Abstract
Highly efficient stereoselective 1,4-addition of racemic α-fluoro-α-nitro(phenylsulfonyl)methane (FNSM) as a fluoromethyl pronucleophile to α,β-unsaturated ketones using a wide range of chiral organobifunctional catalysts under moderate conditions in the absence of an additional base has been achieved. A series of catalysts was screened for the enantioselective addition of FNSM to chalcones and the catalysts CN I, CD I, QN I-IV, and QD I were found to enable this reaction, successfully providing exclusive 1,4-addition products stereoselectively in high yields (conversion, diastereomeric ratio, and enantiomeric excess). Studies involving a model reaction and systematic analysis of the absolute configuration support the suggested mechanism.
Keywords: fluorinated substituents, H bonding, stereoselective catalysis, steric effect
Enantioselective preparation of fluoromethylated organics is one of the major areas of interest today because fluoromethyl substituted compounds carry great importance in pharmaceutical chemistry, material science and healthcare (1–5). Among the highly active fluoromethylated bioorganics, sevoflurane, an effective fluoromethyl anesthetic, has fast uptake and elimination properties (6). The presence of a monofluoromethyl group at the α-carbon (7) in α-monofluoromethyl-dopa makes it very selective for its peripheral activity. Fluoromethylamino acids such as fluoromethylglutamic acid and 4-amino-5-fluoropentanoic acid are found to inhibit glutamic acid decarboxylase and gamma-aminobutyric acid (GABA) transaminase, respectively (8–11). Transfer of the active fluoro(phenylsulfonyl)methyl group into Knoeveneagel systems or systems carrying other active groups can lead to very efficient synthons for the preparation of various biologically active systems. For over a decade, our group has made significant contribution toward efficient nucleophilic fluoroalkylation (12–18). 1-Fluoro-bis(phenylsulfonyl)methane (FBSM) (19, 20) is a very effective synthetic equivalent of monofluoromethide species and has been used in palladium-catalyzed Tsuji–Trost allylic alkylation conditions for highly enantioselective allylic monofluoromethylation. One of the major recent developments in this area involves the efficient, and highly enantioselective monofluoralkylation of alcohols using the Mitsunobu reaction (21). We found that this methodology works efficiently for a wide variety of alcohols including primary, secondary, allylic, alicyclic and benzylic alcohols. The reaction proceeds through a stereochemical inversion for chiral alcohols producing the adducts with high enantiomeric excess (ee) of up to 98% (Scheme 1).
Scheme 1.
Mitsunobu reaction of alcohols with 1-fluoro-bis(phenylsulfonyl)methane.
Our recent investigations showed that fluorine containing (phenylsulfonyl)methane derivatives such as α-nitro, cyano, ester, or acetyl-substituted fluoro(phenylsulfonyl)methane can be effectively used under mild conditions for the synthesis of a variety of functionalized monofluoromethylated compounds, which are crucial synthons for many valuable compounds in the pharmaceutical arena (Scheme 2) (22). Reports on highly enantioselective 1,4-addition of monofluoromethylenes to α,β-unsaturated compounds are rare (23, 24). Studies by Prakash et al. (22) and Ni et al. (25) show that conjugate addition of acidic fluoro(phenylsulfonyl)methane (FSM) derivatives to α,β-unsaturated ketones is a suitable example of efficient Michael addition reaction for the C–C bond formation.
Scheme 2.
Michael addition of FSM derivatives to α,β-unsaturated compounds.
FBSM-based asymmetric monofluoromethylation was successfully carried out by Shibata and colleagues (19) for allylic monofluoromethylation (20) and Mannich type monofluoromethylation for the synthesis of chiral α-fluoromethylamines (26). Very recently, Shibata and colleagues have achieved a catalytic enantioselective Michael addition of FBSM to chalcones using cinchona-based phase transfer catalysts. Large amounts of base (Cs2CO3, 3 eq) were, however, required and the reaction was carried out at −40 °C (27). The potential of bifunctional catalysts bearing thiourea and chiral tertiary amine functionalities for enantioselective Michael addition is demonstrated in ref. 28. Soós and coworkers (29) have reported enantioselective Michael addition between nitromethane and chalcone with high ee and chemical yields using cinchona alkaloid-derived chiral bifunctional thiourea as an effective organocatalyst. Earlier, Shibasaki and colleagues (30) used lanthanum trisbinaphthoxide catalyst effectively for the efficient enantiomeric addition of nitromethane to chalcone. A few highly enantioselective aza-Michael additions to simple α,β-unsaturated ketones have been developed recently (31–35). Shibasaki and coworkers (32) have developed a highly efficient aza-Michael addition with greater scope and range to afford enantioselective reactions for a wide range of α,β-unsaturated ketones with aryl or alkyl β-substituents.
Results and Discussion
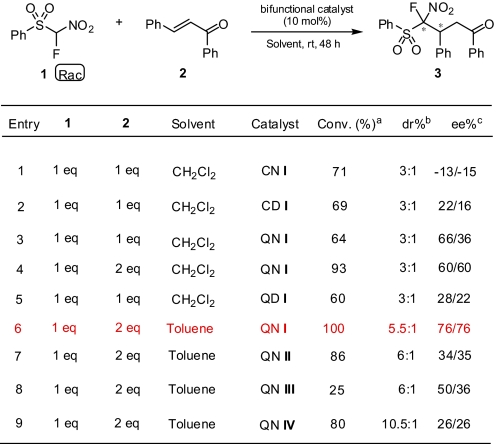
Parallel to our racemic conjugate addition reactions, which we reported earlier (Scheme 2), we have been conducting a series of reactions focusing our attention toward a highly efficient enantioselective 1,4-addition of various fluoromethyl pronucleophiles to α,β-unsaturated ketones using a wide range of organobifunctional catalysts under moderate conditions in the absence of an additional base such as Cs2CO3 or K2CO3. We have screened a series of catalysts for the enantioselective addition of α-fluoro-α-nitro-(phenylsulfonyl)methane (FNSM) to chalocones systematically and we found that catalysts CN I, CD I, QN I-IV and QD I (Fig. 1) enable this reaction to occur successfully in the absence of any base to provide exclusive 1,4-addition products (Scheme 3).
Fig. 1.
Cinchona-based bifunctional chiral catalysts screened for enantioselective 1,4-addition of α-fluoro-α-nitro(phenylsulfonyl)methane [FNSM (1)] to chalcone.
Scheme 3.
Enantioselective 1,4-addition of α-fluoro-α-nitro-(phenylsulfonyl)methane (FNSM) to chalcones.
To find the best catalyst and the conditions, reactions were carried out with different combination of parameters such as catalyst, catalyst loading, solvent, temperature, and ratio of substrates. Preliminary screening of the catalysts was carried out using dichloromethane or toluene as solvent (Table 1). In both solvents, when FNSM (1) and trans-chalcone (2) were taken in a 1:1 stoichiometric ratio, conversion was low irrespective of the catalyst. However, when the ratio was changed to 1:2, higher conversion and stereoselectivity was observed in the presence of QN I in toluene as the solvent (Table 1, entry 6).
Table 1.
Screening of catalysts CN/CD I, QN/QD I, and QN I–IV for enantioselective addition of FNSM (1) to chalcone (2)
a,bDetermined by 19F NMR. dr, diastereomeric ratio.
cDetermined by chiral HPLC. ee, enantiomeric excess.
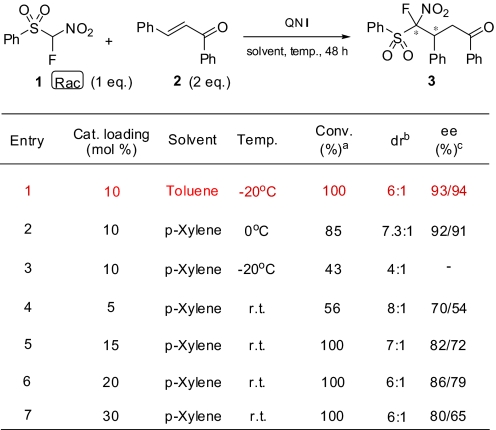
Therefore, among various bifunctional catalysts used for the stereoselective addition of FNSM, QN I has been taken as the model bifunctional catalyst for the Michael addition reaction. To find the optimum conditions for maximum efficiency of the catalyst QN I, 1,4-conjugate addition with FNSM was studied in various solvents at different temperatures (Tables 2 and 3).
Table 2.
Enantioselective 1,4-addition of FNSM (1) to chalcone (2) catalyzed by QN I in various solvents
a,bDetermined by 19F NMR.
cDetermined by chiral HPLC.
Table 3.
Enantioselective 1,4-addition of FNSM (1) to chalcone (2) under different catalyst loading of QN I at different temperatures
a,bDetermined by 19F NMR.
cDetermined by chiral HPLC.
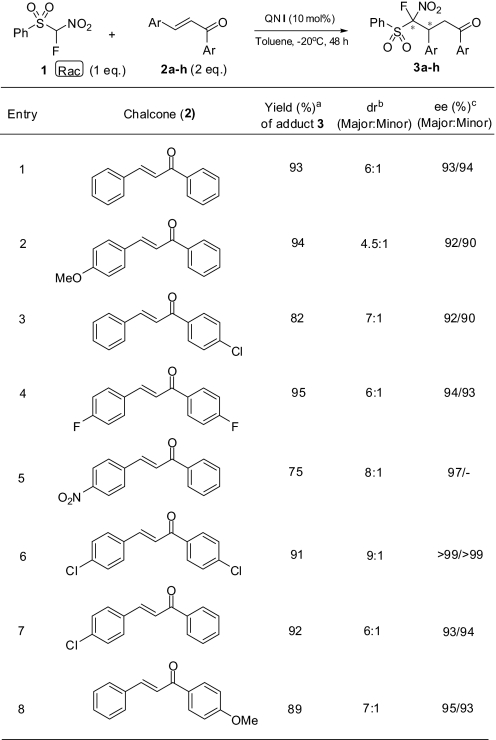
As mentioned, in the case of enantioselective additions using phenylsulfonylmethane derivatives reported earlier (25, 26) strong bases such as CsOH·H2O, Cs2CO3, and K2CO3 were required in excessive amounts for the formation of the corresponding phenylsulfonylmethide nucleophile in the medium. However, FNSM is found to be an efficient Michael-type donor in the absence of a base in all reactions performed in our study. The presence of the nitro group and proper catalyst selection not only eliminate the use of a base, but also add the prospects of further derivatization and modification of the FNSM-adduct to provide very useful biologically active endproducts. Using racemic starting material (FNSM, which acts as a good carbon acid), we have discovered that 1,4-addition products with high diastereoselectivity and enantioselectivity could be achieved under the reaction conditions (Table 3) using cinchona-based bifunctional catalyst QN I. It is known that the resulting carbanion containing fluorine in the α-position assumes a tetrahedral structure rather than a planar one suggested for nonfluorinated α-nitromethane derivatives (36, 37). This helps to enhance the diastereoselectivity by a possible inversion during the addition reaction. Under the optimized conditions, FNSM has been added to a series of chalcone derivatives to obtain the corresponding adducts in high yields (Table 4) with high diastereomeric ratios and excellent enantiomeric excesses (Table 4).
Table 4.
Enantioselective 1,4-addition of FNSM (1) to chalcone (2)
aIsolated yields. Yields were calculated based on FNSM.
bDetermined by 19F NMR.
cDetermined by chiral HPLC.
To understand the mechanistic aspects behind the high stereoselectivity observed in these reactions, we have suggested a possible transition state for these transformations (Fig. 2).
Fig. 2.
Formation of α-fluoro-α-nitro(phenylsulfonyl)methide anion and suggested transition state involving chalcone-QN I assembly during Michael addition.
The remarkable and unprecedented efficacy of the bifunctional catalyst QN I is manifested from the observed deracemization, enantioselectivity, and diastereoselectivity because 4 stereomers were obtained in almost equal amounts in the absence of chiral catalysts. The bifunctional catalysts themselves are capable of deprotonating the FNSM into the corresponding carbanion, which can attack the Michael acceptors in an appropriate configuration by undergoing an inversion (Fig. 2). The high facial selectivity of chalcones is due to the coordination of chalcones and the catalyst QN I via di-hydrogen bonding and aromatic π–π interactions. It is evident from Fig. 2 that the coordination of the carbonyl group in the chalcone and the nitro group in the FNSM anion with the catalyst QN I is more effective with the transition state B because of minimum steric interaction. This helps the transition state B to be involved in the assembly playing a crucial role for providing high diastereoselectivity.
To clarify the suggested mechanism, we carried out a model reaction between FNSM and methyl vinyl ketone (4) under similar conditions (Scheme 4). The substrate 4 is used as the analog of chalcones. It is able to coordinate with QN I in a similar way, nevertheless, the steric interaction between FNSM and 4 has been minimized. As expected, a racemic product was obtained. This demonstrates that the stereoselectivity on the α-carbon does not originate from the “stereoselective deprotonation” of FNSM by the bifunctional catalyst QN I. Rather, the stereoselectivity is derived from the interaction between FNSM and the chalcone-QN I complex.
Scheme 4.
1,4-Addition of α-fluoro-α-nitro(phenylsulfonyl)methane (FNSM) to methyl vinyl ketone with the bifunctional catalyst QN I.
The absolute configuration of the product 3a was unequivocally established by X-ray crystallographic analysis (Fig. 3, see SI Appendix for details).
Fig. 3.
Crystal structure of (3R,4R)-4-fluoro-4-nitro-1,3-diphenyl-4-(phenylsulfonyl)butan-1-one (3a).
Conclusion
In conclusion, we have achieved an enantioselective and diastereoselective 1,4-conjugate addition of FNSM, an effective fluoromethyl pronucleophile to chalcones using cinchona alkaloid-based bifunctional catalysts with highest efficacy observed for QN I. It is worthwhile to note that no additional base was required for the 1,4-addition reactions, which were carried out in a homogeneous catalytic mode. Further explorations involving other α-substituted substrates such as α-cyano, α-ester, or α-acetyl-α-fluoro(phenylsulfonyl)methane for various stereoselective addition reactions are clearly warranted.
Materials and Methods
Unless otherwise mentioned, all other reagents were purchased from commercial sources. All of the bifunctional catalysts were prepared according to Soós and coworkers procedure (29). New catalysts QN II-IV have been completely characterized by spectral and HRMS analysis.
Typical Procedure for Catalytic 1,4-Addition of α-Fluoro-α-nitro(phenylsulfonyl)methane to α,β-Unsaturated Ketones.
To a solution of α-fluoro-α-nitro(phenylsulfonyl)methane (21.9 mg, 0.1 mmol, 1 equivalent) and ketone (0.2 mmol, 2 equivalent) in CH2Cl2, Et3N (10.0 μL of 0.1 mmol, 0.7 equivalent) was added. The reaction mixture was stirred for 12 h at room temperature and the conversion was monitored by 19F NMR before purification (diastereomeric ratios were ≈1:1 in all of the cases). The reaction mixture was loaded on to a preparative TLC plate. In most cases, the diastereomers can be separated with hexane/ethyl acetate (4/1–6/1) to produce the title product in good to excellent yield.
Typical Procedure for Catalytic Enantioselective 1,4-Addition of α-Fluoro-α-nitro-(phenylsulfonyl)methane to Chalcones.
To a solution of α-fluoro-α-nitro(phenylsulfonyl)methane (21.9 mg, 0.1 mmol, 1 equivalent) and ketone (0.2 mmol, 2 equivalent) in precooled toluene (−20 °C, 0.5 mL), catalyst QN I was added (6.0 mg, 0.01 mmol, 10 mol%) in one load. The reaction mixture was stirred for 1 min and placed in freezer (−20 °C) for 2 days without stirring. The reaction mixture was monitored by 19F NMR for conversion and diastereoselectivity, and loaded on to preparative TLC plate. In most cases, the diastereomers can be separated with hexane/ethyl acetate (4/1–6/1) to produce the title product in good to excellent yield.
Products were characterized by spectral analysis (1H NMR, 13C NMR, 19F NMR, and HRMS), and the ee values were determined by chiral HPLC.
Supplementary Material
Acknowledgments.
This work was supported by the Loker Hydrocarbon Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900179106/DCSupplemental.
References
- 1.Kirsh P. Modern Fluoroorganic Chemistry. Weinheim, Germany: Wiley-VCH; 2004. [Google Scholar]
- 2.Smart BE. Fluorine substituent effects (on bioactivity) J Fluorine Chem. 2001;109:3–11. [Google Scholar]
- 3.Soloshonok VA. Enantiocontrolled Synthesis of Fluoroorganic Compounds: Stereochemical Challenges and Biomedical Targets. Chichester, UK: Wiley-VCH; 1999. [Google Scholar]
- 4.Banks RE, Smart BE, Tatlow JC. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum; 1994. [Google Scholar]
- 5.Filler R, Kobayashim Y, Yagupolski LM. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. Amsterdam: Elsevier; 1993. [Google Scholar]
- 6.Hiyama T. Organofluorine Compounds: Chemistry and Applications. Berlin: Springer; 2000. [Google Scholar]
- 7.Welch JT. Tetrahedron report number 221: Advances in the preparation of biologically active organofluorine compounds. Tetrahedron. 1987;43:3123–3197. [Google Scholar]
- 8.Kuo D, Rando RR. Irreversible inhibition of glutamate decarboxylase by α-(fluoromethyl)glutamic acid. Biochemistry. 1981;20:506–511. doi: 10.1021/bi00506a010. [DOI] [PubMed] [Google Scholar]
- 9.Silverman RB, Levy MA. Syntheses of (S)-5-substituted 4-aminopentanoic acids: A new class of γ-aminobutyric acid transaminase inactivators. J Org Chem. 1980;45:815–818. [Google Scholar]
- 10.Silverman RB, Levy MA. Mechanism of inactivation of gamma-aminobutyric acid-alpha-ketoglutaric acid aminotransferase by 4-amino-5-halopentanoic acids. Biochemistry. 1981;20:1197–1203. doi: 10.1021/bi00508a022. [DOI] [PubMed] [Google Scholar]
- 11.Silverman RB, Levy MA. In vivo inactivation of gamma-aminobutyric acid-alpha-ketoglutarate transaminase by 4-amino-5-fluoropentanoic acid. Biochem Biophys Res Commun. 1980;95:250–255. doi: 10.1016/0006-291x(81)91550-3. [DOI] [PubMed] [Google Scholar]
- 12.Prakash GKS, Yudin AK. Perfluoroalkylation with organosilicon reagents. Chem Rev. 1997;97:757–786. doi: 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]
- 13.Prakash GKS, Hu J. Selective fluoroalkylations with fluorinated sulfones, sulfoxides, and sulfides. Acc Chem Res. 2007;40:921–930. doi: 10.1021/ar700149s. [DOI] [PubMed] [Google Scholar]
- 14.Prakash GKS, Hu J, Olah GA. Preparation of tri- and difluoromethylsilanes via an unusual magnesium metal-mediated reductive tri- and difluoromethylation of chlorosilanes using tri- and difluoromethyl sulfides, sulfoxides, and sulfones. J Org Chem. 2003;68:4457–4463. doi: 10.1021/jo030110z. [DOI] [PubMed] [Google Scholar]
- 15.Prakash GKS, Mandal M. Stereoselective synthesis of trifluoromethylated vicinal ethylenediamines with α-amino N-tert-butanesulfinimines and TMSCF3. J Am Chem Soc. 2002;124:6538–6539. doi: 10.1021/ja020482+. [DOI] [PubMed] [Google Scholar]
- 16.Prakash GKS, Hu J. New nucleophilic fluoroalkylation chemistry. ACS Symp Ser. 2005;911:16–56. [Google Scholar]
- 17.Prakash GKS, Mandal M, Olah GA. Stereoselective nucleophilic trifluoromethylation of N-(tert-butylsulfinyl)imines by using trimethyl(trifluoromethyl)silane. Angew Chem Int Ed. 2001;40:589–590. doi: 10.1002/1521-3773(20010202)40:3<589::AID-ANIE589>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Prakash GKS, Hu J, Mathew T, Olah GA. Difluoromethyl phenyl sulfone as a selective difluoromethylene dianion equivalent: One-pot stereoselective synthesis of anti-2,2-difluoropropane-1,3-diols. Angew Chem Int Ed. 2003;42:5216–5219. doi: 10.1002/anie.200352172. [DOI] [PubMed] [Google Scholar]
- 19.Fukuzumi T, et al. Fluorobis(phenylsulfonyl)methane: A fluoromethide equivalent and palladium-catalyzed enantioselective allylic monofluoromethylation. Angew Chem Int Ed. 2006;45:4973–4977. doi: 10.1002/anie.200600625. [DOI] [PubMed] [Google Scholar]
- 20.Ni C, Li Y, Hu J. Nucleophilic fluoroalkylation of epoxides with fluorinated sulfones. J Org Chem. 2006;71:6829–6833. doi: 10.1021/jo060955l. [DOI] [PubMed] [Google Scholar]
- 21.Prakash GKS, et al. Stereoselective monofluoromethylation of primary and secondary alcohols by using a fluorocarbon nucleophile in a Mitsunobu reaction. Angew Chem Int Ed. 2007;46:4933–4936. doi: 10.1002/anie.200700834. [DOI] [PubMed] [Google Scholar]
- 22.Prakash GKS, et al. Efficient 1,4-addition of α-substituted fluoro(phenyl sulfonyl)methane derivatives to α,β-unsaturated compounds. Beils J Org Chem. 2008;4:1–7. doi: 10.3762/bjoc.4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitazume T, Nakayama Y. Synthetic approach to versatile chiral molecules containing a fluorine atom. J Org Chem. 1986;51:2795–2799. [Google Scholar]
- 24.Bridge CF, O'Hagan D. The synthesis of α-fluoroketones by 1,4-additions of mono-fluorinated enamines to Michael acceptors. J Fluorine Chem. 1997;82:21–24. [Google Scholar]
- 25.Ni C, Zhang L, Hu J. Nucleophilic fluoroalkylation of α,β-enones, arynes, and activated alkynes with fluorinated sulfones: Probing the hard/soft nature of fluorinated carbanions. J Org Chem. 2008;73:5699–5713. doi: 10.1021/jo702479z. [DOI] [PubMed] [Google Scholar]
- 26.Mizuta S, et al. Cinchona alkaloid-catalyzed enantioselective monofluoromethylation reaction based on fluorobis(phenylsulfonyl)methane chemistry combined with a Mannich-type reaction. J Am Chem Soc. 2007;129:6394–6395. doi: 10.1021/ja071509y. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa T, et al. Catalytic enantioselective Michael addition of 1-fluoro-bis(phenylsulfonyl)methane to α,β-unsaturated ketones catalyzed by cinchona alkaloids. Angew Chem Int Ed. 2008;47:8051–8054. doi: 10.1002/anie.200802904. [DOI] [PubMed] [Google Scholar]
- 28.Okino T, Hoashi Y, Takemoto Y. Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J Am Chem Soc. 2003;125:12672–12673. doi: 10.1021/ja036972z. [DOI] [PubMed] [Google Scholar]
- 29.Vakulya B, Varga S, Csámpai A, Soós T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org Lett. 2005;7:1967–1969. doi: 10.1021/ol050431s. [DOI] [PubMed] [Google Scholar]
- 30.Funabishi K, et al. Catalytic asymmetric Michael addition of nitromethane to enones controlled by (R)-LPB. Tetrahedron Lett. 1998;49:7557–7558. [Google Scholar]
- 31.Lu X, Deng L. Asymmetric aza-Michael reactions of α,β-unsaturated ketones with bifunctional organic catalysts. Angew Chem Int Ed. 2008;47:7710–7713. doi: 10.1002/anie.200802785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamagiwa N, Qin H, Matsunaga S, Shibasaki M. Lewis acid-Lewis acid heterobimetallic cooperative catalysis: Mechanistic studies and application in enantioselective aza-Michael reaction. J Am Chem Soc. 2005;127:13419–13427. doi: 10.1021/ja054066b. [DOI] [PubMed] [Google Scholar]
- 33.Taylor MS, Zalatan DN, Lerchner AM, Jacobsen EN. Highly enantioselective conjugate additions to α,β-unsaturated ketones catalyzed by a (salen)Al complex. J Am Chem Soc. 2005;127:1313–1317. doi: 10.1021/ja044999s. [DOI] [PubMed] [Google Scholar]
- 34.Yamagiwa N, Matsunaga S, Shibasaki M. Heterobimetallic catalysis in asymmetric 1,4-addition of O-alkylhydroxylamine to enones. J Am Chem Soc. 2003;125:16178–16179. doi: 10.1021/ja038688d. [DOI] [PubMed] [Google Scholar]
- 35.Jin XL, et al. Chiral rare earth metal complex-catalyzed conjugate addition of O-alkylhydroxylamines. An efficient synthetic entry into optically active 2-acyl aziridines. Tetrahedron. 2002;58:8321–8329. [Google Scholar]
- 36.Lorand JP, Urban J, Overs J, Ahmed QA. Fluoronitromethane: Synthesis and estimation of acid strength. J Org Chem. 1969;34:4176–4178. [Google Scholar]
- 37.Castejon HJ, Wiberg KB. Effect of fluorine substitution on the carbon acidity of methane, methyl isocyanide, acetonitrile, acetaldehyde, and nitromethane. J Org Chem. 1998;63:3937–3942. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.