Abstract
Overproduction of the reactive oxygen species (ROS) superoxide (O2−) and hydrogen peroxide (H2O2) are increasingly implicated in human disease and aging. ROS are also being explored as important modulating agents in a number of cell signaling pathways. Earlier work has focused on development of small catalytic scavengers of O2−, commonly referred to as superoxide dismutase (SOD) mimetics. Many of these compounds also have substantial abilities to catalytically scavenge H2O2 and peroxynitrite (ONOO−). Peroxides have been increasingly shown to disrupt cell signaling cascades associated with excessive inflammation associated with a wide variety of human diseases. Early studies with enzymatic scavengers like SOD frequently reported little or no beneficial effect in biologic models unless SOD was combined with catalase or a peroxidase. Increasing attention has been devoted to developing catalase or peroxidase mimetics as a way to treat overt inflammation associated with the pathophysiology of many human disorders. This review will focus on recent development of catalytic scavengers of peroxides and their potential use as therapeutic agents for pulmonary, cardiovascular, neurodegenerative and inflammatory disorders.
Keywords: Catalytic antioxidants, Cell signaling, Drug development, Hydrogen peroxide, Inflammation, Oxidative stress
1. Endogenous catalytic hydrogen peroxide scavengers
Hydrogen peroxide (H2O2) is generated directly from superoxide (O2−) through a rapid dismutation reaction that can occur either enzymatically with superoxide dismutases (SOD) or spontaneously. This means that wherever O2− is generated there is also formation of H2O2. In addition, H2O2 is formed enzymatically as a by-product of lipid metabolism in peroxisomes. H2O2 is stable at biological pH and easily crosses lipid membranes. H2O2 can participate in hydroxyl radical (HO•) formation in the presence of reduced transition metals. Oxidative stress is traditionally defined as an imbalance between reactive oxygen species (ROS) production and antioxidant defense against these ROS. A consequence of oxidative stress is an increase in the formation of oxidized cellular macromolecules. Critical cysteinethiol groupson proteins are a common site ofoxidation and many of these cysteines are important in maintaining proteins in a proper conformation for catalytic function. This is a common mechanism proposed for how oxidative stress can disrupt cell signaling pathways leading to unregulated inflammatory responses [1]
SODs and catalase are metalloproteins that catalyze “dismutation” reactions, which detoxify O2− and H2O2, respectively. SODs catalyze the formation of oxygen and H2O2 from two O2−, whereas catalase catalyzes the formation of oxygen and water from two H2O2 molecules.
Because these efficient reactions do not require additional reducing equivalents, no energy is required from the cell. The overall goal of cellular antioxidant defenses is to reduce ROS to water. Mammalian catalase is a tetramer in which each monomer contains an iron heme (porphyrin) group bound to the catalytic site [2]. The heme groups are protected, being buried in a non-polar pocket with narrow hydrophobic channels to aid in H2O2 selectivity. It should be noted that catalase also possesses peroxidase activity and is known to oxidize short chain alcohols to their corresponding aldehydes [3]. Overexpression of SOD and catalase in cultured cells and whole animals has provided protection against the deleterious effects of a wide range of oxidative stress paradigms [4].
Another class of endogenous catalytic H2O2 scavengers is the selenium-containing peroxidases [5]. This is a broad group of enzymes that utilize H2O2 as a substrate along with an endogenous source of reducing equivalence. One of the best studied families of peroxidases are the glutathione peroxidases (GPx). GPxs are tetrameric proteins where each monomer contains one atom of selenium at the catalytic site. The active site of GPx contains a selenocysteine where the sulfur in cysteine has been replaced by selenium (R-SeH). During the catalytic cycle, a selenol (protein-Se−) reacts with peroxide (H2O2 or lipid peroxide, LOOH) resulting in a selenenic acid (protein-SeOH). The selenenic acid group is reduced back to a selenol by two glutathiones (GSH) which are in turn oxidized to disulfide (GSSG) and LOOH is reduced to its corresponding alcohol (LOH).
The GSSG is converted back to two GSH by glutathione reductase that uses reducing equivalents derived from β-nicotinamide adenine dinucleotide phosphate (NADPH). Overexpression of GPx has been shown to be protective against oxidative stress in cultured cells and whole animals [6,7]. Not all of the peroxidases detoxify H2O2. A number of non-specific peroxidases, such as myleoperoxidase, eosinophil peroxidase and lactoperoxidase, actually form more reactive produces such as hypochlorite, hypothiocyanite, and hypobromous acid [8].
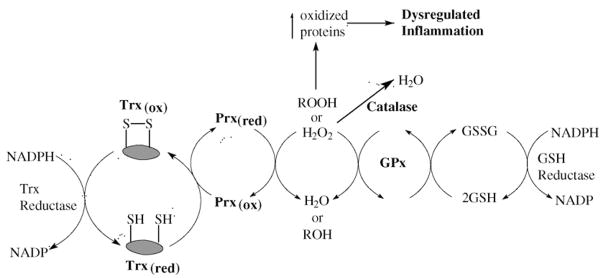
A new family of proteins have been discovered that are known as the thioredoxin-dependent peroxidases or peroxiredoxins (Prx). These proteins can directly reduce peroxides and the oxidized protein is regenerated indirectly by thioredoxin (Trx) reductase [9,10]. There are at least 13 mammalian Prxs and they are widely abundant in mammalian cells. Prx have been found to be induced in response to oxidative stress [11] and are protective against oxidative stress when over-expressed in animals [12]. The Prxs and Trxs work in tandom with the GPxs to maintain cellular peroxide steady-state levels and also maintain cellular protein cysteines in a reduced state (Fig. 1). During oxidative stress, where cellular peroxides are elevated, there is an increased level of oxidized protein cysteines that leads to inactivation of phosphatases and transcription factors that are thought to drive dysregulated inflammatory reactions [13]. This is the drug target and rationale for the development of catalase and GPx mimics.
Fig. 1.
Endogenous scavenging of cellular peroxide. The cell’s steady-state peroxide (ROOH) levels are largely maintained by the activities of catalase, glutathione peroxidases (GPx) and the thioredoxin-assisted peroxidases (peroxiredoxins, Prx). This system also maintains cellular protein cysteines in a reduced (red) state. During oxidative stress, where cellular peroxides are elevated, there is an increased level of oxidized (ox) protein cysteines that leads to inactivation of phosphatases and transcription factors and dysregulated inflammatory reactions. This is the drug target and rationale for the development of catalase and GPx mimics.
2. Development of catalytic hydrogen peroxide scavengers
There are two main design strategies to detoxify peroxides. One approach models after the catalase dismutation reaction and focuses on compounds with redox-active metal centers that often containing either manganese (Mn) or iron (Fe). Another strategy models after the GPx enzymes using either selenium or tellurium active sites. An ideal mimetic is stable and non-toxic at therapeutically efficacious concentrations. The size and charge of the mimetic is often exploited to target cellular sites of oxidant production, such as the mitochondria, and to improve their pharmacodynamic properties.
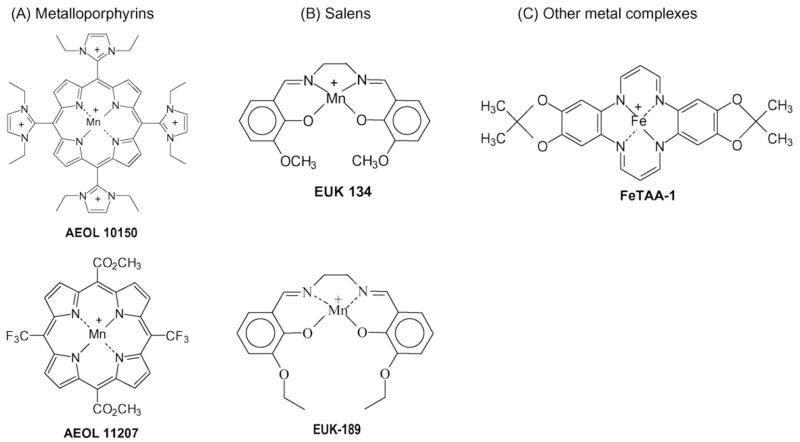
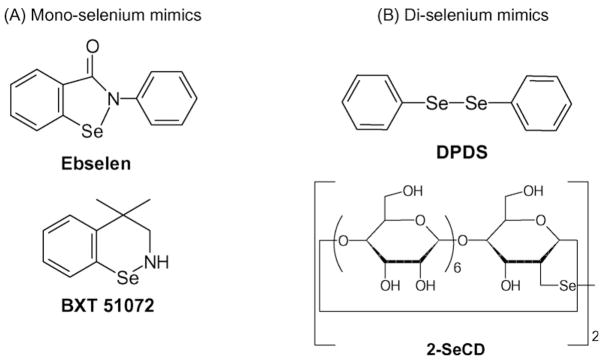
Many simple metal chelates readily react with H2O2. However, the rates of reaction with these chelates are generally low and the complexes formed are relatively unstable. Recent developments in the field have yielded more stable and active metal chelates (Fig. 2). These include the salens, metalloporphyrins, and other metal complexes that can dismutate H2O2 under highly defined conditions. Likewise, a number of selenium-containing compounds have been developed around the GPx mechanism of H2O2 decomposition (Fig. 3).
Fig. 2.
Examples of catalase-like mimics chemical structures: (A) metalloporphyrins; (B) salens; and (C) other metal complexes.
Fig. 3.
Examples of glutathione peroxidase-like mimics chemical structures: (A) mono-selenium mimics; and (B) di-selenium mimics.
All of the reported catalytic H2O2 scavengers react with a wide range of ROS and are not specific for H2O2. The potencies of these peroxide scavengers are based on rate constants derived under very defined and often non-biologically relevant conditions. For example, most compounds are routinely screened in buffers using very high H2O2 (1–10 mM) levels that are two orders of magnitude higher than the 1–100 nM levels that occur physiologically [14]. The findings that many of these diverse compounds are effective in similar oxidative stress models confirms the basic concept that small, efficient, catalytic antioxidants show promise in the treatment of ROS-mediated conditions associated with injury and tissue dysfunction.
3. Antioxidant properties of catalase-like hydrogen peroxide scavengers
3.1. Metalloporphyrins
Metalloporphyrins [AEOL series is currently being developed by Aeolus Pharmaceuticals, Laguna Niguel, CA (http://www.aeoluspharma.com)] are structurally different from endogenous protoporphyrins and are classified as synthetic meso-substituted porphyrins. Metalloporphyrins have been shown to possess at least four distinct antioxidant properties, which include scavenging O2− [15], H2O2 [16], ONOO− [17], and LOOH [18]. Most metalloporphyrins contain either a Fe or Mn moiety that is coordinated by four nitrogen axial ligands. The catalase-like activity of metalloporphyrins is thought to be due to their extensive conjugated ring system that can undergo reversible one-electron transfers in addition to the one-electron transfers on the metal center. This mechanism is similar to that proposed for the heme prosthetic groups of endogenous catalase and peroxidases. There are two classes of metalloporphyrins wherein one group the SOD activities track with their catalase activities and another group that has very little SOD activity with high catalase activity. Examples of a manganese porphyrins with both high SOD and catalase-like activities are the pyridinium and imidazolium-substituted meso-porphyrins such, as AEOL 10113 and 10150 [19], whereas examples of compounds with low SOD activity and high catalase activity are MnTBAP [16] and AEOL 11207 [20]. It is still unknown which antioxidant activities are the most important in mediating the protective effects of metalloporphyrins in models of oxidative stress. Metalloporphyrins have been shown to be effective in ameliorating oxidative stress, inflammation and injury in a large number of in vitro [21] and animal models of human disease (Table 1). Metalloporphyrins have plasma half-lives that range from 4 to 48 h. Most metalloporphyrins are not extensively metabolized by the body and are largely excreted unchanged in the urine. A previous limitation of the metalloporphyrin class of compounds has been their poor oral bioavailability, but several compounds in the AEOL 112-series have been shown to have good oral bioavailability and longer plasma half-lives which should make them better candidates for treating chronic diseases [22].
Table 1.
Examples of catalytic antioxidants with H2O2-scavenging activity that are effective in attenuating oxidative stress in in vivo models..
| Model system | Species | Active site | Compound(s) | Reference |
|---|---|---|---|---|
| Pulmonary | ||||
| Bleomycin fibrosis | Mice | Mn | AEOL 10201 | [57] |
| Radiation fibrosis | Rats | Mn | AEOL 10113 | [59] |
| Mn | AEOL 10150 | [60] | ||
| Mn | EUK 189 | [61] | ||
| Cigarette smoke | Rats | Se | DPDS | [54] |
| Mn | AEOL 10150 | [55] | ||
| Endotoxin | Pigs | Mn | EUK 8 | [66] |
| Bronchopulmonary dysplasia | Preterm baboons | Mn | AEOL 10113 | [64] |
| Antigen-induced asthma | Mice | Mn | AEOL 10113 | [50] |
| Hemorrhage | SOD3 KO mice | Mn | AEOL 10150 | [65] |
| Cardiovascular | ||||
| Heart ischemia/reperfusion | Mice | Se | BXT 51072 | [68] |
| Aged rats | Mn | EUK 8 | [110] | |
| Rats | Mn | AEOL 10113 | [69] | |
| Dilated cardiomyopathy | SOD2 KO mice | Mn | AEOL 10201 | [111] |
| Harlequin mutant mice | Mn | EUK 8 | [83] | |
| Sepsis | Rats | Mn | AEOL 10113 | [84] |
| Mn | EUK 8 | [81] | ||
| Hemorrhage | Rats | Mn | EUK 8 | [67] |
| Mn | EUK 134 | |||
| Nervous system | ||||
| Neurofibromatosis | Flies | Mn | AEOL 10201 | [112] |
| Mn | AEOL 10150 | |||
| MPTP | Monkeys | Se | Ebselen | [97] |
| Mice | Mn | AEOL 11207 | [22] | |
| ALS | Mutant SOD1 | Mn | AEOL 10150 | [105] |
| Tg mice | Mn | EUK 8 | [106] | |
| Mn | EUK 134 | |||
| Stroke | Rats | Se | Ebselen | [74] |
| Mn | EUK 134 | [73] | ||
| Mice | Mn | AEOL 10113 | [72] | |
| Mn | AEOL 10150 | |||
| Humans | Se | Ebselen | [75] | |
| Spinal cord trauma | Rats | Mn | AEOL 10201 | [113] |
| Se | Ebselen | [114] | ||
| Mice | Mn | AEOL 10150 | [115] | |
| Cerebral vasoconstriction | Amyloid Tg mice | Mn | AEOL 10201 | [108] |
| Rats | Mn | AEOL 10201 | [109] | |
| Cognitive function | Aged mice | Mn | EUK 189 | [103] |
| Mn | EUK 207 | |||
| Hepatic/gastrointestinal/renal | ||||
| Ischemia/reperfusion | Rat | Mn | AEOL 10150 | [70] |
| Mn | EUK 134 | [76] | ||
| Se | Ebselen | [71] | ||
| Endotoxin | Mice | Se | Ebselen | [85] |
| Mn | AEOL 10113 | [82] | ||
| Rats | Mn | EUK 134 | [86] | |
| Fas | Mice | Mn | AEOL 10201 | [116] |
| Ethanol | Rats | Se | Ebselen | [117] |
| Carbon tetrachloride | Rats | Se | Ebselen | [118] |
| Hyperthermia | Aged rats | Mn | EUK 189 | [119] |
| Colitis | Rats | Mn | AEOL 11201 | [120] |
3.2. Salens
The salen class of catalytic antioxidants (EUK series) is currently being developed by Proteome Systems, North Ryde, Australia (http://www.proteomesystems.com). Generically, salens are aromatic, substituted ethylenediamine metal complexes. The Mn(III)-containing salen complexes have both O2− and H2O2 dismutation activities [23]. However, like all the small molecular weight scavengers, these compounds are not selective and can react with O2− and other peroxides and ONOO−. The Mn moiety of the salen is coordinated by four axial ligands. One of the unique features of these compounds is that the metal center is coordinated to oxygen and nitrogen atoms which is in contrast to the porphyrins where the metal is only coordinated to nitrogen atoms. The coordination of Mn by four axial ligands results in the formation of several possible valence states that give these compounds their broad ROS scavenging capabilities. The rates at which reported salens scavenge H2O2 are similar to those reported for metalloporphyrins, but are many orders less than those documented for catalase under similarly defined conditions [23]. Salens have also been shown to protect cells against H2O2-mediated injury [24]. Salens have been shown to be efficacious in a large number of animal models of human diseases (Table 1). One of the current limitations of the salens is the stability of the parent compounds in biological matrix which makes it difficult to determine tissue levels and half-lives.
3.3. Other metal complexes
There are a number of other metal containing macrocyclic compounds that have been described as catalase mimics. Iron complexes of 14-membered macrocycles have been shown to catalytically scavenge H2O2 to oxygen and water [25]. These compounds have been shown to be effective only in cell culture systems [26]. It is unclear how stable the complexes will be in more complex biological systems. Another group of compounds with H2O2-scavenging activity are the dimanganese complexes. A number of bacteria have catalase enzymes that use a dimanganese center to dismutate H2O2 and a number of investigators have tried to emulate this strategy with small molecules and peptides. Some examples of the small molecules are the nitro and chloro-substituted dimanganese complexes of 1,5-bis(5-salicylidenamino) pentan-3-ol (5-NO2-salpent & 5-Cl-sal-pent), 1,5-bis(2-hydroxybenzophylideneamino)pentan-3-ol (2-OH-benzpent), and 1,5-bis(2-hydroxynaphtylideneamino)pentan-3-ol (2-OH-Napthpent) [27]. Very limited data is currently available whether they have protective properties in biological systems. It should be noted that free manganese is an efficient scavenger of H2O2 and so stability is an important feature to establish for any claim of catalytic H2O2 scavenging by a metal complex.
4. Antioxidant properties of glutathione peroxidase-like hydrogen peroxide scavengers
4.1. Ebselen
One of the best studied GPx-like mimics is 2-phenyl-1,2-benzisoselenazol-3(2H)-one also known as ebselen or PZ51. Ebselen was one of the first selenium-based GPx mimics developed. It catalytically scavenges peroxides in the presence of reducing equivalents such as GSH, N-acetylcysteine (NAC), and dihydrolipoate (DHLA) [28]. The mechanism by which this occurs is still debated and may differ under different conditions. Ebselen has also been shown to stimulate the decomposition of a number of ROS including hypochlorous acid (HOCl) [29], singlet oxygen [30], and ONOO− [31]. Ebselen can readily bind cellular thiol groups on proteins which may complicate the interpretation of biological effects since many cellular proteins have reactive thiols in their catalytic domains. In fact, it has been documented that ebselen can inhibit lipoxygenases [32], NADPH oxidases [33], and nitric oxide synthases [34]. All of these enzymes are also potential sources of endogenous ROS. Ebselen has been shown to be protective in a number of cell culture systems [28] and animal models of human disease (Table 1). Ebselen is orally active and appears to be well tolerated in animals and humans.
Newer analogs of ebselen have been developed including BXT-51072, which has increased activity and potency in cell systems. These analogs [BXT-series are being developed by Oxis International, Foster City, CA (http://www.oxis.com)] have been shown to be protective in a limited number of cell culture systems [35] and animal models of human disease (Table 1).
4.2. Diselenide and ditelluride compounds
A number of diselenide and ditelluride containing compounds have been reported to catalytically scavenge peroxides with higher GPx-like activity than ebselen [36]. Sulfur, selenium, and tellurium belong to group VI of the periodic table and have similar chemical properties. Early compounds, such as the diphenyl diselenide (DPDS), were electrophilic agents that have cytotoxic, genotoxic, and mutagenic effects [37,38]. Many previously reported diselenide compounds release free selenium during the catalytic cycle, which may be problematic in their development as therapeutic agents. A unique aspect of a newer series of these compounds is the cyclodextrin (CD) group which may help in directing hydrophobic peroxides towards the selenium or tellurium active site. The diselenide, 2,2′-deseleno-bis-β-cyclodextrin (2-SeCD) can scavenge a variety of peroxides including H2O2, tert-butyl hydroperoxide, and cumenyl hydroperoxide using GSH as a cofactor [39]. Only a limited number of cell culture studies have been reported for these compounds [40], and it is still unclear whether these compounds can be successfully used in animal models of human disease.
4.3. Peptide compounds
Many proteins and peptides contain cysteine residues that can be readily modified to contain selenium and a number of investigators have examined whether these types of compounds have GPx-like activities. Examples of this include seleno-subtilisin which was produced by chemical modification of the serine protease, substilisin. This compound was found to have GPx-like activity [41]. Another strategy used a phage library of random 15-mer selenopeptides that was screened for GPx activity and generated some active GPx mimics (15SeP and 15SeP1). The peptides were found to increase the GPx-like activity of treated cultured cells and protected them against H2O2-mediated lipid peroxidation and cytotoxicity [42]. In order to increase the selectivity of the selenoprotein toward GSH, a number of selenium-containing monoclonal antibodies (i.e. Se-4A4 and Se-scFv2F3) were raised against GSH-S-2,4-dinitrophenyl t-butyl ester and found to have GPx-like activity [43]. The selenium-containing antibody Se-4A4 has been shown to protect isolated cardiac mitochondria against xanthine oxidase-induced oxidative modification [44]. Very limited data exist on whether any of these approaches have produced compounds with biological activity in more complex biological systems.
5. Catalytic antioxidants are effective in ameliorating oxidative stress in vitro
5.1. Cytotoxicity
In vitro models of oxidative stress have proved useful in verifying utility of catalytic antioxidants under more complex biological conditions [21]. Members of all classes of catalytic antioxidants with H2O2-scavenging activities have been shown to be effective in blocking oxidative stress in a variety of in vitro cytotoxicity models involving oxidant production [21,24,28]. The mechanism(s) by which these catalytic antioxidants produce their protective effects are still highly debated and largely unknown. As discussed earlier, many of the compounds are capable of scavenging a number of different ROS and many may also decrease ROS by inhibiting endogenous ROS production [19]. These issues cloud the utility of H2O2-scavenging screens to select potentially biologically potent compounds. Overall, many of the catalytic antioxidant H2O2 scavengers at μmolar levels appear non-toxic and show similar efficacy in protecting a wide variety of different types of cultured cells against the toxicity of ROS.
5.2. Apoptosis
Apoptosis is a form of cell death that is biochemically and morphologically distinct from necrosis and has physiological and pathological roles in biological systems. There is an increasing body of literature that supports the involvement of ROS in some apoptotic pathways. The release of pro-apoptotic factors by mitochondria, which are a major source of ROS, lends further credence to this argument. Delivery of catalase and GPx to cells is protective, whereas paucity of either is deleterious, also supporting H2O2 involvement in cellular apoptosis [45]. A wide assortment of apoptosis paradigms can be ameliorated by catalytic antioxidants with H2O2-scavenging activity [21,24,28]. Apoptosis can be limited by catalytic antioxidants with catalase or GPx-like activities in a number of different cell types. It is not clear from these studies whether the catalytic antioxidants affect a particular point in the intrinsic and/or extrinsic apoptotic pathways. Some studies suggest that ROS might regulate the expression of pro-apoptotic factors, and antioxidants may directly block apoptosis by increasing the expression of anti-apoptotic factors, such as bcl-2 [46].
5.3. Inflammation
A number of recent studies have suggested that catalytic antioxidants with the ability to scavenge H2O2 can attenuate markers of inflammation such as cytokines, chemokines and adhesion molecules. H2O2 has been shown to activate a number of transcription factors including NFkB, AP-1 and Nrf2 [47]. In addition, peroxides and other oxidants can inactivate kinase signaling pathways through the inhibition of protein phosphatases [48]. These pathways likely contribute to the protective effects of many catalytic H2O2 scavengers in a variety of cell models of inflammation [21,24,28].
6. Catalytic antioxidants are effective in ameliorating oxidative stress in vivo
The beneficial effects of catalytic antioxidants with H2O2-scavenging capabilities have been demonstrated in numerous in vivo model systems (Table 1). These model systems cover diseases associated with the pulmonary system (such as fibrosis, asthma, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), bronchopulmonary dysplasia (BPD), and pleurisy), cardiovascular system (including sepsis, hypertension, and myocardial infarction), neurologic system (including amyotrophic lateral sclerosis (ALS), migraine, spinal cord injury, stroke, Parkinson’s disease (PD), and dementia), digestive system (including liver injury, transplantation, hepatitis, and colitis), endocrine system (diabetes), and the renal system (injury, sepsis, and transplantation). The diversity of the various models systems by which these catalytic antioxidants have shown efficacy speaks to the important role ROS/reactive nitrogen species (RNS) play in animal models of human disease.
6.1. Pulmonary models
The lung functions at higher oxygen tensions than most other organs creating a unique relationship with ROS. There is increasing evidence that ROS have an important role in several lung diseases [49]. A common feature of many lung diseases is an inappropriate or dysfunctional inflammatory response. Catalytic antioxidants decrease airway hyperreactivity and inflammation in antigen-induced mouse models of asthma [50]. This is in contrast to a recent finding which reported high overexpression of catalase (8-fold) in the lung actually increased airway reactivity in a mouse model of asthma [51]. A potential explanation for this paradox is that many catalytic antioxidants have bell-shaped dose-responses and that very high levels can actually give the opposite response. An important goal of antioxidant therapy is to restore redox balance. Given the role of H2O2 in cell signaling pathways, it is conceivable that excessive scavenging of H2O2 may be detrimental under certain conditions.
COPD, which includes emphysema and bronchitis, is strongly associated with cigarette smoke that is a rich source of ROS [52]. In fact, cigarette smoke has been shown to inhibit catalase activity [53]. The diselenide GPx-like mimic diphenyl diselenide has been shown to protect rat pup lungs from oxidative changes associated with exposure to cigarette smoke [54]. Likewise, the metalloporphyrin AEOL 10150, which has both high SOD and catalase-like activities, attenuated inflammation and protected rat lung epithelium from cigarette smoke-induced precancerous lesions [55]. These data support the development of catalytic antioxidants for the treatment of COPD.
Interstitial lung disease is also associated with oxidative stress and many of the animal models of lung fibrosis use agents that overproduce or stimulate the production of ROS. Bleomycin is a redox-cycling chemotherapeutic agent that produces lung fibrosis in rodents and humans. Bleomycin-induced lung fibrosis can be attenuated by a liposomal mixture of SOD and catalase [56]. The metalloporphyrin AEOL 10201, also known as MnTBAP, has low SOD activity and moderate catalase activities and attenuated bleomycin-induced lung fibrosis in mice [57]. Ionizing radiation is also known to produce lung fibrosis in animals and man. Administration of a mixture of polyethylene glycol-tagged SOD and catalase has been shown to attenuate radiation-induced lung fibrosis in mice without affecting radiation-induced tumor killing [58]. Similarly, a couple of different metalloporphyrins (AEOL 10113 & 10150) have been shown to decrease radiation-induced lung fibrosis in rats [59,60]. The manganese-containing salen EUK-189 also was found to ameliorate early DNA damage in a rat model of lung irradiation [61]. These studies illustrate the potential utility of catalytic antioxidants in the treatment of interstitial lung disease.
ARDS is associated with sepsis and shock and both of these conditions involve the over production of ROS and reactive nitrogen species. A related lung disorder is BPD that occurs in premature infants where the lung is not fully developed and requires supplemental oxygen for adequate gas exchange. Both ARDS and BPD animals models commonly use either hyperoxia or endotoxin exposures, both of which are known to elevate lung ROS production. Both hyperoxia and endotoxin models have been shown to be responsive to modulation of endogenous SOD and catalase lung levels [62,63]. The metalloporphyrin AEOL 10113 was found to be beneficial in a preterm baboon model of BPD [64] and AEOL 10150 was found to attenuate hemorrhage-induced acute lung injury in SOD3 KO mice [65]. The manganese containing salen EUK 8 was found to be protective in an endotoxin-mediated swine model of ARDS [66]. These finding support the further development of catalytic antioxidants for the treatment of ARDS and BPD.
6.2. Cardiovascular models
Cardiovascular disease is a major cause of death in humans. A common cause of tissue injury directly related to the cardiovascular system is ischemia-reperfusion (IR). IR is associated with hemorrhage [67], myocardial infarction, arrhythmias, angina, myocardial stunning and transplantation. The role of excessive ROS production during IR and the protective effects of endogenous antioxidants have been well documented. Catalytic antioxidants with H2O2-scavenging activities are effective in animal models of heart IR [68,69]. In addition, several other organ systems have been shown to benefit from catalytic antioxidant treatments in IR, including the liver [70,71], lung [65], brain [72–75] and kidney [76].
Hypertension is a well characterized risk factor for cardiovascular disease. A number of systems are involved in the complex regulation of blood pressure and include cardiac output, fluid balance, vasodilatation and renal function. An important system in blood pressure regulation is the renin/angiotensin system which has also been shown to regulate vascular ROS production [77]. A well known anti-hypertensive agent captopril is both an angiotensin-converting enzyme inhibitor and antioxidant [78]. Catalytic antioxidants with H2O2-scavenging activities are effective in animal models of hypertension [79,80]. These studies suggest that catalytic antioxidants may be useful antihypertensive agents.
Systemic infection or sepsis frequently results in the overproduction of ROS and RNS that has devastating consequences for the cardiovascular system. A serious consequence of sepsis is the loss of vascular tone and its responsiveness to vasoconstrictive agents in a condition referred to as shock. A number of catalytic antioxidants with H2O2-scavenging activities are effective in animal models of sepsis induced by bacteria or endotoxin [66,81–86]. The well-established role of ROS and RNS in these conditions and the abundance of literature supporting a protective role of the endogenous antioxidant defenses make this a very attractive arena for development of catalytic antioxidants.
6.3. Nervous system models
The brain consumes a large amount of oxygen and is particularly sensitive to ROS-mediated damage. Factors that are thought to contribute to this phenomena are the presence of autooxidizable neurotransmitters, the high levels of polyunsaturated fatty acids in neuronal membranes, and modest levels of endogenous antioxidants. Collectively, these factors make catalytic antioxidants with H2O2-scavenging activities good candidates for several acute and chronic neuronal disorders that involve the overproduction of ROS such as PD [87], Huntington’s disease (HD) [88], Alzheimer’s disease (AD) [89], ALS [90], stroke [91], and trauma [92].
PD is a chronic neurodegenerative disorder where a large body of literature supports a role for ROS and oxidative stress and neuronal loss in the substantia nigra. Much of this data is linked to the mitochondrial dysfunction of complex I associated with this disease [93]. There have been numerous reports of increases in lipid, DNA and protein oxidative changes in PD and animal models of PD [94]. In addition, there are reports of decreases in antioxidants such as GSH, increased iron content and activation of NFkB [95]. Toxins that increase ROS in the substantia nigra such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) also produce PD-like symptoms and pathology in man and animals [96]. Catalytic antioxidants with H2O2-scavenging activities are effective in animal models of PD [22,97]. Recent studies with the orally active metalloporphyrin AEOL 11207 demonstrated neuroprotection in a mouse MPTP model that was also associated with decreased oxidative stress [22]. Currently available PD therapies are largely limited to palliative treatments with unpleasant side effects and opens PD as an attractive target for the development of catalytic antioxidants.
A number of dementias, such as AD and HD, are memory disorders that have been associated with protein aggregation and increased oxidative stress in the cortex and hippocampus [98]. Some investigators have suggested that amyloid may increase H2O2 production through metal-catalyzed reactions [99]. A few studies have shown that catalytic antioxidants can protect neurons from amyloid-induced cytotoxicity [100]. Increased production of ROS and RNS may also be an indirect consequence of amyloid protein deposits stimulating an inflammatory response from microglial cells [101]. In addition, there is some evidence that NO plays a role in memory and that catalytic antioxidants can be protective [102]. Aging is also a factor in the loss of cognitive function and catalytic antioxidants have also shown to be effective at slowing this process [103]. These data suggest that dementias may be a fruitful area for the further development of catalytic anti-oxidants.
ALS is a motor neuron disorder that leads to progressive loss of motor function, muscle atrophy and death within a few years. Most cases of ALS are sporadic, but about 10% are familial and some of these are associated with genetic mutations. Some familial forms of ALS are associated with mutations in SOD1, and transgenic mice overexpressing one of the SOD1 mutations develop a progressive degenerative disease of motor neurons [104]. It is thought that there is a gain of function associated with the mutant SOD1 that leads to the motor neuron loss in these transgenic mice, and the mechanisms by which this occurs are highly debated. The importance of oxidative stress in ALS is also highly debated even though ample evidence exists that it occurs in this disease [90]. A few studies have shown that catalytic antioxidants with H2O2-scavenging activities can prolong survival in the SOD1 mutant transgenic mice [105,106]. AEOL 10150 has completed phase I safety testing in ALS patients.
Stroke is a leading cause of death in humans with few treatment options. Stroke is an acute neurodegenerative condition that often involves tissue injury due to IR events. Stroke is associated with increased ROS production, and injury can be enhanced or attenuated by modulation of endogenous antioxidants [107]. A number of catalytic antioxidants with H2O2-scavenging activities are effective in animal vessel occlusion models of stroke [72–74]. Catalytic antioxidants are also effective in attenuating cerebral vasoconstriction that is commonly associated with hemorrhagic stroke and migraines [108,109]. Ebselen has had some success in human phase II clinical trials [75] and is currently being evaluated in human phase III clinical trials for stroke in Japan sponsored by Daiichi Sankyo Pharmaceuticals. These studies suggest that stroke may be a potential target for catalytic antioxidants.
7. Implications for the use of catalytic antioxidants as modulators of human disease
The postulated role of ROS as a terminal mediator of tissue injury and dysfunction in diseases of diverse etiologies emphasizes the wide range of therapeutic opportunities for catalytic antioxidant development. Pathologies that are most likely to benefit from catalytic antioxidant therapy include conditions in which a clear role for ROS has been established. Inflammation, which is a pivotal etiological factor in many human pathophysiologic processes involving multiple organ systems, is a major therapeutic opportunity for catalytic antioxidant development given the increasingly important role ROS are being given in modulating cell signaling pathways. The role of ROS has been well documented in host defense mechanisms involving phagocytosis, cytokines, chemokines and immune complex formation, all of which can contribute to auto-immune disorders. Inflammatory lung, intestinal, and cardiovascular diseases all are potentially important targets for catalytic antioxidant therapy.
An important unaddressed issue in the development of catalytic antioxidants is the mechanism by which these agents diminish oxidative stress and injury in animals and humans. To date, catalytic antioxidants are defined by their chemistry under highly defined and largely non-biological conditions. The relevance of using this classification system is still yet to be verified. In fact, over time the potential number of mechanisms these agents possess in biological systems has increased substantially. A more important fact is that these compounds have potent biological effects that often track with their ability to suppress oxidative stress in a large variety of animal models of human disease. However, definitive proof that these compounds are effective in human disease is still needed.
8. Conclusion
Emerging research is strengthening the role of ROS in redox-mediated cell signaling pathways that have well-established roles in human disease and aging. A novel class of compounds that efficiently scavenge cellular peroxides are being developed to modulate cellular ROS and alter some of the aberrant cell signaling associated with many human disorders. There is promising data in the literature that these compounds may have potential therapeutic use in pulmonary, cardiovascular, neurodegenerative and inflammatory disorders.
Acknowledgments
The author wishes to thank Dr. Carl White for his helpful comments and suggestions. This work was supported in part by NIH grants HL075523, HL084469, ES012504, ES015678 and an unrestricted research grant from Aeolus Pharmaceuticals. Dr. Day is a consultant for and holds equity in Aeolus Pharmaceuticals that is commercially developing metalloporphyrins as therapeutic agents.
Abbreviations
- AP-1
activator protein 1
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ARDS
acute respiratory distress syndrome
- BPD
bronchopulmonary dysplasia
- COPD
chronic obstructive pulmonary disease
- CD
cyclodextran
- DHLA
dihydrolipoate
- DPDS
diphenyl diselenide
- GSH
glutathione
- GSSG
glutathione disulfide
- GPx
glutathione peroxidase
- HD
Huntington’s disease
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- HOCl
hypochlorous acid
- IR
ischemia-reperfusion
- LOOH
lipid peroxide
- MnTBAP
manganese(III) tetrakis (4-benzoic acid) porphyrin
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAC
N-acetylcysteine
- NADPH
β-nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- O2
oxygen
- PD
Parkinson’s disease
- ROOH
peroxides
- Prx
peroxiredoxin
- ONOO−
peroxynitrite
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- O2−
superoxide
- SOD
superoxide dismutase
- TAA
tetraaza macrocycle
- Trx
thioredoxin
- H2O
water
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11(1):1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 2.Deisseroth A, Dounce AL. Catalase: physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50(3):319–75. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 3.Oshino N, Oshino R, Chance B. The characteristics of the “peroxidatic” reaction of catalase in ethanol oxidation. Biochem J. 1973;131(3):555–63. doi: 10.1042/bj1310555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner HR. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994;17(3):249–58. doi: 10.1016/0891-5849(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Zachara BA. Mammalian selenoproteins. J Trace Elem Electrolytes Health Dis. 1992;6(3):137–51. [PubMed] [Google Scholar]
- 6.Mirault ME, Tremblay A, Furling D, Trepanier G, Dugre F, Puymirat J, et al. Transgenic glutathione peroxidase mouse models for neuroprotection studies. Ann N Y Acad Sci. 1994;738:104–15. doi: 10.1111/j.1749-6632.1994.tb21795.x. [DOI] [PubMed] [Google Scholar]
- 7.Comhair SA, Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal. 2005;7(1–2):72–9. doi: 10.1089/ars.2005.7.72. [DOI] [PubMed] [Google Scholar]
- 8.Kettle AJ, van Dalen CJ, Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep. 1997;3(5–6):257–8. doi: 10.1080/13510002.1997.11747120. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signalling. Free Radic Biol Med. 2005;38(12):1543–52. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–26. doi: 10.1016/s0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Manevich Y, Feinstein SI, Pak JH, Ho YS, Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L363–9. doi: 10.1152/ajplung.00078.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Manevich Y, Feinstein SI, Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1188–93. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- 13.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signalling. Am J Respir Crit Care Med. 2002;166(12 Part 2):S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 14.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 15.Pasternack RF, Skowronek WR. Catalysis of the disproportionation of superoxide by metalloporphyrins. J Inorg Biochem. 1979;11:261–7. doi: 10.1016/s0162-0134(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 16.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347(2):256–62. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. Fed Eur Biochem Soc Lett. 1996;381(1–2):82–6. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 18.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med. 1999;26(5–6):730–6. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 19.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67(1):77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Castello PR, Drechsel DA, Day BJ, Patel M. Inhibition of mitochondrial hydrogen peroxide production by lipophilic metalloporphyrins. J Pharmacol Exp Ther. 2008;324(3):970–6. doi: 10.1124/jpet.107.132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today. 2004;9(13):557–66. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 22.Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27(16):4326–33. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, et al. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv Pharmacol. 1997;38:247–69. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- 24.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45(20):4549–58. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 25.Sicking W, Korth HG, Jansen G, de Groot H, Sustmann R. Hydrogen peroxide decomposition by a non-heme iron(III) catalase mimic: a DFT study. Chemistry. 2007;13(15):4230–45. doi: 10.1002/chem.200601209. [DOI] [PubMed] [Google Scholar]
- 26.Rauen U, Li T, Sustmann R, De Groot H. Protection against iron- and hydrogen peroxide-dependent cell injuries by a novel synthetic iron catalase mimic and its precursor, the iron-free ligand. Free Radic Biol Med. 2004;37(9):1369–83. doi: 10.1016/j.freeradbiomed.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Moreno D, Palopoli C, Daier V, Shova S, Vendier L, Sierra MG, et al. Synthesis, structure and catalase-like activity of dimanganese(III) complexes of 1,5-bis(X-salicylidenamino)pentan-3-ol (X = 3- and 5-methyl). Influence of phenyl-ring substituents on catalytic activity. Dalton Trans. 2006;43:5156–66. doi: 10.1039/b609366c. [DOI] [PubMed] [Google Scholar]
- 28.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med. 1993;14(3):313–23. doi: 10.1016/0891-5849(93)90028-s. [DOI] [PubMed] [Google Scholar]
- 29.Biewenga GP, Bast A. Reaction of lipoic acid with ebselen and hypochlorous acid. Methods Enzymol. 1995;251:303–14. doi: 10.1016/0076-6879(95)51133-4. [DOI] [PubMed] [Google Scholar]
- 30.Scurlock R, Rougee M, Bensasson RV, Evers M, Dereu N. Deactivation of singlet molecular oxygen by organo-selenium compounds exhibiting glutathione peroxidase activity and by sulfur-containing homologs. Photochem Photobiol. 1991;54(5):733–6. doi: 10.1111/j.1751-1097.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 31.Masumoto H, Sies H. The reaction of ebselen with peroxynitrite. Chem Res Toxicol. 1996;9(1):262–7. doi: 10.1021/tx950115u. [DOI] [PubMed] [Google Scholar]
- 32.Safayhi H, Tiegs G, Wendel A. A novel biologically active seleno-organic compound–V. Inhibition by ebselen (PZ 51) of rat peritoneal neutrophil lipoxygenase. Biochem Pharmacol. 1985;34(15):2691–4. doi: 10.1016/0006-2952(85)90569-6. [DOI] [PubMed] [Google Scholar]
- 33.Cotgreave IA, Duddy SK, Kass GE, Thompson D, Moldeus P. Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem Pharmacol. 1989;38(4):649–56. doi: 10.1016/0006-2952(89)90211-6. [DOI] [PubMed] [Google Scholar]
- 34.Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ. Inhibition of endothelial nitric oxide synthase by ebselen, Prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J Pharmacol Exp Ther. 1993;267(3):1112–8. [PubMed] [Google Scholar]
- 35.Moutet M, d’Alessio P, Malette P, Devaux V, Chaudiere J. Glutathione peroxidase mimics prevent TNFalpha- and neutrophil-induced endothelial alterations. Free Radic Biol Med. 1998;25(3):270–81. doi: 10.1016/s0891-5849(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Dong Z, Liu J, Mao S, Xu J, Luo G, et al. Selenium-mediated micellar catalyst: an efficient enzyme model for glutathione peroxidase-like catalysis. Langmuir. 2007;23(3):1518–22. doi: 10.1021/la061727p. [DOI] [PubMed] [Google Scholar]
- 37.Adams WJ, Jr, Kocsis JJ, Snyder R. Acute toxicity and urinary excretion of diphenyldiselenide. Toxicol Lett. 1989;48(3):301–10. doi: 10.1016/0378-4274(89)90057-x. [DOI] [PubMed] [Google Scholar]
- 38.Rosa RM, Sulzbacher K, Picada JN, Roesler R, Saffi J, Brendel M, et al. Genotoxicity of diphenyl diselenide in bacteria and yeast. Mutat Res. 2004;563(2):107–15. doi: 10.1016/j.mrgentox.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Luo G, Ren X, Mu Y, Bai Y, Shen J. A bis-cyclodextrin diselenide with glutathione peroxidase-like activity. Biochim Biophys Acta. 2000;1481(2):222–8. doi: 10.1016/s0167-4838(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Mu Y, Ma S, Gong P, Yan G, Liu J, et al. The molecular mechanism of protecting cells against oxidative stress by 2-selenium-bridged beta-cyclodextrin with glutathione peroxidase activity. Biochim Biophys Acta. 2005;1743(3):199–204. doi: 10.1016/j.bbamcr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Bell IM, Fisher ML, Wu ZP, Hilvert D. Kinetic studies on the peroxidase activity of selenosubtilisin. Biochemistry. 1993;32(14):3754–62. doi: 10.1021/bi00065a030. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Li T, Chen H, Zhang K, Zheng K, Mu Y, et al. Selenium-containing 15-mer peptides with high glutathione peroxidase-like activity. J Biol Chem. 2004;279(36):37235–40. doi: 10.1074/jbc.M403032200. [DOI] [PubMed] [Google Scholar]
- 43.Ren X, Gao S, You D, Huang H, Liu Z, Mu Y, et al. Cloning and expression of a single-chain catalytic antibody that acts as a glutathione peroxidase mimic with high catalytic efficiency. Biochem J. 2001;359(Part 2):369–74. doi: 10.1042/0264-6021:3590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding L, Liu Z, Zhu Z, Luo G, Zhao D, Ni J. Biochemical characterization of selenium-containing catalytic antibody as a cytosolic glutathione peroxidase mimic. Biochem J. 1998;332(Part 1):251–5. doi: 10.1042/bj3320251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahl R, Kampkotter A, Watjen W, Chovolou Y. Antioxidant enzymes and apoptosis. Drug Metab Rev. 2004;36(3–4):747–62. doi: 10.1081/dmr-200033488. [DOI] [PubMed] [Google Scholar]
- 46.Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100(25):15035–40. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11(3):301–12. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- 48.Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, et al. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273(49):32554–60. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 49.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7(1–2):42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 50.Chang LY, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33(3):379–86. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 51.Reynaert NL, Aesif SW, McGovern T, Brown A, Wouters EF, Irvin CG, et al. Catalase overexpression fails to attenuate allergic airways disease in the mouse. J Immunol. 2007;178(6):3814–21. doi: 10.4049/jimmunol.178.6.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pryor WA, Dooley MM, Church DF. The inactivation of alpha-1-proteinase inhibitor by gas-phase cigarette smoke: protection by antioxidants and reducing species. Chem Biol Interact. 1986;57(3):271–83. doi: 10.1016/0009-2797(86)90002-5. [DOI] [PubMed] [Google Scholar]
- 53.Mendez-Alvarez E, Soto-Otero R, Sanchez-Sellero I, Lopez-Rivadulla Lamas M. In vitro inhibition of catalase activity by cigarette smoke: relevance for oxidative stress. J Appl Toxicol. 1998;18(6):443–8. doi: 10.1002/(sici)1099-1263(199811/12)18:6<443::aid-jat530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Luchese C, Stangherlin EC, Ardais AP, Nogueira CW, Santos FW. Diphenyl diselenide prevents oxidative damage induced by cigarette smoke exposure in lung of rat pups. Toxicology. 2007;230(2–3):189–96. doi: 10.1016/j.tox.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 55.Smith KR, Uyeminami DL, Kodavanti UP, Crapo JD, Chang LY, Pinkerton KE. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic Biol Med. 2002;33(8):1106–14. doi: 10.1016/s0891-5849(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 56.Ledwozyw A. Protective effect of liposome-entrapped superoxide dismutase and catalase on bleomycin-induced lung injury in rats. I Antioxidant enzyme activities and lipid peroxidation. Acta Vet Hung. 1991;39(3–4):215–24. [PubMed] [Google Scholar]
- 57.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25(2):164–9. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 58.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81(2):196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–63. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 60.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 62.White CW, Jackson JH, Abuchowski A, Kazo GM, Mimmack RF, Berger EM, et al. Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol. 1989;66(2):584–90. doi: 10.1152/jappl.1989.66.2.584. [DOI] [PubMed] [Google Scholar]
- 63.Olson NC, Grizzle MK, Anderson DL. Effect of polyethylene glycol-superoxide dismutase and catalase on endotoxemia in pigs. J Appl Physiol. 1987;63(4):1526–32. doi: 10.1152/jappl.1987.63.4.1526. [DOI] [PubMed] [Google Scholar]
- 64.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, et al. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167(1):57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 65.Bowler RP, Arcaroli J, Abraham E, Patel M, Chang LY, Crapo JD. Evidence for extracellular superoxide dismutase as a mediator of hemorrhage-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L680–7. doi: 10.1152/ajplung.00191.2002. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, et al. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock. 1996;6(Suppl 1):S23–6. [PubMed] [Google Scholar]
- 67.Izumi M, McDonald MC, Sharpe MA, Chatterjee PK, Thiemermann C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock. 2002;18(3):230–5. doi: 10.1097/00024382-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Blum S, Asaf R, Guetta J, Miller-Lotan R, Asleh R, Kremer R, et al. Haptoglobin genotype determines myocardial infarct size in diabetic mice. J Am Coll Cardiol. 2007;49(1):82–7. doi: 10.1016/j.jacc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 69.Lubbers NL, Polakowski JS, Crapo JD, Wegner CD, Cox BF. Preischemic and postischemic administration of AEOL10113 reduces infarct size in a rat model of myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2003;41(5):714–9. doi: 10.1097/00005344-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Hines IN, Hoffman JM, Scheerens H, Day BJ, Harada H, Pavlick KP, et al. Regulation of postischemic liver injury following different durations of ischemia. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G536–45. doi: 10.1152/ajpgi.00400.2002. [DOI] [PubMed] [Google Scholar]
- 71.Ozaki M, Nakamura M, Teraoka S, Ota K. Ebselen, a novel anti-oxidant compound, protects the rat liver from ischemia-reperfusion injury. Transpl Int. 1997;10(2):96–102. doi: 10.1007/s001470050019. [DOI] [PubMed] [Google Scholar]
- 72.Sheng H, Enghild JJ, Bowler R, Patel M, Batinic-Haberle I, Calvi CL, et al. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radic Biol Med. 2002;33(7):947–61. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 73.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284(1):215–21. [PubMed] [Google Scholar]
- 74.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32(9):2149–54. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 75.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group Stroke. 1998;29(1):12–7. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 76.Chatterjee PK, Patel NS, Kvale EO, Brown PA, Stewart KN, Mota-Filipe H, et al. EUK-134 reduces renal dysfunction and injury caused by oxidative and nitrosative stress of the kidney. Am J Nephrol. 2004;24(2):165–77. doi: 10.1159/000076547. [DOI] [PubMed] [Google Scholar]
- 77.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagchi D, Prasad R, Das DK. Direct scavenging of free radicals by captopril, an angiotensin converting enzyme inhibitor. Biochem Biophys Res Commun. 1989;158(1):52–7. doi: 10.1016/s0006-291x(89)80175-5. [DOI] [PubMed] [Google Scholar]
- 79.Kastenbauer S, Koedel U, Becker BF, Pfister HW. Pneumococcal meningitis in the rat: evaluation of peroxynitrite scavengers for adjunctive therapy. Eur J Pharmacol. 2002;449(1–2):177–81. doi: 10.1016/s0014-2999(02)01980-5. [DOI] [PubMed] [Google Scholar]
- 80.Sui H, Wang W, Wang PH, Liu LS. Effect of glutathione peroxidase mimic ebselen (PZ51) on endothelium and vascular structure of stroke-prone spontaneously hypertensive rats. Blood Press. 2005;14(6):366–72. doi: 10.1080/08037050500210781. [DOI] [PubMed] [Google Scholar]
- 81.McDonald MC, d’Emmanuele di Villa Bianca R, Wayman NS, Pinto A, Sharpe MA, Cuzzocrea S, et al. A superoxide dismutase mimetic with catalase activity (EUK-8) reduces the organ injury in endotoxic shock. Eur J Pharmacol. 2003;466(1–2):181–9. doi: 10.1016/s0014-2999(03)01538-3. [DOI] [PubMed] [Google Scholar]
- 82.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003;284(3):F532–7. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 83.van Empel VP, Bertrand AT, van Oort RJ, van der Nagel R, Engelen M, van Rijen HV, et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48(4):824–32. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 84.Nin N, Cassina A, Boggia J, Alfonso E, Botti H, Peluffo G, et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30(12):2271–8. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 85.Wendel A, Tiegs G. A novel biologically active seleno-organic compound—VI. Protection by ebselen (PZ 51) against galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1986;35(13):2115–8. doi: 10.1016/0006-2952(86)90578-2. [DOI] [PubMed] [Google Scholar]
- 86.Bianca RV, Wayman NS, McDonald MC, Pinto A, Shape MA, Chatterjee PK, et al. Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med Sci Monit. 2002;8(1):BR1–7. [PubMed] [Google Scholar]
- 87.Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal. 2005;7(5–6):685–93. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- 88.Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal. 2006;8(11–12):2061–73. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 89.Forero DA, Casadesus G, Perry G, Arboleda H. Synaptic dysfunction and oxidative stress in Alzheimer’s disease: emerging mechanisms. J Cell Mol Med. 2006;10(3):796–805. doi: 10.1111/j.1582-4934.2006.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762(11–12):1051–67. doi: 10.1016/j.bbadis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38(11):1433–44. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 92.Shohami E, Beit-Yannai E, Horowitz M, Kohen R. Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab. 1997;17(10):1007–19. doi: 10.1097/00004647-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 93.Mortiboys HJ, Schaefer J, Reichmann H, Jackson S. Mitochondrial dysfunction in Parkinson’s disease—revisited. Neurol Neurochir Pol. 2007;41(2):150–9. [PubMed] [Google Scholar]
- 94.Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci. 2003;991:189–98. [PubMed] [Google Scholar]
- 95.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s disease. Biochem Pharmacol. 2002;64(5–6):1037–48. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 96.Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG. Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacol Ther. 2007;114(3):327–44. doi: 10.1016/j.pharmthera.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Moussaoui S, Obinu MC, Daniel N, Reibaud M, Blanchard V, Imperato A. The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson’s disease. Exp Neurol. 2000;166(2):235–45. doi: 10.1006/exnr.2000.7516. [DOI] [PubMed] [Google Scholar]
- 98.Moreira PI, Honda K, Liu Q, Santos MS, Oliveira CR, Aliev G, et al. Oxidative stress: the old enemy in Alzheimer’s disease pathophysiology. Curr Alzheimer Res. 2005;2(4):403–8. doi: 10.2174/156720505774330537. [DOI] [PubMed] [Google Scholar]
- 99.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38(24):7609–16. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 100.Bruce AJ, Malfroy B, Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci U S A. 1996;93(6):2312–6. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 102.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80(1):452–7. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 103.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100(14):8526–31. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A. 1996;93(7):3155–60. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crow JP, Calingasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann Neurol. 2005;58(2):258–65. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 106.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001;304(3):157–60. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 107.Chan PH, Epstein CJ, Li Y, Huang TT, Carlson E, Kinouchi H, et al. Transgenic mice and knockout mutants in the study of oxidative stress in brain injury. J Neurotrauma. 1995;12(5):815–24. doi: 10.1089/neu.1995.12.815. [DOI] [PubMed] [Google Scholar]
- 108.Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20(12):1659–68. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Aladag MA, Turkoz Y, Sahna E, Parlakpinar H, Gul M. The attenuation of vasospasm by using a sod mimetic after experimental subarachnoidal haemorrhage in rats. Acta Neurochir (Wien) 2003;145(8):673–7. doi: 10.1007/s00701-003-0052-z. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol. 2004;287(1):H165–71. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]
- 111.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, et al. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18(2):159–63. doi: 10.1038/ng0298-159. [see comments] [DOI] [PubMed] [Google Scholar]
- 112.Tong JJ, Schriner SE, McCleary D, Day BJ, Wallace DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nat Genet. 2007;39(4):476–85. doi: 10.1038/ng2004. [DOI] [PubMed] [Google Scholar]
- 113.Leski ML, Bao F, Wu L, Qian H, Sun D, Liu D. Protein and DNA oxidation in spinal injury: neurofilaments—an oxidation target. Free Radic Biol Med. 2001;30(6):613–24. doi: 10.1016/s0891-5849(00)00500-1. [DOI] [PubMed] [Google Scholar]
- 114.Kalayci M, Coskun O, Cagavi F, Kanter M, Armutcu F, Gul S, et al. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem Res. 2005;30(3):403–10. doi: 10.1007/s11064-005-2615-2. [DOI] [PubMed] [Google Scholar]
- 115.Sheng H, Spasojevic I, Warner DS, Batinic-Haberle I. Mouse spinal cord compression injury is ameliorated by intrathecal cationic manganese(III) porphyrin catalytic antioxidant therapy. Neurosci Lett. 2004;366(2):220–5. doi: 10.1016/j.neulet.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 116.Malassagne B, Ferret PJ, Hammoud R, Tulliez M, Bedda S, Trebeden H, et al. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121(6):1451–9. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- 117.Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001;30(4):403–11. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- 118.Wasser S, Lim GY, Ong CN, Tan CE. Anti-oxidant ebselen causes the resolution of experimentally induced hepatic fibrosis in rats. J Gastroenterol Hepatol. 2001;16(11):1244–53. doi: 10.1046/j.1440-1746.2001.02621.x. [DOI] [PubMed] [Google Scholar]
- 119.Zhang HJ, Doctrow SR, Oberley LW, Kregel KC. Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. J Appl Physiol. 2006;100(4):1385–91. doi: 10.1152/japplphysiol.01046.2005. [DOI] [PubMed] [Google Scholar]
- 120.Choudhary S, Keshavarzian A, Yong S, Wade M, Bocckino S, Day BJ, et al. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig Dis Sci. 2001;46(10):2222–30. doi: 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]