–6. doi: 10.1021/jf052993w. [DOI] [PubMed] [Google Scholar]
- 12.Francois F, Park J, Bini EJ. Colon pathology detected after a positive screening flexible sigmoidoscopy: a prospective study in an ethnically diverse cohort. Am J Gastroenterol. 2006;101:823–30. doi: 10.1111/j.1572-0241.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 14.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–90. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 15.Saijo N. Recent trends in the treatment of advanced lung cancer. Cancer Sci. 2006;97:448–52. doi: 10.1111/j.1349-7006.2006.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–42. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 17.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]


