Abstract
It is now established that native language affects one's perception of the world. However, it is unknown whether this effect is merely driven by conscious, language-based evaluation of the environment or whether it reflects fundamental differences in perceptual processing between individuals speaking different languages. Using brain potentials, we demonstrate that the existence in Greek of 2 color terms—ghalazio and ble—distinguishing light and dark blue leads to greater and faster perceptual discrimination of these colors in native speakers of Greek than in native speakers of English. The visual mismatch negativity, an index of automatic and preattentive change detection, was similar for blue and green deviant stimuli during a color oddball detection task in English participants, but it was significantly larger for blue than green deviant stimuli in native speakers of Greek. These findings establish an implicit effect of language-specific terminology on human color perception.
Keywords: cognition, cultural differences, event-related potentials, linguistic relativity, visual mismatch negativity
The ability to organize the experienced world into categories is a fundamental property of human cognition. The extent to which these categories are influenced by one's language and culture (1–3) has been fiercely debated in the fields of linguistics, anthropology, psychology, and philosophy for at least a century (4). Color perception has been a traditional test-case of Whorf's principle of linguistic relativity (5–7), i.e., the idea that speakers of different languages perceive and process reality and the world differently, influenced by lexical and grammatical distinctions specific to their language. The vast majority of empirical research in the past 15 years has supported the notion that language acts as an attention-directing mechanism in the cognitive processing of color, in both offline similarity judgments (6, 8) and online perceptual discrimination (9–11).
Despite the evidence in favor of Whorf's formulation of the linguistic relativity principle, critics remain unconvinced. Scholars such as Pinker (12, 13) and Munnich and Landau (14) consider effects of language on decision making and similarity judgments as “banal” and ultimately noninformative with regards to the question of whether language affects thought: “Speakers of different languages tilt in different directions in a woolly task, rather than having differently structured minds” (see ref. 13, p. 148). Thus at the heart of the current debate lies the extent and nature of the observed cross-linguistic effects. Specifically, differences in memory and perceptual judgments between speakers of different languages may result from high-level attentional and cognitive processes, overlaid on a perceptual system that is language independent and universal. However, language may fundamentally shape and affect automatic, low-level, unconscious perception of the experienced world.
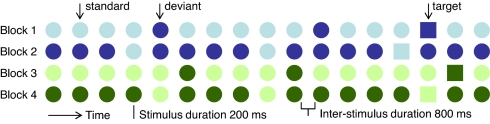
Here, we recorded brain potentials in Greek and English native speakers performing a basic oddball shape discrimination task (Fig. 1) to test the extent to which preattentive and unconscious aspects of perception are affected by an individual's native language. Greek differentiates the blue region of color space into a darker shade called ble and a lighter shade called ghalazio. In 2 experimental blocks, all stimuli were light or dark blue and in 2 others, they were light or dark green. We instructed the participants to press a button when and only when they saw a square shape (target, probability 20%) within a regularly paced stream of circles (probability 80%). Within one block the most frequent stimulus was a light or dark circle (standard, probability 70%) and the remaining stimuli were circles with a contrasting luminance (deviant, probability 10%), i.e., dark if the standard was light or vice versa. Importantly, we provided no instruction regarding differences in luminance between the stimuli nor did we instruct participants to attend or respond to the circle stimuli.
Fig. 1.
Experimental design and sample of stimulus sequences presented in the 4 experimental blocks. Note that targets were not systematically deviant in color as well as in shape; half of them had the color of the standard stimulus.
From previous naming experiments (15) that established the ble–ghalazio boundary in Munsell color space we selected one stimulus from each color category. Three Greek speakers who did not take part in the main experiment confirmed that the colors chosen were prototypical exemplars of ghalazio and ble. The 2 green stimuli (one light green, one dark green) were matched to the blues in terms of the difference in luminance between light and dark instances. Furthermore, the blue and green stimuli were equally distant from the middle gray background in terms of saturation and luminance. After the experiment, all participants were asked to name the experimental stimuli in their native language. All Greek participants named the dark blue stimulus ble, the light blue stimulus ghalazio, and light and dark green stimuli prasino (green). All English participants named both blue stimuli blue, and both green stimuli green.
We expected luminance deviants to elicit a visual mismatch negativity (vMMN) in all blocks, indexing preattentive change detection, which requires no active response on the part of the participants (16–18). The vMMN is considered a visual equivalent of the auditory MMN (16). It is elicited by deviant (rare) stimuli in visual oddball paradigms, independently of the direction of focused attention (18) and is therefore considered automatic and preattentive (17–18). Given the physical matching of green and blue stimuli, we expected a vMMN effect of similar magnitude for blue and green contrasts in English monolinguals. Furthermore, we hypothesized that the existence of 2 basic color terms distinguishing light and dark blue in Greek would lead Greek participants to perceive luminance deviants as more different in the blue than in the green blocks and would therefore induce a greater vMMN effect for blues.
Results
We systematically analyzed main effects of the 3 factors manipulated in this study: deviancy (deviant vs. standard luminance), color (green vs. blue), and participant group (English and Greek) and their interactions.
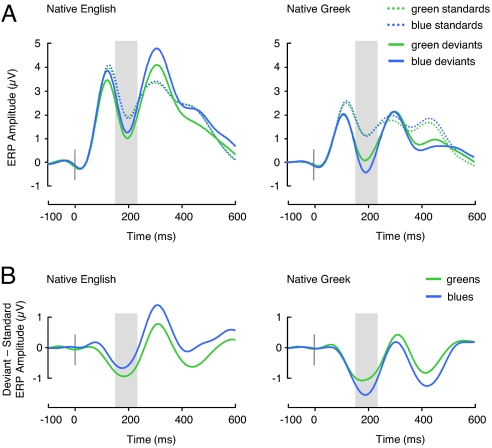
Deviant circles elicited the expected vMMN effect for both colors and in both participant groups, as indicated by a deviancy main effect (F[1, 38] = 40.1, P < 0.0001; Fig. 2A). In addition, we found a deviancy by participant group interaction (F[1, 38] = 7.8, P < 0.01) induced by a greater overall deviancy effect in Greek than English participants.
Fig. 2.
Event-related potential (ERP) results. (A) ERPs elicited by standard circles (standards) and passive deviant circles (deviants) irrespective of luminance over parietooccipital electrodes where the vMMN was maximal (linear derivation of IZ, O1, O2, OZ, PO7, PO8, PO9, and PO10). Mean brain potential amplitude was significantly more negative for deviants than standards between 162 and 232 ms (shaded interval). (B) Amplitude difference between ERPs elicited by deviants and standards irrespective of luminance over the same group of electrodes. The data are filtered at 8 Hz low pass for graphic illustration only.
Critically, we found no overall main effect of color (P > 0.1) or participant group (P > 0.1) and no significant color by group interaction on the mean amplitude of the vMMN but, as predicted, a significant, triple interaction between participant group, color, and deviancy (F[1, 38] = 4.8, P < 0.05; Fig. 2B). Post hoc tests confirmed that this interaction was generated by a differential vMMN response pattern in Greek and English participants, such that the vMMN effect was numerically (but not significantly) greater for green than blue deviants in the English participants (F[1, 38] = 0.9, P > 0.1) but significantly greater for blue than green deviants in Greek participants (F[1, 38] = 7.1, P < 0.02), whereas the vMMN effect for green deviants was of similar magnitude in both the participant groups (F[1, 38] = 0.27, P > 0.1).
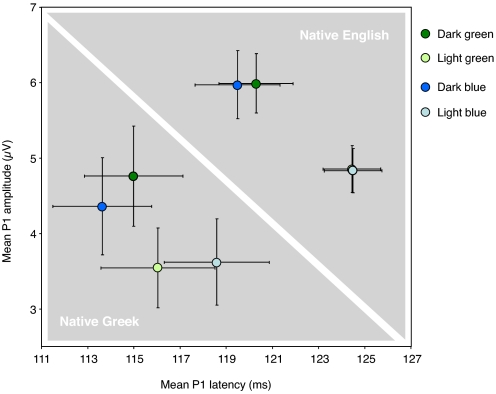
We subsequently explored differences at earlier latencies, focusing on the so-called P1, that is, the first positive peak elicited by visual stimuli over parietooccipital regions of the scalp, to test for potential differences between participant groups in early stages of visual perception. We averaged brain potentials for all standard circle stimuli sorted by color to obtain a light blue, a dark blue, a light green, and a dark green standard brain potential (Fig. 3). Both groups of participants differed significantly in terms of P1 mean amplitude between 100 and 130 ms (F[1, 38] = 5.21, P < 0.03) and peak latency (F[1, 38] = 6.39, P < 0.02). Analyses of variance in English participants showed that the P1 mean amplitude was significantly modulated by luminance (F[1, 19] = 34.24, P < 0.0001) but not color (F[1, 19] = 0, P > 0.1), and the same pattern was found for P1 latency (luminance: F[1, 19] = 29.61, P < 0.0001, color: F[1, 19] = 0.33, P > 0.1). The same analyses in Greek participants revealed significant interactions between luminance and color for both P1 mean amplitude (F[1, 19] = 7.98, P < 0.02) and P1 latency (F[1, 19] = 4.83, P < 0.05), showing that amplitude differences between light and dark blue P1s were smaller than differences between light and dark green P1s, whereas latency differences showed the opposite pattern. In sum, the P1 amplitude/latency pattern was overall different in the 2 participant groups on 3 different accounts: (i) There was no difference in P1 latency between light and dark green standards in the Greek participants (P > 0.1), although significant differences in P1 latency were found for both green and blue stimuli in the English participants (both Ps < .001); (ii) there was a smaller, but significant, difference in P1 amplitude between light and dark blue stimuli in the Greek participants (P < 0.05); and (iii) the variance in both the latencies and mean amplitudes of individual P1 peaks was substantially greater in the Greek participants (Fig. 3).
Fig. 3.
P1 mean amplitudes and latencies elicited by the standard circles (light blue, dark blue, light green, dark green) at electrode of maximal amplitude (PO8). Error bars depict the SEM.
Discussion
This study tested potential effects of color terminology in different languages on early stages of visual perception using the vMMN, an electrophysiological index of perceptual deviancy detection. The vMMN findings show a greater distinction between different shades of blue than different shades of green in Greek participants, whereas English speakers show no such distinction. To our knowledge, this is the first demonstration of a relationship between native language and unconscious, preattentive color discrimination rather than simply conscious, overt color categorization (9).
Surprisingly, analysis of mean peak latencies and mean signal amplitudes between 100 and 130 ms revealed that the P1 peak, traditionally associated with low-level perceptual processing, followed a pattern of differences compatible with—and possibly underlying—the differences found in the vMMN. Indeed, whereas P1 latencies and amplitudes elicited by light and dark stimuli were generally overlapped for blues and greens in English controls, they were different for blues and greens in Greek participants. Such P1 effects are consistent with the established sensitivity of this component to categorical color boundaries (19) and are compatible with its responsiveness to complex categorical contrasts such as faces versus cars or butterflies (20). Nevertheless, it must be kept in mind that the P1 findings reported here were not predicted and therefore fundamentally exploratory in nature. The critical point regarding the P1 results is that the difference between groups was driven by more than just the difference between light and dark green standards: it was determined by both the absence of differences in latency between light and dark green and the smaller difference in mean amplitude between light and dark blue, whereas all differences between light and dark stimuli where highly significant in English participants. It is not yet possible to interpret the direction of P1 differences in relation to fundamental stages of color perception.
There is also a possibility that exposure to shades of blue in the natural environment may account for a greater “sensitivity” to differences in luminance in the blue range in native Greek participants. However, it would be difficult to account for the fact that the deviancy effect observed was very similar in blue and green contexts in English-native participants who are arguably exposed to many shades of green, and certainly more so than shades of blue. Indeed, the vMMN effect was highly comparable for blue and green in the English participants, and the P1 amplitude/latency patterns were remarkably overlapped for blue and green standard stimuli. It is therefore unlikely that the effects seen in either of the 2 groups are solely driven by personal history of exposure to particular colors. Furthermore, behavioral studies reveal robust categorical perception effects along the lightness dimension of the blue area of color space in populations with diverse cultural backgrounds and natural environments but who all have 2 terms to distinguish between a darker and a lighter shade of blue in their respective language, e.g., Greek (15), Turkish (21), and Russian (9).
To conclude, our electrophysiological findings reveal not only an effect of the native language on implicit color discrimination as indexed by preattentive change detection but even electrophysiological differences occurring as early as 100 ms after stimulus presentation, a time range associated with activity in the primary and secondary visual cortices (22). We therefore demonstrate that language-specific distinctions between 2 colors affect early visual processing, even when color is task irrelevant. At debriefing, none of the participants highlighted the critical stimulus dimension tested (luminance) or reported verbalizing the colors presented to them. The findings of the present study establish that early stages of color perception are unconsciously affected by the terminology specific to the native language. They lend strong support to the Whorfian hypothesis by demonstrating, for the first time, differences between speakers of different languages in early stages of color perception beyond the observation of high-level categorization and discrimination effects strategically and overtly contingent on language-specific distinctions. Future studies will shed more light on the relationships between language, environment, and cognition, and will determine whether such early and implicit effects generalize to other domains of human perception.
Materials and Methods
Participants.
Twenty native English speakers and 20 native Greek speakers with normal or corrected-to-normal vision gave written consent to take part in the experiment that was approved by the ethics committee of the School of Psychology, Bangor University. Participants were matched in age (20–23), level of education (university), and handedness (right) across groups. The Greek participants were studying at a British university, had lived in the U.K. for a mean time of 18 months (SD = 18, range 5–60), and were first exposed to English at the age of 9 years on average (SD = 3, range 5–14). To minimize the possibility that knowledge of English might affect performance on the task, participants were selected from courses that do not require an advanced level of English proficiency on the International English Language Testing System (maximum 6). In addition, proficiency in English was tested by means of both a questionnaire and an objective vocabulary test (23). The vast majority of participants self-reported that they had intermediate proficiency in English, and their performance on the vocabulary test indicated lower-intermediate to intermediate English proficiency (mean = 66/90, SD = 14, range 39–84).
Stimuli and Procedure.
The filled circle and square shapes subtending ≈2° of visual angle were presented on a middle gray background on a calibrated CRT monitor. Chromaticity was measured using a Minolta CS-100 Colorimeter. The following Munsell colors were used (CIE 1931 Y, x, y chromaticity coordinates are given in parentheses): dark blue: 5PB/value 4 (Y = 10.7, x = 0.234, y = 0.230), light blue: 5PB/value 7 (Y = 41.5, x = 0.259, y = 0.264), dark green: 5G/value 4 (Y = 10.7, x = 0.259, y = 0.397), light green: 5G/value 7 (Y = 41.7, x = 0.279, y = 0.377). Munsell chroma (saturation) was held constant across stimuli (chroma 6). Participants viewed 4 blocks (2 blue and 2 green) of 540 stimuli. Within each block, one stimulus was frequent (light or dark circle, 70%) and 3 stimuli were infrequent: luminance deviant (circle with a luminance opposed to that of the frequent stimulus, 10%), standard target (light square, 10%), and deviant target (dark square, 10%). Presentation order was pseudorandomized, such that 2 deviants or targets never appeared in immediate succession, and there were at least 3 standards in a row between 2 infrequent stimuli. Stimuli were flashed for 200 ms with an interstimulus interval of 800 ms. Participants were instructed to detect squares by pressing the spacebar of a keyboard. Block order was fully counterbalanced between participants. The proportion of hits was high (mean = 95% ± 5).
Event-Related Potentials.
Electrophysiological data were recorded in reference to Cz at a rate of 1 kHz from 64 Ag/AgCl electrodes placed according to the extended 10–20 convention. Impedances were kept below 7 kΩ. EEG activity was filtered online with a band pass between 0.01 Hz and 200 Hz, and refiltered offline with a 20-Hz low-pass zero phase shift digital filter (slope 48 db/Oct). Eye blinks were mathematically corrected, and epochs with activity exceeding ±75 μV at any cap electrode site were automatically discarded. There was a minimum of 120 valid epochs per condition in every subject. Epochs ranged from −100 to 1,000 ms after the onset of the stimulus. Baseline correction was performed in reference to prestimulus activity and individual averages were digitally rereferenced to the global average reference. The vMMN analysis was conducted on individual ERPs elicited by passive standard and deviant circles irrespective of luminance (light and dark circles combined) to discard luminance effects. The vMMN was maximal over the parietooccipital scalp and studied at electrodes IZ, O1, O2, OZ, PO7, PO8, PO9, and PO10. The P1 analysis was conducted on individual ERPs elicited by the 4 standard circles in each of the 4 blocks (Fig. 3) at electrode PO8.
Acknowledgments.
We thank Ljubica Damjanovic, Chris Frith, Noriko Hoshino, James Intriligator, Aneta Pavlenko, Bob Rafal, Eirini Sanoudaki, Steve Tipper, Marilyn Vihman, and Simon Watt for useful discussions, and Anna Franklin, I-Fan Su, and Simon Watt for technical assistance. G.T. and J.K. are supported by the Economic and Social Research Council U.K. (RES-E024556–1); G.T. is supported by the European Research Council (ERC-209704).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Whorf BL. In: Language, Thought, and Reality: Selected Writings of Benjamin Lee Whorf. Carroll JB, editor. Cambridge, MA: MIT Press; 1940. pp. 220–232. [Google Scholar]
- 2.Whorf BL. In: Language, Thought, and Reality: Selected Writings of Benjamin Lee Whorf. Carroll JB, editor. Cambridge, MA: MIT Press; 1956. [Google Scholar]
- 3.Vygotsky L. Thought and Language. Cambridge, MA: MIT Press; 1934. [Google Scholar]
- 4.Hunt E, Agnoli F. The Whorfian hypothesis: A cognitive psychology perspective. Psych Rev. 1991;98:377–389. [Google Scholar]
- 5.Heider ER, Olivier DC. The structure of the colour space in naming and memory for two languages. Cogn Psychol. 1972;3:337–354. [Google Scholar]
- 6.Davidoff J, Davies I, Roberson D. Colour categories in a stone-age tribe. Nature. 1999;398:203–204. doi: 10.1038/18335. [DOI] [PubMed] [Google Scholar]
- 7.Brown RW, Lenneberg EH. A study in language and cognition. J Abnorm Psychol. 1954;49:454–462. doi: 10.1037/h0057814. [DOI] [PubMed] [Google Scholar]
- 8.Roberson D, Davies I, Davidoff J. Color categories are not universal: Replications and new evidence from a stone-age culture. J Exp Psychol Gen. 2000;129:369–398. doi: 10.1037//0096-3445.129.3.369. [DOI] [PubMed] [Google Scholar]
- 9.Winawer J, et al. Russian blues reveal effects of language on color discrimination. Proc Natl Acad Sci USA. 2007;104:7780–7785. doi: 10.1073/pnas.0701644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert AL, Regier T, Kay P, Ivry RB. Whorf hypothesis is supported in the right visual field but not the left. Proc Natl Acad Sci USA. 2006;103:489–494. doi: 10.1073/pnas.0509868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drivonikou GV, et al. Further evidence that Whorfian effects are stronger in the right visual field than the left. Proc Natl Acad Sci USA. 2007;104:1097–1102. doi: 10.1073/pnas.0610132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinker S. The Language Instinct: How the Mind Creates Language. New York: William Morrow & Co.; 1994. [Google Scholar]
- 13.Pinker S. The Stuff of Thought: Language as a Window into Human Nature. New York: Viking; 2007. [Google Scholar]
- 14.Munnich E, Landau B. In: Language in Mind: Advances in the Study of Language and Thought. Gentner D, Goldin-Meadow S, editors. Cambridge MA: MIT Press; 2003. pp. 112–155. [Google Scholar]
- 15.Athanasopoulos P. Cognitive representation of colour in bilinguals: The case of Greek blues. Biling Lang Cogn. 2009;12:83–95. [Google Scholar]
- 16.Czigler I, Balazs L, Winkler I. Memory-based detection of task-irrelevant visual changes. Psychophysiology. 2002;39:869–873. doi: 10.1111/1469-8986.3960869. [DOI] [PubMed] [Google Scholar]
- 17.Czigler I, Balazs L, Pato LG. Visual change detection: Event-related potentials are dependent on stimulus location in humans. Neurosci Lett. 2004;364:149–153. doi: 10.1016/j.neulet.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Winkler I, et al. Preattentive binding of auditory and visual stimulus features. J Cogn Neurosci. 2005;17:320–339. doi: 10.1162/0898929053124866. [DOI] [PubMed] [Google Scholar]
- 19.Fonteneau E, Davidoff J. Neural correlates of colour categories. Neuroreport. 2007;18:1323–1327. doi: 10.1097/WNR.0b013e3282c48c33. [DOI] [PubMed] [Google Scholar]
- 20.Thierry G, Martin CD, Downing P, Pegna AJ. Controlling for interstimulus perceptual variance abolishes N170 face selectivity. Nat Neurosci. 2007;10:505–511. doi: 10.1038/nn1864. [DOI] [PubMed] [Google Scholar]
- 21.Özgen E, Davies IRL. Turkish color terms: Tests of Berlin and Kay's theory of color universals and linguistic relativity. Linguistics. 1998;36:919–956. [Google Scholar]
- 22.Mangun GR, Buonocore MH, Girelli M, Jha AP. ERP and fMRI measures of visual spatial selective attention. Hum Brain Mapp. 1998;6:383–389. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nation P. Teaching and Learning Vocabulary. New York: Newbury House/Harper Row; 1990. [Google Scholar]