Abstract
Hydrogen sulfide (H2S) is synthesized by 2 enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). l-Cysteine (l-Cys) acts as a natural substrate for the synthesis of H2S. Human penile tissue possesses both CBS and CSE, and tissue homogenates efficiently convert l-Cys to H2S. CBS and CSE are localized in the muscular trabeculae and the smooth-muscle component of the penile artery, whereas CSE but not CBS is also expressed in peripheral nerves. Exogenous H2S [sodium hydrogen sulfide (NaHS)] or l-Cys causes a concentration-dependent relaxation of strips of human corpus cavernosum. l-Cys relaxation is inhibited by the CBS inhibitor, aminoxyacetic acid (AOAA). Electrical field stimulation of human penile tissue, under resting conditions, causes an increase in tension that is significantly potentiated by either propargylglycine (PAG; CSE inhibitor) or AOAA. In rats, NaHS and l-Cys promote penile erection, and the response to l-Cys is blocked by PAG. Our data demonstrate that the l-Cys/H2S pathway mediates human corpus cavernosum smooth-muscle relaxation.
Keywords: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), H2S, penile erection, L-cysteine
Hydrogen sulfide (H2S), like nitric oxide (NO), was best known as a toxic pollutant until recent years when it has been proposed to be a gaseous neurotransmitter. In the recent literature, H2S is becoming recognized as a mediator of physiological and/or pathological processes (1–3). H2S is present in micromolar concentrations in blood (4), and it can be synthesized from l-cysteine (l-Cys), which acts as a substrate for two pyridoxal-5′-phosphate-dependent enzymes: cystathionine β-synthase (CBS; EC 4.2.1.22) and cystathionine γ-lyase (CSE; EC 4.4.1.1). These enzymes are responsible for the majority of the endogenous production of H2S in mammalian tissues. l-Cys can be derived from alimentary sources or can be liberated from endogenous proteins. It can also be synthesized endogenously from l-methionine through the transsulfuration pathway, with homocysteine being an intermediate in the process (5, 6). CBS and CSE were detected in human and other mammalian cells (7), and their expression is thought to be tissue-specific (4). Indeed, CBS was expressed in hippocampus, cerebellum, cerebral cortex, and brainstem, and its activity is 30-fold greater than CSE (8). However, CSE expression and activity were shown to be higher than CBS in aorta, mesenteric artery, portal vein, and other vascular tissue (4, 9).
Penile corpus cavernosum is a highly vascularized tissue whose function depends on an equilibrium between vasodilatory and vasoconstrictory tone. Indeed, it is now widely accepted that erectile dysfunction (ED) is predominantly a vascular disease, and ED is considered an early sign of cardiovascular disease (10, 11). On this basis, preliminary animal studies have suggested the involvement of H2S in facilitating erectile function (12, 13). However, the involvement of a functionally intact l-Cys/H2S pathway in human penile erection has not yet been demonstrated.
In the present work, by using human corpus cavernosum (HCC) obtained by a standardized surgical procedure (14), we have demonstrated that human penile tissue expresses both CBS and CSE, and tissue homogenates efficiently convert l-Cys to H2S. Functional studies, performed in vitro, confirm that the l-Cys/H2S pathway plays a functional role in human tissue. Indeed, either sodium hydrogen sulfide (NaHS), an exogenous source of H2S, or l-Cys, the substrate for CBS/CSE, relaxed HCC strips in a concentration-related manner. Pharmacological modulation of CBS and CSE by using a CSE inhibitor [proparglycine (PAG)] and/or a CBS inhibitor [aminoxyacetic acid (AOAA)] confirmed the involvement of the l-Cys/H2S pathway both in vitro and in vivo in rats. The intracavernous administration of either NaHS or l-Cys to rats elicited penile erection, and the response to l-Cys was blocked by PAG. Collectively, these observations indicate that a functional l-Cys/H2S pathway may be involved in mediating penile erection in humans and other mammals.
Results
Real-Time Quantitative RT-PCR and Western Blot Studies.
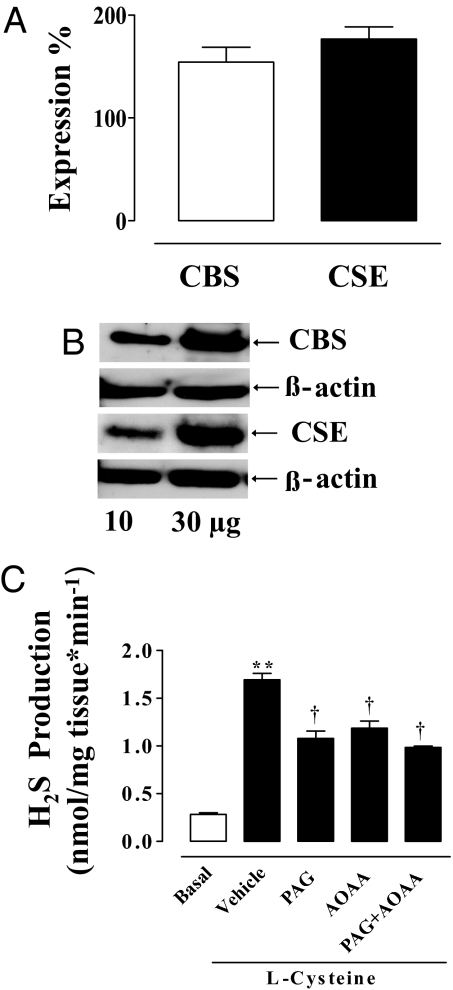
Quantitative RT-PCR demonstrated the presence of both CBS and CSE mRNA in HCC tissue (Fig. 1A). CBS and CSE were also expressed as proteins as demonstrated by Western blot studies (Fig. 1B).
Fig. 1.
CBS and CSE: activity, Western blot analysis, and qRT-PCR of human penile tissue. (A) HCC-expressed mRNA for both CBS and CSE as determined by qRT-PCR. (B) Representative Western blot analysis for CBS and CSE. (C) HCC homogenate produced H2S under basal conditions (open bar). Incubation of HCC homogenate with 10 mM l-Cys caused a significant increase in the H2S production compared with basal values (**, P < 0.001). PAG (10 mM), 1 mM AOAA, or 10 mM PAG plus 1 mM AOAA significantly inhibited the l-Cys-induced increase in H2S production (†, P < 0.01). Data represent the mean ± SEM from 3 or 4 different human specimens.
H2S Production in HCC.
HCC generated detectable amounts of H2S (Fig. 1C). The biosynthesis of H2S was increased by 3- fold over basal values after incubation of tissue homogenates with l-Cys, the CBS/CSE substrate (Fig. 1C). PAG (10 mM), AOAA (1 mM), or the combination of both inhibitors significantly inhibited the increase in H2S production stimulated with l-Cys. Therefore, HCC is capable of synthesizing H2S from l-Cys.
Immunohistochemistry of CBS and CSE.
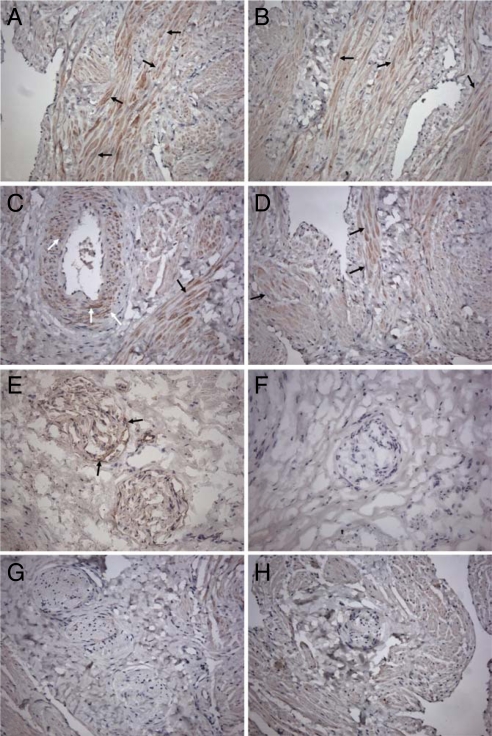
The immunohistochemistry study shows that HCC tissue expressed a robust signal for CSE and to a lesser extent for CBS (Fig. 2). Bundles of muscular tissue in trabeculae showed a clear immunoreactivity for CSE (Fig. 2 A and B) and CBS (Fig. 2D). In addition, immunoreactivity for CSE was also present in the vascular smooth-muscle cells of penile arteries (Fig. 2C). When we attempted to perform an immunohistochemistry study on peripheral nerves to visualize CBS and CSE, we found a negative staining (Fig. 2 G and H). Because nerve morphology was particularly affected by the antigen retrieval in paraffin sections, we performed the immunohistochemistry study in cryostat sections. Under those experimental conditions we found a positive staining for CSE only (Fig. 2E). Thus, CSE might play a role in the HCC by triggering the H2S pathway both in smooth-muscle cells and peripheral nerves.
Fig. 2.
Immunochemistry for CBS and CSE in HCC. (A–D) Immunohistochemical detection of CSE and CBS in HCC tissue. Immunoreactivity and nuclear staining appear brown (DAB) and blue (hematoxylin counterstain), respectively. CSE was detected in trabecular muscular tissue (A and B, black arrows) and vascular smooth-muscle cells (C, white arrows). Immunoreactivity for CBS was mostly observed in trabecular muscular tissue (D, black arrows). Results illustrated are from a single experiment and are representative of 3 different specimens. (Original magnification, 200×.) (E–H) Immunohistochemical detection of CSE and CBS in HCC nerve fibers. Immunoreactivity and nuclear staining appear brown (DAB) and blue (hematoxylin counterstain), respectively. CSE was detected in nerve fibers in cryostat (E, arrows) and not in paraffin (G) sections. Both cryostat (F) and paraffin (H) sections lacked immunoreactivity for CBS. Results illustrated are from a single experiment and are representative of 3 different specimens. (Original magnification, 200×.)
Effect of NaHS in HCC Strips.
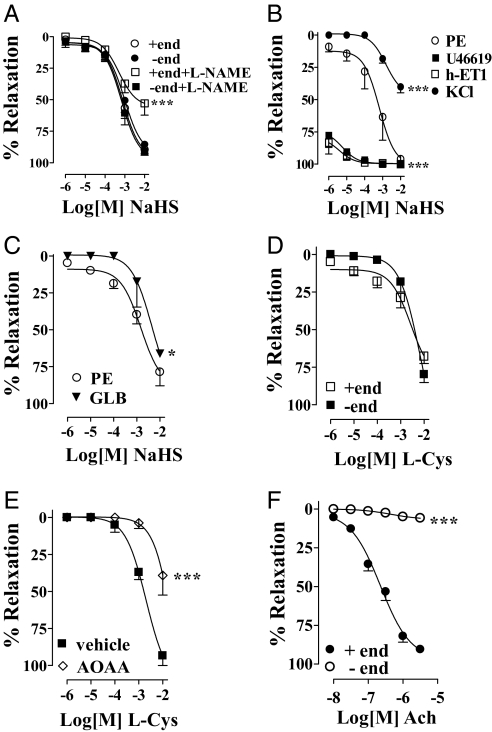
In the presence of stable tone elicited by phenylephrine (PE; 3 μM), the H2S donor, NaHS (1 μM–10 mM), caused a concentration-dependent relaxation in an endothelium-independent manner (Fig. 3A). To assess further the potential involvement of endogenous NO in the NaHS effect, the strips were incubated with 100 μM l-NAME. l-NAME slightly reduced NaHS-induced relaxation but only at the highest NaHS concentration (Fig. 3A). Then, to investigate further the underlying mechanism involved in the relaxation caused by NaHS, we precontracted HCC with U46619 (10 nM) or h-endothelin-1 (h-ET1, 30 nM), 2 known modulators of the Rho kinase pathway. In this setting, the NaHS-induced relaxation was robustly enhanced in HCC precontracted with U46619 compared with the results obtained with HCC precontracted with PE (Fig. 3B). Similarly, in the presence of h-ET1, we observed a significant increase in NaHS-induced relaxation (Fig. 4B). In contrast, the relaxant effect mediated by NaHS was inhibited by KCl-induced precontraction (80 mM, Fig. 3B). Additionally, glibenclamide (GLB, 150 μM), a KATP channel inhibitor, significantly attenuated the relaxation caused by NaHS in HCC strips precontracted with 3 μM PE (Fig. 3C). Fig. 3F reveals that Ach relaxed HCC strips only in the presence of endothelium.
Fig. 3.
NaHS or l-Cys effect on HCC strips. (A) NaHS-relaxed HCC strips with (+end) or without (−end) endothelium; 100 μM l-NAME inhibited NaHS-induced relaxation at higher concentrations tested and in the presence of endothelium only (***, P < 0.0001). (B) HCC strips without endothelium precontracted with 10 nM U46619 or 30 nM h-ET1 displayed a significantly increased relaxant response to NaHS compared with 3 μM PE (***, P < 0.0001). NaHS-induced relaxation in HCC strips without endothelium contracted with 80 mM KCl was significantly reduced compared with 3 μM PE contractions (***, P < 0.0001). (C) Incubation of HCC with 150 μM glibenclamide (GLB) before 3 μM PE reduced NaHS-induced relaxation (*, P < 0.05). (D) l-Cys relaxed HCC strips precontracted with 3 μM PE with (+end) or without (−end) endothelium. (E) One millimolar AOAA inhibited l-Cys-induced relaxation in HCC strips without endothelium (−end) precontracted with 3 μM PE (***, P < 0.0001). (F) Ach caused relaxation of HCC strips with (+end) but not without (−end) endothelium precontracted with 3 μM PE (***, P < 0.0001). Experiments were performed on 8 strips for each of the NaHS experiments, 5 strips for each of the l-Cys experiments, and 15 strips for the Ach experiment.
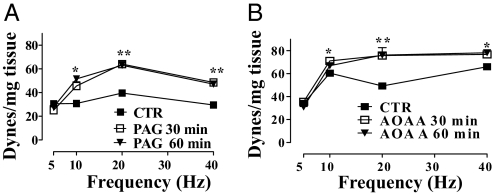
Fig. 4.
EFS of HCC strips. EFS caused a frequency-related increase in basal tone. Incubation of HCC strips with 10 mM PAG for 30 or 60 min (A) or with 1 mM AOAA for 30 or 60 min (B) significantly increased the EFS-induced contraction [*, P < 0.05; **, P < 0.01 vs. control (CTR)]. Tissue responses to EFS are expressed as force in dynes per milligram of tissue. Experiments were performed on 3 different specimens.
Effect of l-Cys in HCC Strips.
To verify the involvement of the l-Cys/H2S pathway in HCC, we challenged the strips with l-Cys. l-Cys relaxed HCC strips in an endothelium-independent manner (Fig. 3D). AOAA (1 mM) incubation significantly inhibited l-Cys-induced relaxation (Fig. 3E).
HCC Strips and Electric Field Stimulation (EFS).
EFS elicited contraction of HCC strips under resting conditions. Addition of the vehicle did not modify the contraction at 30 or 60 min. Incubation with 10 mM PAG (Fig. 4A) or 1 mM AOAA (Fig. 4B) at 30 min and 60 min caused a significant increase in contraction to EFS at 10, 20, and 40 Hz.
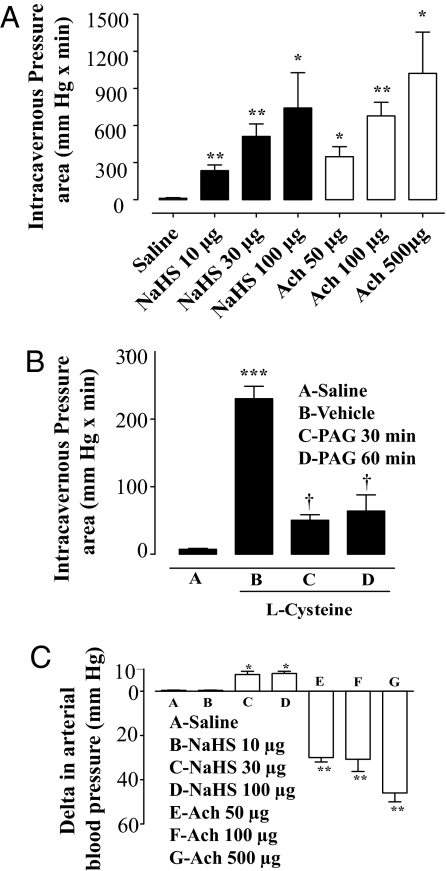
NaHS Increases Intracavernosal Pressure in Anesthetized Rats.
To evaluate whether NaHS causes penile erection in vivo, we monitored the intracavernous pressure after NaHS administration into the rat corpus cavernosum. To validate the experimental procedure, we used acetylcholine (Ach, 50, 100 or 500 μg per rat), which caused a dose-dependent increase in intracavernous pressure (Fig. 5A). NaHS at doses of 10, 30, or 100 μg per rat induced a significant increase in intracavernous pressure as shown in Fig. 5A. The NaHS administration at a lower dose (10 μg per rat) failed to modify the mean arterial blood pressure, whereas the higher doses tested (30 and 100 μg per rat) caused a small dose-unrelated but significant increase in mean arterial blood pressure (Fig. 5C). Ach caused a dose-dependent decrease in mean arterial blood pressure (Fig. 5C). Injection of 50 μL of saline (vehicle) caused no appreciable effect (Fig. 5).
Fig. 5.
Effect of NaHS or l-Cys on intracavernous pressure in anesthetized rats. (A) NaHS (10, 30, and 100 μg per rat) caused significant dose-dependent increases in intracavernous pressure. Ach (50, 100, and 500 μg/ per at) caused a dose-dependent increase in intracavernous pressure. (B) l-Cys at a dose of 30 μg per rat caused an increase in intracavernous pressure. Intravenous administration of 50 mg/kg PAG significantly inhibited l-Cys-induced penile erection (***, P < 0.0001; †, P < 0.05). (C) NaHS at a dose of 10 μg per rat did not cause any change in arterial blood pressure whereas 30 and 100 μg of NaHS per rat caused a significant increase in blood pressure. Ach (50, 100, and 500 μg per rat) caused a dose-dependent significant decrease in blood pressure (**, P < 0.01; *, P < 0.05 vs. saline). Data were obtained from 7 separate experiments for NaHS and 4 for l-Cys.
Intracavernous administration of l-Cys (30 μg per rat) caused a significant increase in intracavernous pressure (Fig. 5B). Intravenous administration of PAG (50 mg/kg) at 30 and 60 min significantly reduced l-Cys-elicited rat penile erection (Fig. 5B). These data support the possibility that H2S is a natural mediator of penile erection in the rat.
Discussion
H2S is a new emerging gaseous signaling molecule (15–17). H2S is normally present in humans and can be generated endogenously from l-Cys in a reaction catalyzed by either CBS or CSE. Historically, H2S was known as a toxic gas, and mammalians have evolved a fine-tuned regulatory system to control H2S generation at the cellular level by catabolism of the gas through oxidation in mitochondria or by methylation followed by scavenging by metalloproteins and heme-containing compounds (18). The role of H2S in vascular homeostasis is a new concept, and in recent years data have accumulated suggesting a vasorelaxant role for H2S (15–17, 19), and a possible cross-talk with the NO pathway has been proposed (20). H2S was recently shown to be a physiologic vasorelaxant in mice with deletion of CSE (19).
In penile erection there is a strong involvement of the vascular system, and the l-Arg/NO pathway plays a major role (21–24). Following the parallelism between NO and H2S recently established in other cardiovascular areas, we addressed the question of whether H2S could act as a mediator in HCC and thus be involved in human erectile function. Previous studies have indicated the possible involvement of this gaseous mediator in rodents and monkeys (12, 13). However, there are no published studies using human tissue. To address this issue, we used HCC obtained from patients undergoing sex change, which we have already shown to be a reliable tissue source to study receptors and mediators involved in human physiology (14, 25–27). Human tissue expressed CBS and CSE both as protein and mRNA. The quantitative (q) RT-PCR studies clearly showed that mRNA levels of CBS and CSE are similar in HCC. Next, we addressed the question of the tissue localization of both enzymes. HCC tissue stained strongly for CSE and to a lesser extent for CBS. Interestingly, a clear immunoreactivity for CSE and CBS was detected in bundles of muscular tissue and in trabeculae. Immunoreactivity for CSE was also observed in the vascular smooth-muscle cells of penile vessels and in the penile artery. These data are in line with the current belief that CSE rather than CBS is more important in H2S production at the vascular level (28). In addition, peripheral nerves showed a positive stain to CSE, in contrast to CBS, suggesting that CSE may modulate the l-Cys/H2S pathway in peripheral cavernous nerves of man. Next, we demonstrated that HCC tissue homogenates under basal conditions produces ≈0.3 nmol of H2S per mg of protein and that addition of exogenous l-Cys boosts the production of H2S by 3-fold. The specificity of the assay and the involvement of both CBS and CSE were confirmed by the finding that H2S production was prevented by either PAG, an inhibitor of CSE, or AOAA, an inhibitor of CBS. These data indicate that HCC can synthesize H2S from l-Cys via the catalytic actions of CBS and CSE.
Having determined that (i) HCC possesses both CBS and CSE protein and mRNA, (ii) homogenates of HCC convert l-Cys (the substrate) to H2S, (iii) PAG and AOAA inhibit l-Cys conversion, and (iv) both enzymes are localized in the smooth-muscle and vascular component of the corpus cavernosum, we performed a functional study by using isolated HCC strips. EFS of HCC strips, under resting conditions, caused a contraction that increased in a frequency-dependent manner. Subtraction of the H2S component, by incubation of HCC strips with PAG or AOAA, produced a significant increase in EFS-induced contraction, confirming the involvement of the l-Cys/H2S pathway in maintaining basal tone. In line with this finding, HCC strips precontracted with PE relaxed to l-Cys in an endothelium-independent and concentration-dependent manner, and this effect was significantly inhibited by AOAA. The capacity of HCC strips to respond to H2S was also confirmed by the fact that HCC strips relaxed in a concentration-dependent manner to NaHS, an H2S donor. This effect was endothelium-independent, but at the higher concentration of NaHS tested, there was a significant inhibition of HCC relaxation by l-NAME. This effect may or may not be important. Because it has been suggested that there is a possible cross-talk between NO and H2S (29), all of the other experiments in vitro, using NaHS, were performed by using HCC strips without endothelium present.
To gain further insight into the possible mechanism(s) underlying the H2S effect, the HCC strips were precontracted with h-ET1 or U46619, a stable analog of thromboxane. These 2 stimuli were selected because the contractile mechanisms they trigger strongly rely on activation of the Rho kinase pathway (30, 31). RhoA is a monomeric GTPase that is inactive when GDP is bound but becomes active after binding to GTP. Activated RhoA stimulates Rho kinase, a serine/threonine kinase. Rho kinase phosphorylates the myosin light chain (MLC) phosphatase at the myosin-binding subunit, thereby causing its inactivation. This results in an increased expression of phosphorylated MLC leading to myosin binding to α-actin and smooth-muscle contraction. This pathway is particularly relevant in the erectile mechanism(s) because selective inhibition of the RhoA/Rho kinase pathway has been shown to promote erectile responses in the rat (32). When NaHS was used to relax HCC strips contracted with either U46619 or h-ET1, there was a marked increase in the H2S vasorelaxant effect, suggesting that H2S may interfere with this contractile mechanism. Interestingly, when HCC strips were precontracted with KCl there was a marked inhibition of the NaHS vasorelaxant effect, suggesting that part of the action could be mediated by potassium conductance channels as also suggested by the effect glibenclamide, an inhibitor of KATP channels (1).
Experiments using CBS knockout mice were not considered because they have serious hyperhomocysteinemia (40- to 50-fold elevated plasma homocysteine) and need to be treated with homocysteine-lowering drugs to survive a reduced life span (33–36). These mice have growth retardation, reduced survival and altered vascular responsiveness to cholinergic and bradykinin stimulation, making their use of questionable value in the present experiments. During revision of this manuscript, a study in which CSE knockout mice were developed revealed that such mice had much reduced serum H2S levels, developed age-related hypertension, and showed vasorelaxant effects to administered H2S (19). Such mice were fertile, which may be attributed to a more dominant NO-mediated erectile system or development of alternate pathways for erectile function.
The present in vitro data strongly support the case for a role of H2S as a natural signaling molecule in HCC. However, the question of whether the l-Cys/H2S pathway is involved in penile erection in vivo needed to be addressed. In vivo animal models of measuring the cavernous pressure in the rat or mouse have been widely used to study the effects of phosphodiesterase 5 inhibitors and to define the relevance of the other pathways in penile erection (32, 37). When we challenged anesthetized rats with NaHS, there was a dose-dependent increase in intracavernous pressure, implying that exogenous H2S can cause penile erection. In parallel, we performed experiments with acetylcholine to be certain that the experimental model used does respond to a well-established physiological exogenous stimulus. Interestingly, whereas administration of acetylcholine caused, as expected, a systemic hypotension, NaHS caused a small but not dose-dependent hypertension. This slight hypertension is difficult to explain because i.v. or intraarterial administration of H2S should cause hypotension (4). A possible explanation could be related to the observation that low concentrations of NaHS tested in vitro can cause vascular smooth-muscle contraction followed by relaxation at higher concentrations (29). To address further the role of this pathway in penile erection, we sought to determine whether the intracavernous injection of l-Cys could cause a change in intracavernous pressure. l-Cys increased the intracavernous pressure, and this effect was prevented by in vivo administration of PAG. These data indicate that l-Cys elicits an erectile response in rats that is blocked by an inhibitor of H2S formation from l-Cys.
In conclusion, the data presented in this study demonstrate collectively that the l-Cys/H2S signaling pathway is involved in mediating HCC smooth-muscle relaxation. Therefore, it is possible that H2S may function to mediate penile erection in humans, as it appears to do in rats. To what extent this pathway complements the l-Arg/NO signaling pathway in promoting erectile function is presently unknown. These observations may help to unravel the complex mechanisms underlying the pathophysiology of human penile erection and may lead to the development of therapeutic approaches in the treatment of ED and sexual arousal disorders.
Materials and Methods
Human Tissue.
In male-to-female transsexual surgical procedures, the penis and testicles are amputated, and a neovagina is created to simulate female external genitalia. Patients undergo appropriate hormonal pretreatment with antiandrogens and estrogens to adapt to female appearance, and the therapy is discontinued 2 months before surgery. The corpora cavernosa were carefully excised from the penis immediately after amputation and placed in ice-cold oxygenated Krebs solution and washed extensively with heparinized Krebs solution. After the lavage, the corpora cavernosa were placed in ice-cold Krebs solution and kept on ice until the experiments were conducted (14). All patients were informed of all procedures and gave their written consent. The protocol was approved by the Ethics Committee of the Medical School of the University of Naples Federico II. Corpus cavernosum specimens were obtained from 6 different individuals.
Real-Time Quantitative RT-PCR.
The presence of CBS and CSE was determined by PCR. Total mRNA from HCC was extracted by using TRIzol reagent (Invitrogen, according to the manufacturer's recommendations). Reverse transcription was performed, and 100 ng of the RNA samples described above was used for qPCR. Samples were run in triplicate in 50-μL reactions by using an ABI PRISM 5700 sequence detector system (Applied Biosystems). Samples were incubated at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. SYBR Green oligonucleotides to detect human CBS and CSE were specifically designed by using primer express software (Applied Biosystems) and validated for their specificity. Relative quantification of target cDNA was determined by arbitrarily setting the control value at 100, and changes in cDNA content of a sample were expressed as a multiple thereof. Differences in cDNA input were corrected by normalizing signals obtained with primers specific for GAPDH. mRNA copy differences were corrected by using human GAPDH endogenous control predeveloped assay reagent (Applied Biosystems). To exclude nonspecific amplification and/or the formation of primer dimers, control reactions were performed in the absence of target cDNA. All of the experiments were run in triplicate.
The primer sequences were as follows: CBS forward, 5′-cctggcaccgttatccctg-3′; CBS reverse, 5′-ctgtgcagtcattgcctgtgt-3′; CSE forward, 5′-gcaagtggcatctgaatttg-3′; and CSE reverse, 5′-cccattacaacatcactgtgg-3′.
Western Blotting.
HCC was homogenized in modified RIPA buffer [50 mM Tris·HCl (pH 7.4), 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mmol/L PMSF, 10 μg/mL aprotinin, 20 μmol/L leupeptin, 50 mmol/L NaF) by using a Polytron homogenizer (2 cycles of 10 s at maximum speed). After centrifugation of homogenates at 10,000 rpm for 10 min, 10–30 μg of the denatured proteins were separated on 10% SDS/polyacrylamide gels and transferred to a PVDF membrane. Membranes were blocked by incubation in PBS containing 0.1% vol/vol Tween 20 and 5% nonfat dry milk for 2 h, followed by overnight incubation at 4 °C with mouse polyclonal CBS (Abnova Novus Biologicals) antibody (1:500) or mouse monoclonal CSE (Abnova Novus Biologicals) antibody (1:500). The filters were washed extensively in PBS containing 0.1% vol/vol Tween 20 before incubation for 2 h with anti-horseradish peroxidase conjugate secondary antibody. Membranes were then washed and developed using enhanced chemiluminescence substrate (ECL; Amersham Pharmacia Biotech).
Immunohistochemistry.
HCC samples were snap frozen in liquid nitrogen in OCT embedding medium (Tissue Tek) and stored at −80 °C or fixed overnight in 4% buffered formalin and paraffin-embedded. Cross-sections were cut (6 μm) and used for CSE and CBS detection by immunohistochemistry. Paraffin sections, after being dewaxed and rehydrated, were boiled for 30 min in citrate buffer for antigen retrieval. Cryostat sections were incubated in acetone for 10 min, air dried, and rehydrated with PBS. For staining, sections were incubated with 3% H2O2 in methanol for 10 min, and protein block serum-free (DakoCytomation) was added for 30 min. Sections were stained with 3 μg/mL mouse monoclonal Ab against CSE (M02 Clone; Abnova) or 1/500 mouse polyclonal Ab against CBS (A01; Abnova), both diluted in 1% blocking reagent (PerkinElmer)/0.3% Triton X-100 (MP Biomedicals) in PBS overnight before washing in TNT buffer [Tris·HCl, 50 mM (pH 7.5), 0.15 M NaCl, and 0.05% Tween 20 (Sigma)]. Sections incubated with no primary antibody were used as negative controls. Subsequently, sections were incubated with biotinylated anti-mouse secondary antibody (1/1,000; DakoCytomation) for 15 min before washing. Streptavidin–horseradish peroxidase (LSAB kit; DakoCytomation) was added for 15 min before washing as described above. Enzymatic activity was detected with 3,3′-diaminobenzidine substrate (DAB; DakoCytomation) before washing in dH2O. Hematoxylin was used to counterstain before rinsing in H2O. Sections were subsequently dehydrated and mounted in Entellan (Merck). Images were taken with the aid of a Leica DFC320 video camera connected to a Leica DM RB microscope using Leica Application Suite software V2.4.0.
Measurement of H2S in HCC.
H2S determination was performed according to Stipanuk and Beck (37) with modifications. The tissue was homogenized in a lysis buffer [100 mM potassium phosphate buffer (pH 7.4), 10 mM sodium orthovanadate, and protease inhibitors]. Protein concentration was determined by using the Bradford assay (Bio-Rad). Homogenates were added in a reaction mixture (total volume 500 μL) containing 20 μL of 2 mM piridoxal 5′-phosphate, 20 μL of 10 mM l-Cys, and 30 μL of saline. The reaction was performed in parafilmed Eppendorf tubes and initiated by transferring tubes from ice to a water bath at 37 °C. After incubation of 30 min, 250 μL of 1%, Zn(Ac)2 was added followed by 250 μL of 10% trichloroacetic acid. Subsequently, 133 μL of 20 mM N,N-dimethyl-p-phenylendiamine-sulphate (DPD) in 7.2 M HCl and 133 μL of 30 mM FeCl3 in 1.2 M HCl were added, and the absorbance of the solution was measured after 20 min at a wavelength of 650 nm. The H2S synthesis inhibitors PAG (10 mM), AOAA (1 mM), or a combination of both were added 5 min before addition of l-Cys. All samples were assayed in duplicate, and H2S concentrations were calculated against a calibration curve of NaHS (3.12–250 μM). Results were expressed as nmoles per milligram of protein*min−1 and calculated as mean ± SEM from 4 specimens. Data were analyzed by using Student's t test.
HCC Strips.
Longitudinal strips (2 cm) of HCC were dissected from the trabecular structure of the penis and isolated (14). Krebs solution had the following composition: 115.3 mM NaCl, 4.9 mM KCl, 1.46 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, 11.1 mM glucose (Carlo Erba). HCC strips were mounted in a 2-mL organ bath containing oxygenated (95% O2 and 5% CO2) Krebs solution at 37 °C. HCC strips were connected to isometric force-displacement transducers (model 7002; Ugo Basile), and changes in tension were recorded continuously by using a polygraph linearcorder (WR3310; Graphtec). Tissues were preloaded with 2 g of tension and allowed to equilibrate for 90 min in Krebs solution that was changed at 15-min intervals. After equilibration, tissues were standardized by performing repeated 3 μM PE (Sigma) contractions until 3 equal responses were obtained. After standardization, endothelial integrity was assessed by using 0.01–10 μM Ach (Sigma) (see Fig. 3F). Strips without a functional (Ach-responsive) endothelium were obtained by incubating in distilled water for 15 s. A concentration–response curve to 1 μM–10 mM NaHS (Sigma) was obtained in the presence or absence of endothelium, by using HCC strips precontracted with 3 μM PE. To assess the involvement of NO, we incubated the strips for 20 min with 100 μM l-NAME (Sigma) before NaHS challenge. KATP channel involvement (4) in NaHS-induced relaxation was assessed by incubating HCC-denuded strips with 150 μM glibenclamide (Sigma) for 20 min. In another set of experiments, HCC strips without endothelium were precontracted with 10 nM U46619 (Alexis), 30 nM h-ET1 (Tocris) or 80 mM potassium chloride (Carlo Erba). In another set of experiments, HCC strips, under resting conditions, were electrically stimulated for 10 s with platinum wire electrodes connected to a digital stimulator LE12106 (Pan Lab-2B), which provided square wave pulses at the stimulation parameters of 5, 10, 20, and 40 Hz (frequency), 1 ms (pulse width), and 90 V (amplitude). This contractile response was repeated after 30 and 60 min of incubation with 10 mM PAG or 1 mM AOAA or vehicle (saline). The tissue responses to EFS were expressed as force in dynes per milligram of tissue. Experiments were performed on 3 different specimens. Finally, a concentration–response curve to 1 μM–10 mM l-Cys (Sigma) was obtained by using HCC strips with or without endothelium. In another set of experiments, in the absence of endothelium, the strips were incubated with 1 mM AOAA and challenged with 1 μM–10 mM l-Cys. Data were analyzed by using ANOVA followed by Bonferroni's post hoc test. The data represent the mean ± SEM from 8 separate specimens for NaHS or 5 for l-Cys.
Monitoring Intracavernous Pressure in Anesthetized Rats.
The present work was performed in accordance with the guidelines of Italian law (No. 116/1992) and European Council law (No. 86/609/CEE) for animal care. Male Wistar rats weighing 200–250 g were used (Charles River). Animals were kept under laboratory conditions (temperature 23 ± 2 °C, humidity range 40–70%, 12-h light/dark cycle). Food and water were fed ad libitum. Rats were anesthetized with an i.p. injection of urethane (1 g/kg), so that the rats breathed spontaneously during the experiment. For continuous systemic blood pressure measurements, a heparinized (5 units/mL) polyethylene catheter was introduced into the carotid artery connected to a pressure transducer (BLPR-2; 2Biological Instruments). With a midline perineal incision, followed by blunt dissection of the overlying striated muscles, entrance to the tunica albuginea of the crus corpus cavernosum was achieved. A 26-gauge needle attached to a heparinized (50 units/mL) polyethylene catheter was inserted into the crus corpus cavernosum, and the intracavernous pressure was monitored with a pressure transducer (BLPR-2). These parameters were recorded, and data acquisition and calculations were performed by using a computer system (Biopac; 2Biological Instruments). For pharmacological evaluation via the intracavernous route, a 26-gauge needle was placed at the other crus for drug injection. NaHS dissolved in 50 μL of saline was given at doses of 10, 30, and 100 μg per rat. To validate the experimental model we used Ach in 50 μL of saline at doses of 50, 100, and 500 μg per rat. In another set of experiments we assessed the involvement of H2S in penile erection by using l-Cys in 50 μL of saline at a dose of 30 μg per rat. Saline (50 μL) served as the control vehicle. l-Cys was injected intracavernously, and PAG (CSE inhibitor) was administered at a dose of 50 mg/kg i.v. (30- and 60-min pretreatment). Data were calculated as area under the curve (mmHg × min) and expressed as mean ± SEM from 7 separate experiments for NaHS and 4 for l-Cys. The changes in systemic blood pressure were calculated as differences from basal values after intracavernous drug injection (ΔmmHg) and expressed as mean ± SEM. Data were analyzed by using Student's t test.
Footnotes
The authors declare no conflict of interest.
References
- 1.Li L, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 2.Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, et al. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 6.Fiorucci S, Distrutti E, Cirino G, Wallace J-L. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Levonen A-L, Lapatto R, Saksela M, Raivio K-O. Human cystathionine γ-lyase: Developmental and in vitro expression of two isoforms. Biochem J. 2000;347:291–295. [PMC free article] [PubMed] [Google Scholar]
- 8.Awata S, Nakayama K, Suzuki I, Sugahara K, Kodama H. Changes in cystathionine γ-lyase in various regions of rat brain during development. Biochem Mol Biol Int. 1995;35:1331–1338. [PubMed] [Google Scholar]
- 9.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 10.Ganz P. Erectile dysfunction: Pathophysiologic mechanisms pointing to underlying cardiovascular disease. Am J Cardiol. 2005;96:8M–12M. doi: 10.1016/j.amjcard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Hodges L-D, Kirby M, Solanki J, O'Donnell J, Brodie D-A. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61:2019–2025. doi: 10.1111/j.1742-1241.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 12.Srilatha B, Adaikan P-G, Moore P-K. Possible role for the novel gasotransmitter hydrogen sulfide in erectile dysfunction: A pilot study. Eur J Pharmacol. 2006;535:280–282. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Srilatha B, Adaikan P-G, Li L, Moore P-K. Hydrogen sulfide: A novel endogenous gasotransmitter facilitates erectile function. J Sex Med. 2007;4:1304–1311. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 14.Mirone V, et al. A standardized procedure for using human corpus cavernosum strips to evaluate drug activity. J Pharmacol Toxicol Methods. 2000;44:477–482. doi: 10.1016/s1056-8719(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 15.Pearson R-J, Wilson T, Wang R. Endogenous hydrogen sulfide and the cardiovascular system: What's the smell all about? Clin Invest Med. 2006;29:146–150. [PubMed] [Google Scholar]
- 16.Johansen D, Ytrehus K, Baxter G-F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia–reperfusion injury: Evidence for a role of KATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 17.Xiaohui L, et al. Down-regulation of endogenous hydrogen sulfide pathway in pulmonary hypertension and pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Circ J. 2005;69:1418–1424. doi: 10.1253/circj.69.1418. [DOI] [PubMed] [Google Scholar]
- 18.Geng B, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali M-Y, et al. Regulation of vascular nitric oxide in vitro and in vivo: A new role for endogenous hydrogen sulfide? Br J Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005;106:233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Cartledge J, Minhas S, Eardley I. The role of nitric oxide in penile erection. Expert Opin Pharmacother. 2001;2:95–107. doi: 10.1517/14656566.2.1.95. [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 24.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 25.Cirino G, et al. Involvement of β3-adrenergic receptor activation via cyclic GMP- but not NO-dependent mechanisms in human corpus cavernosum function. Proc Natl Acad Sci USA. 2003;100:5531–5536. doi: 10.1073/pnas.0931347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d'Emmanuele di Villa Bianca R, et al. Peripheral relaxant activity of apomorphine and of a D1 selective receptor agonist on human corpus cavernosum strips. Int J Impot Res. 2005;17:127–133. doi: 10.1038/sj.ijir.3901293. [DOI] [PubMed] [Google Scholar]
- 27.d'Emmanuele di Villa Bianca R, et al. Sphingosine 1-phosphate induces endothelial nitric-oxide synthase activation through phosphorylation in human corpus cavernosum. J Pharmacol Exp Ther. 2006;316:703–708. doi: 10.1124/jpet.105.093419. [DOI] [PubMed] [Google Scholar]
- 28.Wallace J-L, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: Contribution to dual modulation of vascular tension. Toxicology. 2007;232:138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Chitaley K, et al. Antagonism of Rho kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Eto M, Steers W-D, Somlyo A-P, Somlyo A-V. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 32.Mills T-M, Chitaley K, Wingard C-J, Lewis R-W, Webb R-C. Effect of Rho kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol. 2001;91:1269–1273. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 33.Ovechkin AV, et al. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol. 2006;291:L905–L911. doi: 10.1152/ajplung.00543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe M, et al. Mice deficient in cystathionine β-synthase: Animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss N, et al. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine β-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 36.Kamath AF, et al. Elevated levels of homocysteine compromise blood–brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn G-J, et al. Chronic administration of phosphodiesterase 5 inhibitor improves erectile and endothelial function in a rat model of diabetes. Int J Impot Res. 2005;17:134–141. doi: 10.1111/j.1365-2605.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 38.Stipanuk M-H, Beck P-W. Characterization of the enzymic capacity for cysteine desulfhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]