Abstract
Francisella tularensis (Ft), a Gram-negative intracellular bacterium, is the etiologic agent of tularemia. Infection of mice with <10 Ft Live Vaccine Strain (Ft LVS) organisms i.p. causes a lethal infection that resembles human tularemia. Here, we show that immunization with as little as 0.1 ng Ft LVS lipopolysaccharide (Ft-LPS), but not Ft lipid A, generates a rapid antibody response that protects wild-type (WT) mice against lethal Ft LVS challenge. Protection is not induced in Ft-LPS-immunized B cell-deficient mice (μMT or JhD), male xid mice, or Ig transgenic mice that produce a single IgH (not reactive with Ft-LPS). Focusing on the cellular mechanisms that underlie this protective response, we show that Ft-LPS specifically stimulates proliferation of B-1a lymphocytes that bind fluorochrome-labeled Ft-LPS and the differentiation of these cells to plasma cells that secrete antibodies specific for Ft-LPS. This exclusively B-1a antibody response is equivalent in WT, T-deficient (TCRαβ−/−, TCRγδ−/−), and Toll-like receptor 4 (TLR4)-deficient (TLR4−/−) mice and thus is not dependent on T cells or typical inflammatory processes. Serum antibody levels peak ≈5 days after Ft-LPS immunization and persist at low levels for months. Thus, immunization with Ft-LPS activates a rare population of antigen-specific B-1a cells to produce a persistent T-independent antibody response that provides long-term protection against lethal Ft LVS infection. These data support the possibility of creating effective, minimally invasive vaccines that can provide effective protection against pathogen invasion.
Francisella tularensis (Ft), the etiologic agent of tularemia, is classified as a Category A agent because it can be disseminated by the aerosol route, has an extremely low infectious dose, and can cause severe morbidity and mortality. Because typhoidal and respiratory forms of human tularemia can have a mortality rate of >30%, most research into the pathogenesis of Ft has used the “Live Vaccine Strain” (LVS). Ft LVS is attenuated for humans, but virulent for mice when introduced by certain routes, and causes disease that resembles human tularemia (reviewed in refs. 1 and 2).
Host defenses against pathogens like Ft have classically been separated into innate and adaptive immune responses. Innate immunity offers a rapid response because of stimulation of germ-line encoded invariant receptors, such as Toll-like receptors (TLRs). Adaptive immune responses, in contrast, occur more slowly and reflect antigen recognition by clonally distributed Ig (Ig) or T cell receptors. However, there are important immune cells that do not conform to this bifurcated model of immunity. Subsets of T cells and B cells (e.g., γδ, CD8αα and NK T, and marginal zone and B-1 cells) are neither fully innate nor adaptive based on classical definitions (reviewed in ref. 3).
B-1 lymphocytes, often referred to as innate immune B cells, constitute a self-renewing population that expresses a largely unique set of Ig receptors. These cells, which are primarily located in spleen, peritoneal cavity (PerC), pleural cavity and intestinal mucosa, are the major producers of “natural” antibodies in serum (4). Although they are known to induce T-dependent responses (5), B-1 cells commonly participate in early defenses against pathogens by rapidly producing antibodies against T-independent antigens (6). They are subdivided based on expression of CD5, i.e., B-1a cells are CD5+ whereas B-1b cells are CD5−.
We previously showed that treatment of mice i.p. or intradermally (i.d.) with Ft-LPS protects against lethal i.p. challenge with Ft LVS (7, 8). Here, we show that antigen-specific B-1a antibody responses are required for this protection and that protection is induced by Ft-LPS, but not Ft-lipid A. We show further that the induction of protective antibody responses proceeds normally in the absence of αβ or γδ T cells or TLR4, but fails when B-1 development is compromised. In addition, we show that although Ft-LPS administration does not induce splenic enlargement or other signs of nonspecific inflammation, it clearly induces the expansion of a specific population of B-1a cells that bind fluorochrome-labeled Ft-LPS and the rapid differentiation of these cells to plasma cells that secrete anti-Ft-LPS antibodies. Thus, we demonstrate a unique and highly specific B-1a antibody response that is induced in the absence of accompanying inflammation and protects against subsequent Ft LVS infection. These studies are the first to demonstrate the unique involvement of B-1a cells in this protective antigen-specific response and to show that this antibody response occurs in the absence of either T cell help or detectable TLR4 stimulation. This response pattern challenges classification of this antibody response as either innate or adaptive.
Results
Protection Against Lethal Ft LVS Infection Is Induced by as Little as 0.1 ng Ft-LPS.
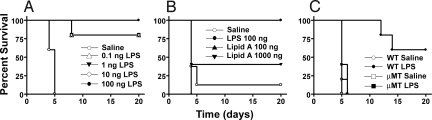
To determine the minimal dose of Ft-LPS required for protection, we pretreated mice with saline or Ft-LPS (0.1 to 100 ng per mouse) i.p. 2 days before challenge with ≈103 CFU Ft LVS. All saline-pretreated mice died (MTD of 4.6 ± 0.5 d), whereas all mice pretreated with 100 ng Ft-LPS i.p. survived (Fig. 1A). As little as 0.1 ng Ft-LPS protected 80% of mice (MTD = 8 d for the mice that succumbed to infection).
Fig. 1.
Survival of saline- or Ft-LPS-pretreated mice after challenge with Ft LVS. (A) WT C57BL/6J mice were injected i.p. with saline or 0.1 - 100 ng Ft-LPS 2 d before challenge with ≈103 CFU Ft LVS i.p. Data shown are from 1 experiment, 5 mice per treatment. (B) 5 WT mice per treatment/experiment were injected i.p. with saline, 100 ng Ft-LPS, 100 ng Ft LVS lipid A, or 1000 ng Ft LVS lipid A 2 d before challenge with ≈104 CFU Ft LVS. Combined results are shown for 2 experiments. (C) 5 WT or μMT mice were injected i.p. with saline or 100 ng Ft-LPS 2 d before challenge with ≈104 CFU Ft LVS. Data in the experiment shown are representative of 7 with similar design and outcome.
Protection induced by immunization with Ft-LPS is long-lived. When challenged i.p. with ≈103 CFU of Ft LVS 72 days after immunization, all 5 Ft-LPS-immunized animals survived. In contrast, all 6 unimmunized control mice died.
Ft-LPS Carbohydrate Component Is Critical for Induction of Protection by Ft-LPS.
LPS is composed of a hydrophobic glycolipid, lipid A, covalently coupled to the “core oligosaccharide” which, in turn, is coupled to the O-polysaccharide antigen. Although administration of Ft-LPS induces strong protection against Ft LVS challenge, lipid A purified from Ft-LPS does not. In a representative experiment (Fig. 1B), Ft lipid A pretreatment provided, at best, partial protection against a challenge dose of ≈104 CFU Ft LVS administered i.p. Pretreatment of mice with 100 ng lipid A per mouse resulted in no survival, and pretreatment with 1,000 ng Ft LVS lipid A resulted in only 40% survival with a MTD = 4.0 ± 0 d.
B Cells Are Required for Ft-LPS Induced Protection Against Lethal Ft LVS Infection.
μMT mice lack mature B cells (9). Ft-LPS fails to provide any protection to μMT mice against lethal challenge with ≈104 CFU Ft LVS (Fig. 1C). In contrast, in this experiment, 60% of the Ft-LPS-pretreated WT mice survived (P < 0.0023). Even when challenged with only ≈102 CFU, 0 of 5 Ft-LPS-pretreated μMT mice survived. Therefore, B cells (and/or the antibody they produce) are required for Ft-LPS-induced protection against lethal Ft challenge.
Experiments with additional B cell- or Ig-deficient mouse strains confirm this conclusion. JhD mice have a deletion of the IgH chain J segments (JH) that impairs B cell development (9). Ft-LPS pretreatment of JhD mice fails to protect against Ft LVS challenge whereas the WT BALB/cByJ mice are fully protected [supporting information (SI) Table S1]. In mIgM-Tg and (m+s)IgM-Tg mice, which express a single transgenic VH186.2 heavy chain that rescues normal B cell development (10), the lack of JH ensures that the transgenic heavy chain is the only one expressed (9), resulting in a very limited Ig repertoire. In addition, the mIgM-Tg mice lack the Ig secretion signal, thereby precluding all antibody secretion (9), whereas (m+s)IgM-Tg mice contain an Ig secretion signal and secrete antibody expressing only VH186.2 heavy chain. Neither mIgM-Tg nor (m+s)IgM-Tg strain produces a detectable antibody response to Ft-LPS (titers < 20), and Ft-LPS immunization does not protect against Ft infection (Table S1).
Bruton's tyrosine kinase (Btk) is required for B cell receptor-induced signaling (11). In mice, the xid mutation in Btk results in impaired BCR signaling accompanied by a severe deficiency in B-1a cells, decreased serum IgM and IgG3, and decreased numbers of mature B-2 cells (12, 13). Because our data pointed to a role for B cells and antibody in Ft-LPS-mediated protection, we compared male WT and mutant (xid) mice for their ability to be protected by Ft-LPS pretreatment (Table S2). None of the mice carrying the defective allele, regardless of pretreatment, survived challenge with as few as 102 CFU Ft LVS. Lowering the challenge dose did not improve survival as Ft-LPS pretreatment protected 0 of 5 xid male mice after a challenge with only 10 CFU Ft LVS i.p. Collectively, these studies demonstrate that antibody responses, perhaps produced by B-1a, are required for the protection induced by Ft-LPS immunization.
Ft-LPS Immunization Evokes Antigen-Specific B-1a Cells in the Spleen and PerC.
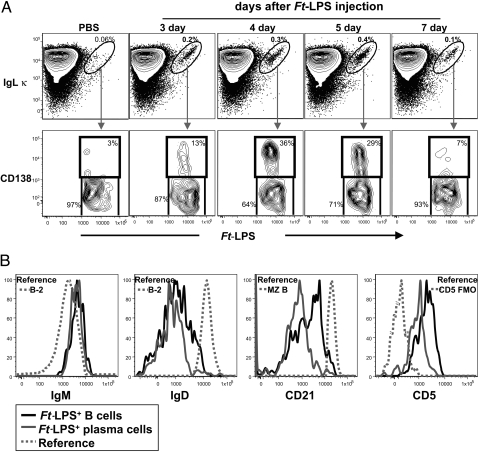
Hi-D FACS analyses of spleen cells from mice immunized with Ft-LPS 1–7 days earlier show that Ft-LPS-binding (Ft-LPS+) B cells become detectable in spleen (Fig. 2A) and in PerC (Fig. S1) 3 days after immunization. Although these Ft-LPS+ cells comprise only ≈0.1% of spleen cells, they are readily detectable as a distinct population in contour plots in which the amount of Ig kappa light chain (Igκ) bound to each cell is plotted against the amount of fluorochrome-coupled Ft-LPS bound to the same cell (Fig. 2A): nearly all of the Ft-LPS+ B cells are located in a tight “diagonal” in the upper right corner of the plot, indicating that the vast majority of the Ft-LPS+ B cells express antibodies containing Igκ. The tightness of this diagonal indicates that the amount of Ft-LPS bound is closely proportional to the amount of surface Igκ (which reports the total amount of Ig on the cells regardless of isotype). In contrast, virtually no Ft-LPS+ Igκ+ cells are detectable in spleens from unimmunized control mice (<0.06% of total spleen cells) (Fig. 2A) or from Ft-LPS-immunized B cell deficient (μMT) mice (<0.001% of total spleen cells). Furthermore, few (if any) such B cells are detected in murine bone marrow or lymph nodes taken from unstimulated mice or from mice immunized with Ft-LPS (data not shown).
Fig. 2.
Ft-LPS induces the appearance and proliferation of splenic Ft-LPS-binding (Ft-LPS+) B cells and plasma cells of the B-1a subset/lineage. (A) (Upper) Spleen cells from C57BL/6J mice immunized i.p. with Ft-LPS (100 ng) were stained with fluorochrome-labeled Ft-LPS and fluorescent antibodies recognizing a panel of B cell surface antigens. The total live cell population was further gated to display splenic B cells (IgM+B220+), for which κ light chain expression and Ft-LPS binding are shown in the figure. The circled cell population contains B cells that bind Ft-LPS; the proportion of the total B cell population represented within this gate is indicated for each day. (Lower) Ft-LPS+ B cells were further gated to distinguish Ft-LPS+ plasma cells, which express CD138. The proportion of cells expressing the plasma cell phenotype is shown. Note: on days 1 and 2, spleens from Ft-LPS immunized animals are equivalent to spleens in control (PBS) animals; i.e., few cells are within the gated region for Ft-LPS+Igκ+. (B) Surface marker expression on live Igκ+ Ft-LPS+ CD138− B cells (black histogram) and Igκ+ Ft-LPS+ CD138+ plasma cells (gray histogram) in spleen 4 days after i.p. injection of 100 ng Ft-LPS. Reference histograms show surface marker expression for the relevant B cell population as indicated. B-2 cells were gated as IgDhi IgMlo CD23hi B cells and Marginal Zone (MZ) B cells were gated as IgDlo IgMhi CD21hi B cells. The reference histogram for the CD5+ cells shows data for the FMO staining control, in which cells were stained with the full reagent mixture from which anti-CD5 antibody has been omitted to reveal the appropriate threshold for identifying CD5+ cells. Similar data were obtained on day 3, day 5 and day 7 (data not shown).
Consistent with this localization pattern, Ft-LPS+ B cells in spleen and PerC express either the typical B-1a cell phenotype (IgMhiIgDlo−negCD21loCD23negCD5+) (Fig. 2B) or a related plasma cell phenotype expressed on a subset of the splenic Ft-LPS+ B-1a cells (see below). Thus, we find no evidence that B-2 cells bind Ft-LPS+ or respond to Ft-LPS immunization.
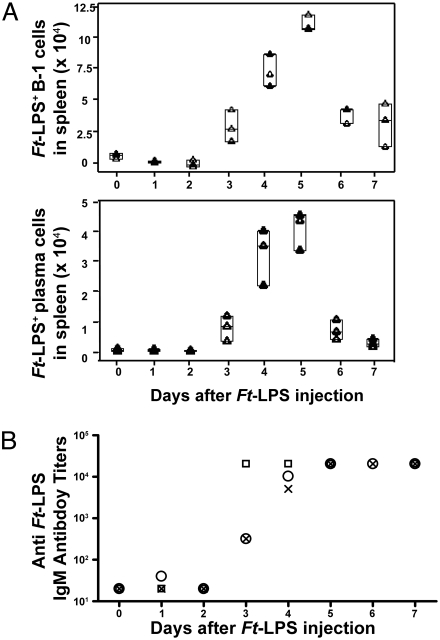
A population of Ft-LPS+ B-1a cells becomes detectable in the PerC at approximately the same time as they become detectable in the spleen. However, the splenic Ft-LPS+ B-1a cells rapidly increase in frequency during the first few days after immunization (from 0.01% of total spleen cells at day 2 to 0.13% at day 5) and then fall to below the level of detection by 7–10 d postimmunization (Fig. 3A). In contrast, the Ft-LPS+ B-1a cells in PerC first become detectable on day 3, reach ≈0.3% of total PerC B cells on days 5–7, and then remain at 0.1% for at least 2 months (Fig. S1).
Fig. 3.
Absolute numbers of Ft-LPS+ B-1a cells and plasma cells (CD138+) in spleen peak on day 5 after immunization, whereas anti-Ft LVS IgM titers in the sera peak at day 5 and are sustained. C57BL/6J mice were killed 1–7 days after i.p. injection of 100 ng Ft-LPS. Mice killed on day 0 were injected with saline only. Tissues and sera were collected from all mice at the time of killing and analyzed by Hi-D FACS (A) and ELISA (B) (n = 3). (A) Each triangle represents Hi-D FACS data from an individual mouse. Horizontal lines in the “quartile box plots” indicate the 25th, 50th and 75th percentile values. (B) ELISAs using Ft-LPS-coated plates were performed on serum from collected from the mice analyzed in A. Each symbol (squares, circles, and crosses) represents the anti-Ft-LPS titer from an individual mouse.
Ft-LPS Immunization Induces Antigen-Specific B-1a Antibody Secreting (Plasma) Cells in Spleen.
Ten-40% of the Ft-LPS+ splenic B cells (Fig. 2A) are typical plasma cells, or plasmablasts, that are similar to typical splenic B-1a cells (IgMhiIgDlo−negCD21loCD23negCD5+) (Fig. 2A) but express little or no surface IgD, CD21, or CD5 (Fig. 2B). Instead, they express high levels of CD138 (syndecan-1, the classic plasma cell surface marker), contain high levels of intracellular IgM (Fig. S2) and secrete anti-Ft-LPS IgM, revealed in ELISPOT assays in which the number of spots detected was equivalent to the number of sorted Ft-LPS+ CD138+ cells seeded (data not shown). Importantly, we never detected any Ft-LPS+ plasma cells (or any other plasma cells) in PerC, despite the emergence and subsequent persistence of a readily detectable numbers of Ft-LPS+ cells at this location.
By 4–5 days after immunization, Ft-LPS+ plasma cells reach peak frequency and represent ≈40% of all splenic Ft-LPS+ cells (Fig. 2A). The absolute number of Ft-LPS+ plasma cells in spleen shows similar kinetics, as does the total number of Ft-LPS+ Igκ+ cells (B-1a plus plasma cells) (Fig. 3A). After day 5, the numbers of both Ft-LPS+ B-1a cells and Ft-LPS+ plasma cells decline and fall by day 7 to below the level of detection (<0.06% of total spleen cells).
In vivo BrdU incorporation studies show that the vast majority (>90%) of Ft-LPS+ B-1a cells undergo at least 1 cell division within 4–5 days of Ft-LPS stimulation whereas only ≈10–15% of total splenic B cells undergo division during this period. Importantly, all of the Ft-LPS+ plasma cells present 4–5 days after immunization (Fig. S3) incorporated BrdU, indicating that they have all divided at least once during, before, or after differentiating to CD138+ plasma cells expressing intracellular IgM.
Plasma Cell Development Is Accompanied by Increases in Anti-Ft-LPS in Serum.
Serum levels of antibodies reactive with Ft-LPS approximately follow the kinetics of plasma cell development in the spleen. The serum antibody levels become detectable within 3 days of immunization, reach peak titers that are 102-103-fold above background by 4–5 days (Fig. 3B), and then fall over the next 2 weeks to low levels that are significantly above background and remain as such for long periods (Fig. S4). BALB/cByJ mice immunized with Ft-LPS still had readily detectable levels of IgM anti-Ft-LPS at 70 days postimmunization, just 2 days before the day when they were given, and survived, a lethal Ft-LVS challenge.
T Cell Participation and TLR4 Stimulation Are Not Required for Antibody Responses to Ft-LPS.
Ft-LPS-immunized mice subsequently depleted of T cells survive i.p. challenge with Ft LVS, suggesting that anti-LPS antibodies or other B cell functions are sufficient to limit infection, even in the absence of T cell-mediated immunity (14). Consistent with the central role that antibodies play in this protection, TCRαβ- or TCRγδ-deficient mice respond to Ft-LPS immunization with splenic plasma cell and serum antibody responses that are equivalent to those observed in WT controls (data not shown).
However, although T cell-deficient, nude mice pretreated with 100 ng Ft-LPS 3 days before challenge survive 2–3 weeks longer than euthymic control mice that were saline-pretreated, the nude mice eventually succumb (8). This indicates that T cells are important for long-term survival even though they are not required for the B-1a antibody responses that allow mice to initially survive the infection.
Similarly, production of anti Ft-LPS antibody responses does not require TLR4 stimulation, because Ft-LPS immunization results in equivalent serum IgM anti-Ft-LPS titers in WT and TLR4−/− mice, i.e., Day 6 anti-LPS ELISA titers, mean (95% confidence interval) in 6 mice per treatment: WT = 15,521 (9,700–24,900); TLR4−/− = 10,240 (5,600–18,800). This finding supports data from a previous study showing that TLR4-deficient (C3H/HeJ) and TLR4-competent C3H/OuJ mice immunized with Ft-LPS were comparably protected against lethal Ft challenge (8).
Discussion
We have shown previously that pretreatment of mice with Ft-LPS protects against an otherwise lethal challenge with Ft LVS (7, 8). Studies presented here show that this pretreatment induces a dramatic B-1a immune response in which (i) B-1a with BCR that bind Ft-LPS proliferate in spleen and PerC; (ii) most of the Ft-LPS+ B-1a cells in spleen differentiate rapidly to plasma cells producing antibodies to Ft-LPS; (iii) serum levels of these antibodies rise shortly after immunization, peak at day 4–5, and then fall slowly to a lower level that is maintained for months; (iv) neither T cells nor TLR4 responsiveness is required for this response; (v) the response is specific for the O-antigen expressed by intact Ft-LPS; and (vi) immunization with Ft-LPS is required for protection against lethal challenge with Ft LVS.
The demonstration that Ft-LPS immunization stimulates B-1a cells to produce a specific immune response is somewhat jarring, because B-1 cells are better known as producers of “innate antibodies” in conventional and germ-free mice (15, 16) than as responders to immunogens. However, as data presented here and in previous studies show, B-1a (6, 17) and B-1b (18, 19) with appropriate BCR can clearly respond to stimulation with cognate antigens, e.g., B-1a produce T15-idiotype anti-phosphorylcholine (PC) in response to Streptococcus pneumoniae infection (6, 17) and B-1b produce antibodies in response to Streptococcus pneumoniae (18) and Borrelia hermsii (19). Studies here add Ft-LPS to the list of B-1 antigens and link the B-1a response to this antigen to long-term protection against lethal Ft LVS challenge.
These studies also introduce a new and far more detailed view of the B-1a response itself. In a pioneering use of Hi-D FACS, we track the antigen-binding B-1 cells participating in this T-independent response, monitoring and characterizing these cells from their first emergence as a detectable splenic B cell population 2–3 days after Ft-LPS immunization through their differentiation to splenic plasma cells and their ultimate long-term residence in PerC.
Ft-LPS immunization results in the expansion of a population of rare Ft-LPS+ B-1a cells that are barely detectable (if at all) in spleen or PerC before immunization. Consistent with their typical B-1a phenotype, these antigen-specific cells are found in immunized animals both in the PerC and spleen, but are not present at detectable frequencies in bone marrow or lymph nodes. The appearance of Ft-LPS+ B-1a cells after immunization is followed rapidly by the appearance of differentiated Ft-LPS+ plasma cells in spleen, but not in PerC. The numbers of both types of cells in spleen then increase concomitantly for ≈2–3 days, peak at day 5 and decline dramatically thereafter (Figs. 2A and 3A). By day 7–8, neither Ft-LPS+ B-1a nor Ft-LPS+ plasma cells are detectable in spleen. In contrast, the number of Ft-LPS+ B-1a cells in PerC rises rapidly after immunization and remains substantially above background for at least 2 months whereas neither Ft-LPS+ (nor any other) plasma cells ever become detectable in PerC (Fig. S1).
Consistent with the rapid expansion of Ft-LPS+ B-1a plasma cells in the spleen, IgM anti-Ft-LPS antibodies rise >100-fold in serum, peak 5–7 days after immunization (Fig. 3B) and stabilize some weeks later at a substantially lower level that is nonetheless clearly above background (8). Thus, WT mice immunized with Ft-LPS continue to produce antibodies for at least 70 days and remain protected against lethal Ft LVS challenge at least until this time. The location of the cells responsible for this sustained antibody production is unclear. However, the persistence of Ft-LPS+ B-1a cells (but not plasma cells) in PerC suggests that these cells may constitute a reservoir of precursors for cells that produce the anti-Ft-LPS antibodies that are found in serum and likely provide protection against lethal Ft LVS challenge.
Our findings also introduce new insights into B-1a responses and the mechanisms that underlie B-1a antibody production. For example, all of the Ft-LPS+ B-1a plasma cells (Fig. S3) stimulated by Ft-LPS immunization incorporate BrdU, indicating that these cells divide at least once during, before, or after differentiating to CD138+ intracellular IgM+ plasma cells. This extensive cell division, contrasts markedly with responses to Typhi-LPS, where a sizable proportion of responding splenic B-1a cells differentiate to plasma cells within 24 h without dividing (20).
Overall, Typhi-LPS induces massive splenic enlargement (4–5-fold increase in the total number of spleen cells) and high level serum antibody production. B-1 cells constitute the major, but clearly not the only, source of B cells and antibody producing cells in this response (20). The antibodies produced collectively react with a wide variety of antigenic determinants, including phosphatidylcholine, phosphorylcholine, and dextran, that are not present Typhi-LPS (20), indicating that TLR4 and perhaps other nonspecific stimulation is largely responsible the Typhi-LPS response.
These functional differences between responses induced by Ft-LPS, a poor TLR4 agonist (7) and typical enterobacterial LPS such as Typhi-LPS (and E. coli LPS), which are potent TLR4 agonists, likely relate to lipid A structural differences between these molecules. Specifically, whereas Typhi-LPS is pentaacylated and hexaacylated, Ft-LPS is tetraacylated and hence only weakly activates TLR4 [(7); reviewed in ref. 21]. Thus, Ft-LPS fails to induce production of inflammatory cytokines in vivo and in vitro (7) and induces only minimal splenic enlargement, i.e., ≈1.2-fold increase in cellularity, which is readily explained by the increase in Ft-LPS+ B-1a cells and Ft-LPS+ plasma cells generated during the response. Furthermore, as we have shown, responses to Ft-LPS are T cell- and TLR4-independent and fail in Ig transgenic mice that express only a single IgH gene that does not recognize Ft-LPS. Thus, our findings argue strongly that unlike responses to Typhi-LPS, responses to Ft-LPS are antigen-specific and depend on the engagement of Ft-LPS-specific BCR expressed in the B-1a repertoire.
The literature concerning TLR dependency for antibody production is inconsistent. TLR signaling has been reported to either be inconsequential (22), required (23), or stimulatory for antibody responses to T-dependent antigens (24). For T-independent responses, reports are equally confounded:, TLR signaling has been shown to induce IgM secretion (25, 26), cellular proliferation (25, 26), B-1 egress from the PerC (27), and B-1 differentiation into plasma cells (25). However, natural antibodies are produced in germ-free mice (15, 16) where TLR signaling from exogenous sources is considered to be minimal.
Findings presented here offer a resolution to this apparent conflict by showing that Ft-LPS induces a highly specific, TLR4- and T-independent response that is restricted to B-1a cells committed to produce antibodies specific for Ft-LPS. Thus, unlike the TLR4-dependent polyclonal antibody responses stimulated by E. coli and Salmonella LPS, Ft-LPS accesses a restricted mechanism that only induces production of antibodies reactive with antigenic determinants displayed by the immunizing antigen.
Our conclusion that the protective B-1a antibody response induced by Ft-LPS immunization is specific for the Ft-LPS O-antigen is supported by the following observations: (i) protection against lethal infection is not elicited by vaccination of mice with purified lipid A (Fig. 1), and (ii) immunization of mice with the LPS purified from wbt I, an Ft mutant that lacks O-antigen but retains the core oligosaccharide (28), fails to induce protection (0 of 5 immunized mice survived challenge). These findings confirm and extend previous reports, which show that i.d. administration of Ft-LPS, but not Ft lipid A, reduced bacterial burdens in the livers, lungs, and spleens of mice after aerosol challenge with Ft LVS (29). Interestingly, i.d. administration of purified core-O-polysaccharide also fails to protect mice, suggesting that the necessary O-antigen epitope is not properly exposed or presented when cleaved from intact Ft-LPS (29).
Similarly, studies with passively administered antibodies show that although antibodies elicited to Ft LVS protect against lethal challenge, antibodies elicited by immunization with the O-antigen-deficient strain, Ft LVS wbtA, do not (30, 31). Furthermore, passively administered rabbit anti-Ft LVS antisera, but not antisera depleted of anti-O antibodies, protected mice against lethal challenge (31). Collectively, these findings strongly support the hypothesis that immunization with intact Ft-LPS induces a protective B-1a response that is O antigen-specific.
In sum, the studies presented here demonstrate that B-1a cells respond to a soluble bacterial product, Ft-LPS, derived from an important bacterial pathogen. This response, which provides subsequent protection against lethal challenge by the pathogen, proceeds in the absence of T cell help or input from the inflammatory processes typically induced by bacteria-based immunizations. This finding emphasizes the possibility for creating effective, minimally invasive vaccines that can provide effective protection against pathogen invasion.
Materials and Methods
Mice.
C57BL/6J, CBA/CaJ, CBA/CaHN-BTK<xid>/J, and B6.129S2-Igh-6<tm1Cgn>/J (μMT) mice, 6–8 wk old, were purchased from the Jackson Laboratory. BALB/cByJ, JhD, mIg-Tg, and (m+s)Ig-Tg mice (9, 10) were bred at CBER/FDA and genotyped by PCR before use. For challenge with Ft LVS, mice were pretreated as indicated with saline or with 0.1–100 ng Ft-LPS 2–3 days before i.p. challenge with the indicated Ft LVS dose. The challenge dose was confirmed by colony counts. SigmaStat Program (Systat Software, Inc. Richmond, CA) was used for data analysis.
For FACS studies, mice were injected with saline or Ft-LPS as above. For BrdU assays, mice were provided water containing 0.8 mg/mL of BrdU 1 day after Ft-LPS injection. At the indicated times, mice were killed, cells were harvested for FACS studies, and sera were collected and frozen. All experiments were conducted with Institutional Animal Care and Use Committee approval.
Reagents.
Ft-LPS was purified as described in ref. 7. Ft LVS lipid A was prepared by acid hydrolysis of LPS as described in ref. 32. Biotin-LC-hydrazide (Pierce) was conjugated to Ft-LPS according to the manufacturer's protocol. Frozen aliquots of Ft LVS (ATCC 29684; American Type Culture Collection) were prepared as described in ref. 33.
Hi-Dimensional (Hi-D) FACS.
Cell suspensions from each tissue were incubated with LIVE/DEAD Aqua (Invitrogen) to identify dead cells. Cells were washed, chilled, and blocked with unconjugated anti-CD16/CD32 (FcγRII/III) mAb. Cells were then incubated on ice for 25 min with biotin-coupled Ft-LPS and the following premixed “mixture” of fluorochrome-conjugated antibodies: FITC-anti-Kappa chain (187.1); PE-anti-CD11b (M1/70) or PE-anti-CD138 (281–2); PECy5.5-anti-CD21 (CR2); PECy7-anti-IgD (11–26); APC-anti-B220 (RA3–6B2); APCCy5.5-anti-CD23 (B3B4); APCCy7-anti-IgM (331); PECy5-anti-CD5 (53–7.3); and Pacific Blue-anti-Gr-1 (RB6–8C5). After washing, cells were incubated with streptavidin-QuantumDot 605 (Invitrogen, Carlsbad, CA) for 15 min on ice to reveal binding of the biotin-coupled Ft-LPS. A BrdU flow kit (BD Bioscience, Franklin Lakes, NJ) was used as directed to stain for BrdU uptake. To distinguish autofluorescent cells from cells that express low levels of individual surface markers, cells were stained with Fluorescence-minus-one (FMO) reagent cocktails that enable definition of autofluorescence thresholds for each reagent in question.
Data were collected for 0.5–1 × 106 cells with a 3-laser FACSAria (BD Bioscience). Data were analyzed with FlowJo software (TreeStar Inc, Ashland, OR).
Detection of Anti-Ft LVS or Anti-Ft-LPS Antibodies.
Antibodies (IgM and IgG) against Ft LVS or Ft-LPS were measured by ELISA (8).
Supplementary Material
Acknowledgments.
The authors thank Suzanne M. Michalek, Jan Cerny, Martin Flajnik and John Mantovani for their thoughtful comments, suggestions, and help. This work was supported in part by National Institutes of Health Grant AI18797 and Subaward U54 AI157168 (to S.N.V.), National Institutes of Health Grant AI076434 (to L.A.H.), Center for Biologics Evaluation and Research/Food and Drug Administration-National Institute of Allergy and Infectious Diseases/National Institutes of Health Interagency Agreement Y1-AI-6153–01/224-06-1322 (to K.L.E.), and National Institutes of Health Grants AI57168 and GM50870 (to N.Q.) and AI43603 (to M.J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813411106/DCSupplemental.
References
- 1.Dennis DT, et al. Tularemia as a biological weapon: Medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 2.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 2003;5:135–142. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 3.Borghesi L, Milcarek C. Innate versus adaptive immunity: A paradigm past its prime? Cancer Res. 2007;67:3989–3993. doi: 10.1158/0008-5472.CAN-07-0182. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: A key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller C, Stedra J, Kelsoe G, Cerny J. Facultative role of germinal centers and T cells in the somatic diversification of IgVH genes. J Exp Med. 1995;181:1319–1331. doi: 10.1084/jem.181.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 7.Cole LE, et al. Immunologic consequences of Francisella tularensis Live Vaccine Strain Infection: Role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 8.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 10.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto S, et al. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK-functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood. 1999;94:2357–2364. [PubMed] [Google Scholar]
- 12.Khan WN, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 13.Rawlings DJ, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 14.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 15.Bos NA, et al. Early development of Ig-secreting cells in young of germ-free BALB/c mice fed a chemically defined ultrafiltered diet. Cell Immunol. 1987;105:235–245. doi: 10.1016/0008-8749(87)90071-2. [DOI] [PubMed] [Google Scholar]
- 16.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 17.Kenny JJ, Yaffe LJ, Ahmed A, Metcalf ES. Contribution of Lyb 5+ and Lyb 5- B cells to the primary and secondary phosphocholine-specific antibody response. J Immunol. 1983;130:2574–2579. [PubMed] [Google Scholar]
- 18.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Alugupalli KR, et al. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann NY Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genestier L, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 26.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha SA, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, et al. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153:3141–3153. doi: 10.1099/mic.0.2007/006460-0. [DOI] [PubMed] [Google Scholar]
- 29.Conlan JW, Vinogradov E, Monteiro MA, Perry MB. Mice intradermally-inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb Pathog. 2003;34:39–45. doi: 10.1016/s0882-4010(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian S, et al. Cellular and humoral immunity are synergistic in protection against types A and B Francisella tularensis. Vaccine. 2009;27:597–605. doi: 10.1016/j.vaccine.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebastian S, et al. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257:11808–11815. [PubMed] [Google Scholar]
- 33.Elkins KL, Winegar RK, Nacy CA, Fortier AH. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.