Abstract
There is considerable epidemiological evidence that shift work is associated with increased risk for obesity, diabetes, and cardiovascular disease, perhaps the result of physiologic maladaptation to chronically sleeping and eating at abnormal circadian times. To begin to understand underlying mechanisms, we determined the effects of such misalignment between behavioral cycles (fasting/feeding and sleep/wake cycles) and endogenous circadian cycles on metabolic, autonomic, and endocrine predictors of obesity, diabetes, and cardiovascular risk. Ten adults (5 female) underwent a 10-day laboratory protocol, wherein subjects ate and slept at all phases of the circadian cycle—achieved by scheduling a recurring 28-h “day.” Subjects ate 4 isocaloric meals each 28-h “day.” For 8 days, plasma leptin, insulin, glucose, and cortisol were measured hourly, urinary catecholamines 2 hourly (totaling ≈1,000 assays/subject), and blood pressure, heart rate, cardiac vagal modulation, oxygen consumption, respiratory exchange ratio, and polysomnographic sleep daily. Core body temperature was recorded continuously for 10 days to assess circadian phase. Circadian misalignment, when subjects ate and slept ≈12 h out of phase from their habitual times, systematically decreased leptin (−17%, P < 0.001), increased glucose (+6%, P < 0.001) despite increased insulin (+22%, P = 0.006), completely reversed the daily cortisol rhythm (P < 0.001), increased mean arterial pressure (+3%, P = 0.001), and reduced sleep efficiency (−20%, P < 0.002). Notably, circadian misalignment caused 3 of 8 subjects (with sufficient available data) to exhibit postprandial glucose responses in the range typical of a prediabetic state. These findings demonstrate the adverse cardiometabolic implications of circadian misalignment, as occurs acutely with jet lag and chronically with shift work.
Keywords: autonomic nervous system, diabetes, glucose metabolism, leptin, obesity
Approximately 8.6 million Americans perform shift work (1), which is associated with increased risk of obesity, diabetes, and cardiovascular disease (2–6). The endogenous circadian timing system, including the suprachiasmatic nucleus (SCN) in the hypothalamus and peripheral oscillators in vital organs, optimally regulates much of our physiology and behavior across the 24-h day when it is properly aligned with the sleep/wake cycle. However, shift work is generally associated with chronic misalignment between the endogenous circadian timing system and the behavioral cycles, including sleep/wake and fasting/feeding cycles (7, 8). Shift workers often experience symptoms akin to jet lag, with gastrointestinal complaints, fatigue, and sleepiness during the scheduled wake periods, and poor sleep during the daytime sleep attempts (9). Moreover, chronic circadian misalignment has been proposed to be the underlying cause for the adverse metabolic and cardiovascular health effects of shift work (10, 11). The SCN regulates circadian rhythms in leptin, plasma glucose, glucose tolerance, corticosteroids, and cardiovascular function via neural and/or humoral signals to the white adipose tissue, liver, pancreas, adrenal cortex, and heart (10). Thus, we assessed in humans the independent effects of the circadian system and the behavioral cycle (including fasting/feeding and sleep/wake cycles) on metabolic, autonomic, and endocrine function. We also tested how these effects on cardiometabolic function interact when circadian and behavioral cycles are misaligned (i.e., when waking and eating during the “biological night”), as typically occurs with shift work.
Results
Separation of the independent effects of the behavioral cycle and of the circadian cycle was achieved in a laboratory study by uniformly distributing the behavioral cycle across all phases of the circadian cycle (Fig. 1).
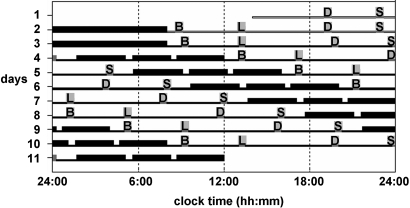
Fig. 1.
Forced desynchrony protocol. Subjects completed 2 baseline days and nights, followed by the FD portion of the study consisting of 7 recurring 28-h “days” in dim light (example subject had habitual bedtime of 24:00). Thick horizontal bars, sleep episodes (interspersed by 2 brief awakenings to perform pulmonary function measurements); gray bars, meal times; B, breakfast; L, lunch; D, dinner; S, snack; thin open horizontal bars, waking episodes of days 1 and 2 at room light intensity (≈90 lux); thin black horizontal bars, waking episodes on days 3–11 in dim light (≈1.8 lux).
Effects of Behavioral Cycle.
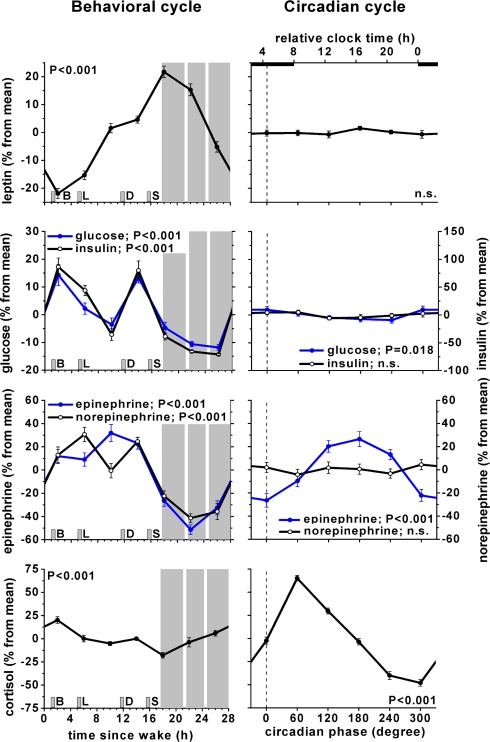
The effects of the behavioral cycle, independent of the circadian cycle, on leptin, glucose, insulin, epinephrine, norepinephrine, and cortisol are shown in Fig. 2, Left panels. Leptin varied significantly across the behavioral cycle, with a trough around breakfast and a peak after the last meal, coinciding with the onset of the scheduled sleep episode (P < 0.001, peak-to-trough 44%). Also, both glucose and insulin varied significantly across the behavioral cycle (glucose: P < 0.001, peak-to-trough 26%; insulin: P < 0.001, peak-to-trough 158%), presumably the result of the timing of meals. Both epinephrine and norepinephrine varied significantly across the behavioral cycle with peaks during the wake episode and troughs during the sleep episode (epinephrine: P < 0.001, peak-to-trough 83%; norepinephrine: P < 0.001, peak-to-trough 72%). Cortisol varied significantly across the behavioral cycle, peaking after awakening and with a trough at the onset of the scheduled sleep episode (P < 0.001, peak-to-trough 38%).
Fig. 2.
Independent influence of circadian cycle and behavioral cycle on metabolic, autonomic, and endocrine function. Left panels: influence of behavioral cycle, independent from circadian cycle. Right panels: influence of endogenous circadian cycle, independent from behavioral cycle. Error bars, SEM; gray areas, scheduled sleep episodes; short vertical gray bars, meal times as in Fig. 1; vertical dotted line, fitted core body temperature minimum; P-values, statistical significance for effect of behavioral cycle (Left panels) and circadian cycles (Right panels). Glucose and epinephrine scales are on the left and insulin and norepinephrine scales are on the right for both Left and Right panels.
Effect of Circadian Cycle.
The effects of the circadian cycle, independent of the behavioral cycle, on leptin, glucose, insulin, epinephrine, norepinephrine, and cortisol, are shown in Fig. 2, Right panels. Glucose had a significant endogenous circadian rhythm (P = 0.018, peak-to-trough 4%), with a peak during the biological night (circadian bin 300° and 0°; equivalent to ≈22:30–06:30 in these subjects). Epinephrine exhibited a significant endogenous circadian rhythm (P < 0.001, peak-to-trough 53%), with a peak during the biological day (circadian bin 180°; equivalent to ≈14:30–18:30). Cortisol had a significant endogenous circadian rhythm (P < 0.001, peak-to-trough 113%), with a peak at the end of the biological night (60°; close to habitual wake time). There were no significant circadian rhythms in leptin, insulin, or norepinephrine.
Interaction Between Behavioral and Circadian Cycles.
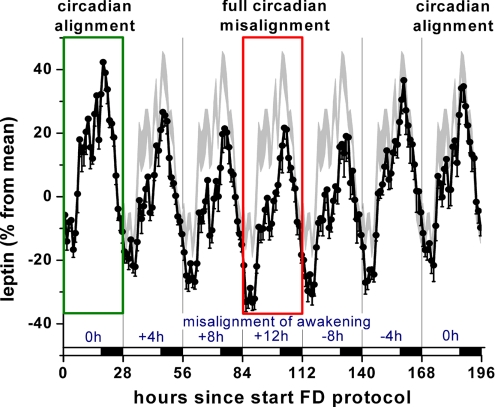
Although the behavioral and circadian influences are presented independently above and in Fig. 2, changes across the behavioral and circadian cycles happen concurrently across the day. Thus, it is necessary to consider the interaction of these factors to understand what happens across a normal or across a misaligned day. There was a clear interaction between the effects of the behavioral cycle and the circadian cycle upon leptin (P < 0.001). Leptin was systematically lower when the behavioral cycle was misaligned with the circadian cycle. This leptin suppression was maximal when the behavioral cycle was ≈12 h misaligned with the circadian cycle, as seen on the fourth 28-h cycle in Fig. 3(red rectangle) and exemplified by the highlighted trajectory in Fig. S1.
Fig. 3.
Circadian misalignment suppressed leptin levels proportionally, with maximum suppression during maximum misalignment (by 12 h on fourth 28-h “day,” indicated in red rectangle) as compared to circadian alignment (indicated in green rectangle). Circles, mean; error bars, SEM; gray area, mean ± SEM on first 28-h day replotted for comparison (circadian alignment); horizontal black bars, scheduled sleep episodes; vertical lines, scheduled awakenings; blue numbers above x-axis, approximate hours of circadian misalignment of the scheduled wake time on each 28-h day.
Effects of Circadian Misalignment on Metabolic, Autonomic, and Endocrine Function.
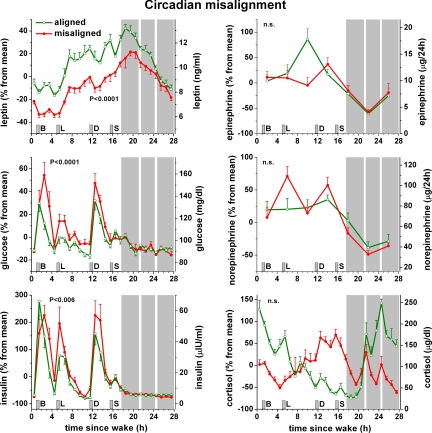
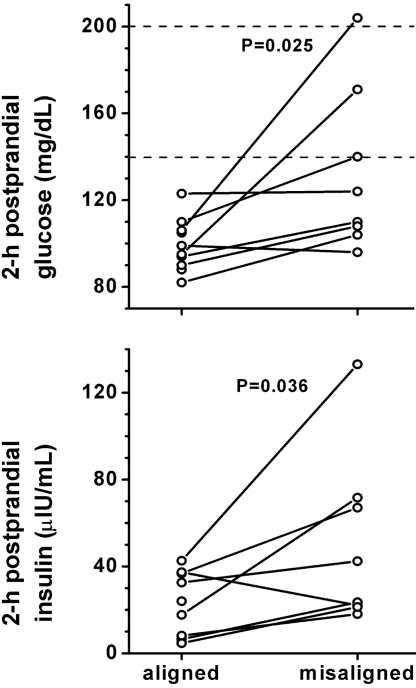
The two 28-h trajectories (aligned normally vs. misaligned) as depicted for leptin in Fig. S1 are shown vs. scheduled time of awakening for leptin and other variables in Fig. 4. Leptin was 17% lower (P < 0.001) across the entire behavioral cycle when misaligned compared to when aligned normally. Glucose was 6% higher (P < 0.001) and insulin was 22% higher (P = 0.006) across the entire behavioral cycle when misaligned. The increase in glucose seemed to be the result of an exaggerated postprandial glucose response (average 3-h postprandial glucose comparing misaligned to aligned: breakfast +21%, lunch +12%, and dinner +11%) and not the result of elevated fasting levels (Fig. 4). Moreover, during normal circadian alignment (wake time ≈08:00), none of the 10 subjects had signs of impaired glucose tolerance, but during maximal misalignment (wake time ≈20:00), 3 of 8 subjects in whom glucose metabolism could be assessed had meal responses consistent with a prediabetic state (>140 mg/dL, 2-h postprandial glucose values) or diabetic state (>199 mg/dL) (Fig. 5, Top panel). Average 2-h postprandial breakfast plasma glucose in these 8 subjects increased from 99.9 ± 4.5 mg/dL when aligned to 132 ± 13 mg/dL when misaligned (P = 0.025; Wilcoxon matched pairs test). The increased glucose levels occurred despite a concomitant increase in insulin values (from 23.3 ± 5.6 to 49.9 ± 14.0 μIU/mL; P = 0.036; Fig. 5, Bottom panel), which implies a decrease in insulin sensitivity and insufficient β-cell compensation during misalignment. In contrast, cortisol, epinephrine, and norepinephrine were not different across the behavioral cycle when subjects were misaligned. For cortisol, the most dramatic change induced by misalignment was a complete inverse pattern across the sleep/wake cycle, resulting in lower levels at the beginning and higher levels at the end of the wake episode (P < 0.001, interaction effects between alignment and behavioral cycle). This is consistent with the dominant role of the circadian system on cortisol regulation relative to the effect of the behavioral cycle. Epinephrine was lower during wakefulness when misaligned (P = 0.002, interaction effect), likely the result of the circadian system inhibiting epinephrine during the biological night even when subjects remained awake. From measurements during wakefulness only, mean arterial blood pressure was 3% higher (3 mm Hg; P = 0.001) when misaligned, while there was no measurable effect of misalignment on oxygen consumption, respiratory exchange ratio, heart rate, or cardiac vagal control [as estimated by logHF (12)]. Similar to leptin, sleep efficiency was significantly lower when misaligned (67% vs. 84%; P = 0.002 Student's t test; n = 9 with complete sleep recordings). Because experimental reduction of sleep can reduce leptin, we attempted to determine whether circadian misalignment itself influences leptin beyond any effect of sleep. From correlation analyses, it appeared that circadian misalignment had a stronger effect on leptin (Spearman's rho = −0.69; P < 0.001) than did sleep efficiency (Spearman's rho = 0.34; P = 0.006). In a complementary analysis when including sleep efficiency as a covariate, circadian misalignment still significantly affected leptin (P < 0.001) without a significant effect of sleep efficiency (P = 0.34). These results suggest that circadian misalignment itself impacts leptin beyond any effect induced by changes in sleep.
Fig. 4.
Consequences of circadian misalignment on metabolic, autonomic, and endocrine function. Data are plotted according to time-since-wake, during normal circadian alignment (open green symbols; scheduled awakening at habitual wake time) and during circadian misalignment (filled red symbols; scheduled awakening 12 h out of phase from habitual wake time). P-values, statistical significance for effect of misalignment [based on 24-h cycle for variable mainly driven by circadian cycle (cortisol) and 28-h cycle for variables mainly driven by behavioral cycle (others)]; gray area, scheduled sleep episode; short vertical gray bars, meal times as in Fig. 1.
Fig. 5.
Circadian misalignment reduces glucose tolerance and insulin sensitivity. During circadian misalignment, 2-h postprandial glucose (Top panel) and insulin (Bottom panel) levels were significantly increased as compared to normal alignment. Dotted lines, 140 mg/dL and 200 mg/dL 2-h postprandial glucose, above which levels are considered prediabetic and diabetic, respectively; P-values, statistical significance for effect of misalignment.
Discussion
Potential Health Consequences of Shift Work and Jet Lag.
We found that short-term circadian misalignment, similar to that which occurs acutely with jet lag and chronically with shift work, results in systematic increases in postprandial glucose, insulin, and mean arterial pressure, systematic decreases in leptin and sleep efficiency, and the complete inversion of the cortisol profile across the behavioral cycle. The abnormally high cortisol at the end of the wake episode and beginning of the sleep episode when misaligned (Fig. 4 Lower Right panel) could contribute to insulin resistance and hyperglycemia (13, 14). Also, decreased leptin stimulates appetite and decreases energy expenditure, which—if maintained chronically—could contribute to the development of obesity. Decreased sleep is associated with increased risk for obesity, diabetes, and hypertension (15–17). These combined effects during circadian misalignment may provide a mechanism underlying the increased risk for obesity, hypertension, and diabetes in shift workers (3, 5, 6).
Others have examined the effect of simulated night shift work on postprandial glucose and insulin with varied results (18, 19). Interpretation of these studies is complicated due to differences in premeal conditions between the night and day shifts (e.g., duration of wakefulness before test meals), such that the effects of circadian misalignment alone could not be assessed. Our study design addressed this limitation by scheduling meals at the same time since awakening and we found systematically increased postprandial glucose and insulin and thereby decreased insulin sensitivity, as previously suggested by the results of a real-life shift work protocol (20).
Recently, the same molecular and genetic factors, including transcription-translation feedback loops that control the SCN outputs have been found in various peripheral organs, which, together with humoral and neural signals, seem to drive organ functions (21). In rodents, such peripheral or extra-SCN circadian oscillators in the liver and brain can be dissociated from the central circadian pacemaker by restricted food access at abnormal times (22, 23). A functional molecular circadian clock selectively in the SCN is sufficient to drive light-entrainment of various circadian rhythms, while a functional clock in the dorsomedial hypothalamus may be sufficient to drive food entrainment [(24) although this is still debated, e.g., (25)]. Whether meals scheduled at abnormal circadian times, as occurs during shift work, also leads to such “internal desynchronization” in humans has yet to be determined. In some animal models, repeated shifts in the light/dark cycle—typical of shift work—leads to premature death, while mutations of circadian clock genes can lead to signs of metabolic syndrome (11, 26, 27), supporting the potential negative health consequences of circadian disturbances.
The 3-mm Hg increase in mean arterial pressure during short-term circadian misalignment is similar in magnitude to the effect of the 3-week Dietary Approaches to Stop Hypertension (DASH) study (28), which has been demonstrated to be clinically important (29). Because blood pressure changes are typically even larger in hypertensive vs. normotensive individuals (28), the hemodynamic effect of chronic misalignment such as occurs with shift work may have greater clinical implications in vulnerable populations (2, 4).
Potential Mechanisms Involved in Effects of Circadian Misalignment.
We measured numerous interacting variables to help determine causal links underlying changes induced by circadian misalignment. The decrease in leptin during circadian misalignment could not be explained by a simultaneous decrease in the measured variables that have been shown to stimulate leptin secretion, namely glucose, insulin, cortisol, vagal tone, or food intake; or by an increase in those measured variables that can inhibit leptin: epinephrine, norepinephrine, or metabolic rate; or by a change in metabolic substrate (as suggested by respiratory exchange ratio) (30–32). Both short-term experimental sleep restriction (33) and chronic self-reported short sleep duration (34) are associated with decreased leptin, so the decrease in sleep during circadian misalignment in the current study may contribute to the decrease in leptin. However, the analyses of the effect of sleep efficiency and circadian misalignment on leptin, separately and combined, suggest that circadian misalignment itself mainly impacts leptin independently of its effects on sleep. It will be useful in future studies to determine the effect of circadian misalignment on other potential effector mechanisms of leptin regulation, such as digestive effectiveness (35), cytokines, free fatty acids, growth hormone and other growth factors (31). For instance, misalignment between meal times and central and/or peripheral (e.g., gastrointestinal) circadian rhythms (35) might result in decreased digestion and energy uptake from meals, leading to a negative energy balance and suppressed leptin. The observations of a decrease in leptin within a few days contrast with the model of leptin as a long-term mediator of energy balance and instead support a model involving a role for leptin as a short-term mediator of energy balance in response to an altered behavioral state.
The increased postprandial glucose in the face of increased insulin implies a decrease in insulin sensitivity and insufficient β-cell compensation during misalignment, which could have directly been caused by the decreased leptin during circadian misalignment or changes in other factors, including other adipokines (31, 36, 37). Notably, the increase in glucose seemed to be due to an exaggerated postprandial glucose response rather than elevated fasting glucose (Fig. 4 Left Middle panel), suggesting that misalignment may impact fat/muscle metabolism or β-cell function more than hepatic gluconeogenesis. Recent evidence in humans of increased risk for type 2 diabetes in a genetic variant of melatonin 1B receptor (Mel1B), expression of Mel1B in pancreatic β-cells, and inhibition of glucose-induced insulin release by melatonin (38, 39), raises the possibility that the reversal of the melatonin profile relative to the feeding/fasting cycle contributed to insufficient β-cell compensation when misaligned (for plasma melatonin data, see Fig. S2). Future mechanistic studies will be needed to fully clarify underlying mechanisms.
The observed increase in blood pressure while awake during circadian misalignment was not associated with increased heart rate and could not be explained by an increase in the measured variables that can increase blood pressure: epinephrine, norepinephrine, cortisol, or by a decrease in vagal tone, or by an underlying circadian rhythm in blood pressure (40). Future studies are required to investigate whether circadian misalignment leads to increased total peripheral resistance, stroke volume, or blood volume.
The observed decrease in sleep efficiency during circadian misalignment has previously been noted and is explained by a circadian rhythm in sleep propensity, with a minimum during the biological day (41).
The forced desynchrony (FD) protocol permits analysis of the separate circadian and behavioral effects, and their interaction. The FD uncovered a large effect of the behavioral cycle independent of the circadian cycle, not only on plasma glucose, insulin, and leptin, but also on epinephrine and norepinephrine, both with peak-to-trough variation of ≈80% peaking during the middle of the wake episode, and on cortisol, with a peak-to-trough variation of ≈45% peaking following scheduled awakening (Fig. 2 Left panels). In addition, the FD uncovered impressive endogenous circadian influences independent of the behavioral cycle on epinephrine and cortisol, with a peak-to-trough variation of ≈50% and ≈110%, respectively (Fig. 2 Right panels). Remarkably, for cortisol the circadian effect was more than twice the size of the combined effects of the sleep/wake cycle, meals, and activity. For epinephrine, the circadian effects were of similar magnitude as these combined behavioral effects. Furthermore, we found a significant circadian rhythm in glucose, with a peak-to-trough variation of 4%, peaking during the biological night, consistent with previous observations (42).
The absence of a significant circadian variation in leptin and insulin could have been caused by masking effects of the large meals in the FD protocol, as opposed to small evenly spaced isocaloric snacks or continuous enteral nutrition in other studies with evidence for circadian control of leptin (43, 44). Alternatively, the feeding/fasting cycle may have entrained peripheral oscillators. In rodents, peripheral oscillators such as in the liver can be dissociated from the master oscillator in the hypothalamus by restricted daytime food access (22). However, it remains to be determined whether meals are also strong zeitgebers in humans (who can survive starvation much longer than rodents). From previous work (e.g., ref. 45) and the current study, it is clear that meals are the most dominant factor controlling the daily leptin profile in humans, but it will require future studies to determine whether these effects are caused by meals directly (masking a circadian cycle) or by entrainment of either central or peripheral circadian oscillators.
Because of nonadditive interactions, the effects of circadian misalignment cannot always be predicted by simply summating the behavioral and circadian cycle effects, as best demonstrated for leptin (Figs. 2, 3, 4, and S1). Knowledge of the differential effects of these mechanisms may be important in determining countermeasures for maladaptation to circadian misalignment, targeting either the circadian system or specific behaviors, or even specific behaviors when they occur at specific circadian phases.
Summary.
Circadian misalignment, a condition that is highly prevalent in shift workers, resulted in a decrease in leptin, increase in glucose and insulin, increase in mean arterial blood pressure, and reduced sleep efficiency. The strengths of the current study include the: (i) experimental evidence supporting epidemiological findings; (ii) within-subject design; (iii) controlled environmental and behavioral conditions; (iv) comprehensive assessment of endocrine, metabolic, and autonomic function and sleep efficiency; and (v) ability to investigate the separate circadian, separate behavioral, and interaction effects on these primary outcome variables. The small number of subjects, the laboratory conditions not mimicking “real life,” and the inclusion of subjects with mild asthma are limitations. Future laboratory and field studies are needed to investigate in more detail the mechanisms and chronic effects of the cardiometabolic changes observed during circadian misalignment.
Materials and Methods
The protocol was approved by the institutional Human Research Committee, and written informed consent was obtained from participants. Cardiopulmonary and cognitive data from the same study have been published elsewhere (46–49).
Subjects.
We studied 10 adult subjects [5 female; mean age 25.5 years (range 19–41 years); mean body mass index 25.1 kg/m2 (20–28 kg/m2)]. Subjects were healthy with no significant medical disorders other than mild asthma, as assessed by history, physical, 12-lead ECG, chest X-ray, complete blood count, blood and urine comprehensive metabolic panel analysis, overnight polysomnography, and psychological assessment. Half of the subjects (n = 5) had mild asthma. However, none of the subjects were on steroid medication and we were unable to detect any impact of the presence of mild asthma on any of the main effects.
Protocol.
To ensure a stable circadian phase angle of entrainment at baseline, subjects initially maintained a regular sleep–wake schedule for >2 weeks, including 8-h sleep opportunity at their average habitual times, and then underwent a 10-day laboratory protocol in an individual suite free of time cues (Fig. 1). The laboratory protocol consisted of 2 baseline days and nights, with 8-h sleep opportunities at habitual times, followed by 7 recurring 28-h sleep–wake cycles under dim light conditions (≈1.8 lux) to minimize any influence of light on the circadian system. This FD protocol desynchronizes, or uncouples, the behavioral cycles from the circadian cycles by distributing sleep and wakefulness across all phases of the circadian cycle, allowing assessment of the independent and interacting effects of the circadian system and behavioral cycles of sleep/fasting and wake/eating (50). During each 28-h “day,” subjects had standardized breakfast, lunch, dinner, and a snack at 1 h, 5 h, 11.5 h, and 15.5 h post-awakening, respectively. Meals were composed of 25% fat, 50% carbohydrate, and 25% protein. The overall calories per unit of time were calculated using the Harris-Benedict formula with an activity factor of 1.4. The 2-h postprandial mixed meal response to breakfast (average ≈670-kcal breakfast) following a 13-h fast was used to assess glucose tolerance during normal circadian alignment (first 28-h sleep/wake cycle; average scheduled wake time ≈08:00) and during circadian misalignment (fourth 28-h sleep/wake cycle; average scheduled wake time ≈20:00). Exercise was prohibited. The ratio of the scheduled sleep (9 h 20 min) to wake (18 h 40 min) was maintained at 1:2, as occurred in the home environment.
Measurements and Analysis.
Most of the laboratory procedures were the same as previously published (44, 50). Blood was sampled hourly during both wakefulness and sleep via an indwelling catheter in a forearm vein. Plasma leptin, insulin, and glucose were assayed as previously described (44). Plasma cortisol was assayed using a chemiluminescent assay with a sensitivity of 0.26 μg/dL. Urinary epinephrine and norepinephrine were assayed using a radioimmunoassay (RIA) with a sensitivity of 12 pg/mL and 24 pg/mL, respectively.
On 4 occasions spread evenly throughout each wake episode, subjects lay semireclined in bed for >1 h while arterial blood pressure, oxygen consumption (indirect calorimetry), and respiratory exchange ratio (CO2 production/O2 consumption) were measured. An ECG was recorded during volitionally controlled breathing at 10 breaths/min for derivation of heart rate and markers of cardiac vagal control [high frequency heart rate variability (HF); 0.15–0.40 Hz] from spectral analyses of interbeat intervals (12). Recordings of 2 electroencephalograms, 2 electrooculograms, and a submental electromyogram were made for quantification of sleep. Core body temperature was measured throughout using a rectal thermistor for assessment of phase and period of the circadian pacemaker (50). All data were assigned a circadian phase depending upon the time from the fitted core body temperature minimum (0°) and the individual's estimated circadian period (mean 24.2 ± 0.1 h). Data were expressed in normalized units (i.e., percentage difference from individual's mean across the FD protocol; as illustrated in Fig. S3 for leptin after correcting for any individual linear trend across the FD). Individuals' normalized data were grouped into 60° circadian bins and into 4-h bins according to time since scheduled awakening. The independent effects of the behavioral cycle, the independent effects of the circadian cycle, and any interaction effects were assessed for leptin, glucose, insulin, epinephrine, norepinephrine, and cortisol using 3-factor mixed model analysis of variance with restricted maximum likelihood (REML) estimates of the variance components (JMP, SAS Institute). To specifically test the effects of misalignment between the behavioral cycles and the circadian cycle, data were grouped into 1-h bins according to time since scheduled awakening and the 28-h profiles of variables were compared when aligned normally (i.e., when waking and eating during the biological day on the first FD cycle, with average scheduled wake time of ≈08:00) and when maximally misaligned as occurs in shift work (i.e., when waking and eating during the biological night on the fourth FD cycle, with average scheduled wake time of ≈20:00). The independent effects of alignment (FD cycle 1 vs. 4), the independent effects of the behavioral cycle, and any interaction effects were assessed for each variable using 3-factor mixed model analysis of variance using REML. All data are presented as mean ± SEM. P-value < 0.05 was considered statistically significant. To correlate leptin with sleep efficiency and circadian misalignment across the whole FD protocol, Spearman's rank correlations were performed between the average leptin levels during each scheduled wake episode and 4 levels of circadian misalignment of awakening (0–30°, 30–90°, 90–150°, 150–180°; 9 subjects contributed to each category) and 4 categories of sleep efficiency in the preceding sleep opportunity (sleep efficiency: 90–100%, 80–90%, 70–80%, <70%; at least 7 subjects contributed to each category). Furthermore, to test whether circadian misalignment resulted in suppression of leptin per se, thus independent of a decrease in sleep efficiency, we analyzed these same variables using mixed model analysis of covariance with circadian misalignment as fixed factor and sleep efficiency as covariate. Conversion factors from conventional and/or metric units to Système International units are listed in the SI Text.
Supplementary Material
Acknowledgments.
We thank the volunteers, research staff, and recruiters who participated in this study, including Heather Evoniuk and Taneisha Benjamin for help with data analysis and Diana Barb for performing the leptin assays. This work was supported by National Heart, Lung and Blood Institute Grants R01-HL64815, R01-HL076409, and K24-HL76446 (to S.A.S.), General Clinical Research Center Grant MO1-RR02635 (to Brigham and Women's Hospital), National Center for Complementary and Alternative Medicine Grant R21-AT002713 (to F.A.J.L.S.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01–57875, and a discretionary grant from Beth Israel Deaconess Medical Center (to C.S.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4069.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808180106/DCSupplemental.
References
- 1.U.S. Department of Labor. Workers on Flexible and Shift Schedules in 2004 Summary. Washington, D.C.: Bureau of Labor Statistics; 2005. [Google Scholar]
- 2.Knutsson A, Åkerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89–91. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–455. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroenke CH, et al. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 6.Morikawa Y, et al. Effect of shift work on body mass index and metabolic parameters. Scand J Work Environ Health. 2007;33:45–50. doi: 10.5271/sjweh.1063. [DOI] [PubMed] [Google Scholar]
- 7.Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–441. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 8.Roden M, Koller M, Pirich K, Vierhapper H, Waldhauser F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol. 1993;265:R261–267. doi: 10.1152/ajpregu.1993.265.1.R261. [DOI] [PubMed] [Google Scholar]
- 9.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 10.Buijs RM, et al. Organization of circadian functions: Interaction with the body. Prog Brain Res. 2006;153:341–360. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 11.Kohsaka A, Bass J. A sense of time: How molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. (see comments) Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 13.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: Impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J Clin Endocrinol Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 14.Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–2290. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangwisch JE, et al. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 17.Kohatsu ND, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 18.Hampton SM, et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 20.Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557–564. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- 21.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 23.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 24.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- 26.Turek FW, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 28.Appel LJ, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 29.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: A meta-analysis. Lancet. 2001;358:1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 30.Mantzoros CS, et al. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 31.Brennan AM, Mantzoros CS. Drug insight: The role of leptin in human physiology and pathophysiology–emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 32.Purnell JQ, Samuels MH. Levels of leptin during hydrocortisone infusions that mimic normal and reversed diurnal cortisol levels in subjects with adrenal insufficiency. J Clin Endocrinol Metab. 1999;84:3125–3128. doi: 10.1210/jcem.84.9.5990. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, et al. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 34.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore JG, Englert E., Jr Circadian rhythm of gastric acid secretion in man. Nature. 1970;226:1261–1262. doi: 10.1038/2261261a0. [DOI] [PubMed] [Google Scholar]
- 36.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 37.Covey SD, et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2008;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2008;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerkhof GA, Van Dongen HP, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens. 1998;11:373–377. doi: 10.1016/s0895-7061(97)00461-5. [DOI] [PubMed] [Google Scholar]
- 41.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 42.Van Cauter E, et al. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 44.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu K, et al. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004;337:307–318. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu K, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shea SA, Scheer FA, Hilton MF. Predicting the daily pattern of asthma severity based on relative contributions of the circadian timing system, the sleep-wake cycle and the environment. Sleep. 2007;30:A65. [Google Scholar]
- 49.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–361. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. (see comments) Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.