While we can't choose our parents, we certainly can choose our friends. And just as we choose our friends, it is also a rite of passage that we break the rules that our parents set, even those that provide healthful benefits. In fact, one of the most common patterns to change with entry into adulthood is the adherence to a “normal” bedtime and disruption in the alignment between periods of activity and sleep with the natural day–night cycle. Indeed, the clock that we learn to disobey is an internal genetically programmed timepiece with near 24-h precision that originally evolved to enable the earliest forms of life on Earth to accurately anticipate the rising and setting of the sun. Importantly, there may even have been a survival benefit to this clock because, at least in plants, misalignment of endogenous period length of the clock with the environmental light cycle reduces survival and diminishes reproduction (1). Interestingly, it has recently been suspected that alignment between behavioral cycles and the light–dark cycle may also provide health benefits to humans, since at clinical and epidemiological levels, a strong correlation has emerged between chronic sleep and circadian disruption and metabolic disease (2). Timing also plays an important role in certain cardiovascular catastrophes and in both hypo- and hyperglycemic crises. Further, the availability of genetically altered animals with mutations in the genes encoding the core molecular clock has supported the idea that clocks are especially important in the regulation of feeding and body weight, in addition to glucose and lipid homeostasis (3–5). Now, with a new report in this issue of PNAS, Scheer et al. (6) provide evidence to support the hypothesis that circadian systems are important to metabolic health not just in mice but also in humans.
It is now well established that 24-h recurring patterns of behavior and physiology are controlled at the molecular level by a transcription–translation feedback loop that is expressed within both the master pacemaker neurons of the brain, located in the suprachiasmatic nucleus (SCN), and also within nearly all peripheral tissues. Although the core components of the clock have emerged over the past 20 years from forward genetic analyses in flies, plants, and mice (7), positional cloning of monogenic circadian disorders in families with the familial advanced sleep phase disorder has proven that conserved clock genes also function to control periodic behavior in humans (8). Moreover, intriguing work in human fibroblasts has shown that, like cell explants from mice, the clock in humans functions as a self-sustained oscillator when maintained in culture (out of the body) and remarkably exhibits a period length that matches each individual's subjective chronotype (e.g., “lark” versus “owl”) (9). As noted above, experimental models in mice have further revealed that a major output of the molecular clock involves the control of neuroendocrine systems involving both brain and peripheral tissues, raising the possibility that alteration of the timing of behavioral cycles (such as the feeding cycle) with the endogenous circadian cycle may adversely affect human health.
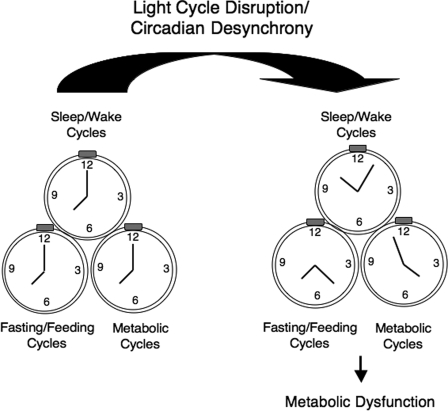
Although there are limitations to comparisons between mouse and humans, an ultimate goal is to establish the function of the clock across all species. To begin to do so, Scheer and colleagues (6) have applied a so-called “forced desynchrony” protocol in human subjects, in essence carefully rebelling against the internal clock, and then asking how internal desynchrony between the preset clock and the shifting behavioral cycle impacts systems involved in glucose and cardiometabolic physiology (Fig. 1). To achieve desynchrony, 10 subjects were admitted to the clinical research center and, over the ensuing 10 days, were subjected to progressive misalignment of behavioral and circadian cycles by extending their behavioral cycle to a 28-h day, under dim light, with 14 h of rest and fasting alternating with 14 h of wakefulness, interspersed with four evenly spaced and isocaloric meals. Simultaneously, the investigators monitored physiological endpoints including sleep, core body temperature, blood pressure, autonomic activity, energetics, melatonin levels, and key markers of glucose and lipid homeostasis (cortisol, leptin, and insulin). By using body temperature as a readout of the endogenous clock, they compared rhythms for each of these clinical endpoints and thereby determined the extent to which each marker conforms to the endogenous circadian cycle versus the overt behavioral cycle. Just as some rhythmic processes in model organisms are driven primarily by the clock (e.g., the temperature rhythm), others appear to follow more the period of feeding and fasting (e.g., leptin). Curiously, shifting the behavioral cycle also changed the phase of certain oscillating clinical markers, especially the glucocorticoid rhythm, with respect to the temperature rhythm. A major effect of circadian misalignment was the suppression of leptin across the entire behavioral cycle, in addition to an elevation of glucose. These effects on glucose and leptin appeared to be independent of alterations in sleep, suggesting that circadian misalignment per se may independently predispose to metabolic disease. Indeed, the observation that leptin is suppressed across the entire behavioral cycle resonates with previous findings of leptin deficiency during sleep deprivation (10, 11). Interestingly, in contrast to findings from human sleep-deprivation studies, the decrease in leptin is not simply due to activation of stress response because neither catecholamine nor cortisol levels were elevated. However, because leptin production is also controlled by insulin, the finding of decreased leptin in the misaligned state is consistent with induction of an “insulin-resistant” phenotype (12). Indeed, the idea that insulin resistance may be exacerbated by circadian disruption, and the reciprocal interplay between insulin action and circadian timing, has some precedent in the observation from animal studies showing that clock genes play a role in adipogenesis, an insulin-regulated process (13). Overall, decreased levels of leptin indicate that misalignment triggers a perceived state of energy deficit, potentially predisposing such individuals to adverse metabolic consequences of leptin deficiency, including increased hepatic gluconeogenesis and increased hunger. Notably, previous studies on sleep restriction have also shown increased levels of ghrelin, so it will be interesting to learn whether circadian misalignment leads to dysregulation of a broader array of incretin hormones.
Fig. 1.
Circadian desynchrony and metabolic disease. Behavioral cycles (including sleep/wake, fasting/feeding, and metabolic cycles) are normally aligned with the light–dark cycle. However, when circadian misalignment occurs, these cycles may become desynchronized from the external light–dark cycle, leading to adverse metabolic physiological consequences. Both clinical and experimental genetic approaches have begun to elucidate how circadian systems may contribute to normal glucose and cardiometabolic homeostasis.
In agreement with findings on altered leptin production, Scheer et al. (6) also observed that postprandial glucose excursion and insulin production was increased following circadian disruption. Because the elevation of insulin was insufficient to prevent increased levels of glucose, we can infer that misalignment creates an acute deficit in the capacity of endocrine pancreas to compensate for insulin resistance. It will be important to learn whether deficits in glucose disposal after a meal arise because of impairment at the level of liver, fat, or muscle, or some combination of these 3 sites. Given the absence of either increased catecholamines in urine or hypercortisolinemia in the misaligned subjects, it is does not appear that the observed disturbance of glucose metabolism arises simply as a consequence of increased activity of autonomic output or hypothalamic-pituitary-adrenal loops. One word of caution is that we still do not know whether misalignment alters the rhythmic increase in growth hormone that occurs during sleep, a factor in regulating hepatic gluconeogenesis at night (14). Since, both hyperglycemia and hyperinsulinemia primarily emerge in the postprandial state, it is possible that meal-associated factors such as deficiency of gut-derived incretin hormones may contribute to impaired glucose metabolism after circadian misalignment (15). Indeed, circadian control of glucose metabolism has been well characterized in previous clinical studies, and it is axiomatic for those who suffer from diabetes and those who care for these individuals to recognize that requirement for insulin and the capacity to metabolize glucose varies profoundly across the day and night. Whereas rodent studies suggest that some of the diurnal variation in glucose control derives from outputs of the central circadian pacemaker via autonomic innervation of liver and fat (16), it will also be important to better understand whether peripheral tissue clock function plays a primary role in glucose homeostasis in both the basal and misaligned conditions in humans.
In addition to the aforementioned disorders of glucose and leptin regulation, Scheer et al. (6) also observed that circadian misalignment resulted in mild but significant hypertension, indicating that over the long term, cumulative cardiovascular risk may increase as a result of circadian misalignment. So what have we now learned regarding human rhythms and cardiometabolic health? A parsimonious summary would be to conclude that periodic variation in behavioral and physiological rhythms is advantageous and even necessary to maintain normal glucose metabolism in otherwise healthy individuals. In fact, the general disregard for timing, in cycles of feeding, sleep, and activity, is equally pervasive in our modern patterns of reduced sleep, shift work, and 24/7 activity. The problem also extends to our analytic approach to studies of metabolism where we frequently overlook the crucial dimension of time. Thus, it should no longer escape us to recognize that we have taken timing for granted, despite its centrality to maintaining metabolic health.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4453.
References
- 1.Dodd AN, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 3.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones CR, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, et al. Molecular insights into human daily behavior. Proc Natl Acad Sci USA. 2008;105:1602–1607. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeigerer A, Rodeheffer MS, McGraw TE, Friedman JM. Insulin regulates leptin secretion from 3T3–L1 adipocytes by a PI 3 kinase independent mechanism. Exp Cell Res. 2008;314:2249–2256. doi: 10.1016/j.yexcr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimba S, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 15.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Cailotto C, et al. Daily rhythms in metabolic liver enzymes and plasma glucose require a balance in the autonomic output to the liver. Endocrinology. 2008;149:1914–1925. doi: 10.1210/en.2007-0816. [DOI] [PubMed] [Google Scholar]