Abstract
The expression of ASPP2 (53BP2L), a proapoptotic member of a family of p53-binding proteins, is frequently suppressed in many human cancers. Accumulating evidence suggests that ASPP2 inhibits tumor growth; however, the mechanisms by which ASPP2 suppresses tumor formation remain to be clarified. To study this, we targeted the ASPP2 allele in a mouse by replacing exons 10–17 with a neoR gene. ASPP2−/− mice were not viable because of an early embryonic lethal event. Although ASPP2+/− mice appeared developmentally normal, they displayed an increased incidence of a variety of spontaneous tumors as they aged. Moreover, γ-irradiated 6-week-old ASPP2+/− mice developed an increased incidence of high-grade T cell lymphomas of thymic origin compared with ASPP2+/+ mice. Primary thymocytes derived from ASPP2+/− mice exhibited an attenuated apoptotic response to γ-irradiation compared with ASPP2+/+ thymocytes. Additionally, ASPP2+/− primary mouse embryonic fibroblasts demonstrated a defective G0/G1 cell cycle checkpoint after γ-irradiation. Our results demonstrate that ASPP2 is a haploinsufficient tumor suppressor and, importantly, open new avenues for investigation into the mechanisms by which disruption of ASPP2 pathways could play a role in tumorigenesis and response to therapy.
Apoptosis-stimulating protein of p53-2 (ASPP2), also known as 53BP2L, encoded by TP53BP2 (1–3), enhances damage-induced apoptosis at least in part through a p53-mediated pathway (2, 4–6). Depending on cell context and type of stress, ASPP2 levels increase via transcriptional or posttranslational mechanisms after cellular damage (4, 6). In addition to interacting with p53 (and family members) (5, 7), ASPP2 protein, and the 123-aa, amino-terminal, truncated splice isoform 53BP2/Bbp, also known as 53BP2S (3), interacts with several proteins involved in modulating apoptosis and cell growth, including Bcl-2, p65/RelA subunit of NF-κB, Yes-associated protein-1, HCV core protein, APCL, and protein phosphatase-1 (8–13). Additionally, ASPP2 is a direct E2F target gene, suggesting that it is a common link between the Rb/E2F and p53/p73 pathways (14–16). ASPP2 expression is suppressed in many human cancers, and it has been associated with poor clinical outcome in patients with aggressive non-Hodgkin's lymphoma treated with chemotherapy (2, 17–24). These findings suggest that ASPP2 is involved in important tumor suppression networks and the cellular damage response. Overexpression of ASPP2 or Bbp/53BP2S can suppress E1A and ras-mediated transformation of rat embryo fibroblasts (25, 26), whereas attenuation of ASPP2 expression promotes clonogenic survival and inhibits apoptosis in cell culture (2, 4, 6) and promotes tumor formation in vivo (27). However, the mechanisms by which reduced ASPP2 expression enhances tumor formation in vivo remain to be elucidated.
In this report, we targeted the ASPP2 allele in a mouse by using homologous recombination to explore the in vivo consequences of attenuated ASPP2 expression. We demonstrate that reduced ASPP2 expression in heterozygous mice results in: (i) an increased incidence of a variety of spontaneous tumors, (ii) the accelerated formation of high-grade thymic T cell lymphomas in γ-irradiated mice, and (iii) after γ-irradiation, an attenuated apoptotic response in ASPP2+/− primary thymocytes and an attenuated G0/G1 checkpoint in ASPP2+/− primary mouse embryonic fibroblasts (MEFs). These data provide significant insight into the observation that ASPP2 expression is reduced in human cancers, and suggest a mechanism by which disruption of ASPP2 pathways may play a role in tumorigenesis and response to therapy.
Results
Generation of ASPP2+/− Mice.
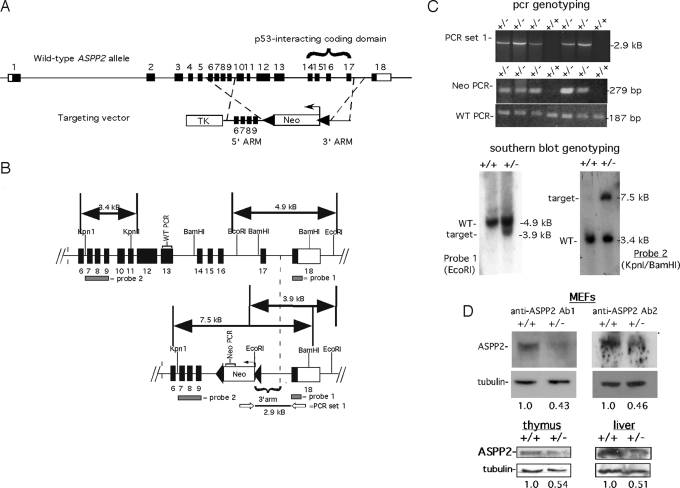
To rigorously test the hypothesis that ASPP2 may have a tumor suppressor function, we targeted ASPP2 in a mouse by using homologous recombination (Fig. 1). The targeting vector was designed to disrupt exons 10–17, which also include codons for the ankyrin repeat and SH3 domain required for interaction with p53 family members (exons 14–17; Fig. 1A). The targeting vector was electroporated into 129/SvJ ES cells, and clones were derived by positive–negative selection in G418/gancyclovir. Approximately 250 clones were screened to identify 4 positive clones by Southern blot analysis (Fig. 1B). Two separate clones were expanded and injected into C57BL/6 blastocysts to generate several highly chimeric mice. Chimeras were crossed with C57BL/6 mice, and offspring were screened with 2 separate PCR strategies and further confirmed by Southern blot analysis (Fig. 1 B and C). Western blot analysis using 2 different antibodies recognizing the amino terminus (anti-ASPP2 Ab1) or the carboxy terminus (anti-ASPP2 Ab2) demonstrated an approximate 2-fold reduction in ASPP2 protein levels in ASPP2+/− MEFs, thymus, and liver (Fig. 1D).
Fig. 1.
Generation of ASPP2+/− mice. (A) Schema of ASPP2 allele and targeting vector. Black boxes indicate coding exons; white boxes indicate untranslated regions. Neo indicates neomycin resistance gene; TK indicates thymidine kinase gene. (B) Strategies for detecting a wild-type ASPP2 allele (Upper) or integration of the targeting vector (Lower). Probe locations for Southern blot analysis are shown as gray bars. Open arrows indicate PCR set 1 primers. Neo-PCR indicates NeoR gene amplicon. WT-PCR indicates ASPP2 exon 13 amplicon. (C) Genotyping using PCR (Upper) and Southern blot analysis (Lower). (D) Western blots on equivalent amounts of total protein from ASPP2+/− and ASPP2+/+ MEFs (Upper), or thymuses and livers (Lower), using anti-ASPP2 Ab1 or anti-ASPP2 Ab2. Fold-expression (relative to +/+) normalized to tubulin.
ASPP2−/− Mice Are Not Viable Because of an Embryonic Lethal Event.
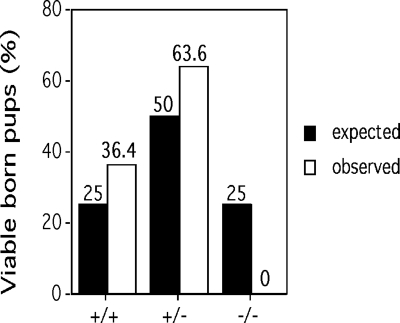
Although ASPP2+/− mice appeared normal and reproduced, we could not identify viable ASPP2−/− pups. Genotyping of newborn litters demonstrated an increased frequency of ASPP2+/+ and ASPP2+/− pups over the expected frequencies, consistent with an embryonic lethal defect (Fig. 2). We performed timed harvests as early as embryonic day 6.5 but were unsuccessful in characterizing ASPP2−/− embryos. To explore whether the lethal ASPP2−/− phenotype could be altered, we generated ASPP2+/−;p53+/− mice and intercrossed them. However, we could not produce ASPP2−/− mice or embryos, regardless of the background p53 genotype, nor could we produce them in an inbred BALB/c background.
Fig. 2.
ASPP2−/− mice are not viable. Indicated genotypes of newborn pups from ASPP2+/− matings are shown. The expected Mendelian frequencies are indicated by black columns; observed ASPP2 genotypes are indicated by white columns.
ASPP2+/− Mice Have an Increased Incidence of Spontaneous Tumors.
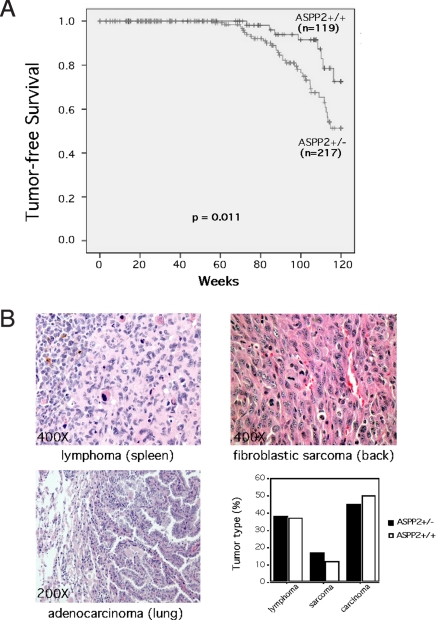
Because human cancers can have reduced ASPP2 levels (2, 17–24), we determined the spontaneous tumor-free survival of ASPP2+/− mice over an extended period (Fig. 3A). ASPP2+/+ mice demonstrated the expected incidence of spontaneous tumors seen in mice of similar background strain (28). In contrast, ASPP2+/− mice had a significant increase in tumor formation (P = 0.011, log-rank test). There was a similar spectrum of tumor types in both genotypes (Fig. 3B). Examination of ASPP2 expression in available tumors arising in heterozygous mice did not reveal loss of expression by quantitative RT-PCR (Fig. S1) or by Western blot analysis (Fig. S2). To further explore whether ASPP2 cooperated with p53 to suppress tumor development, we generated ASPP2+/−;p53+/− and ASPP2+/+;p53+/− mice and determined the incidence of tumor formation as they aged. Although, as expected (28), loss of a p53 allele increased the incidence of spontaneous tumors, we found that an ASPP2+/− background did not further accelerate tumorigenesis in p53+/− mice (Fig. S3).
Fig. 3.
ASPP2+/− mice have an increased incidence of spontaneous tumors. (A) Kaplan–Meier tumor-free survival curves for ASPP2+/+ and ASPP2+/− mice (P = 0.011, log-rank test). (B) H&E-stained microscopic sections of representative tumors found in ASPP2+/− mice and a graph of tumor type frequency between genotypes (P = n.s., Fisher exact test).
ASPP2+/− Mice Have an Increased Incidence of γ-Irradiation-Induced High-Grade Lymphomas.
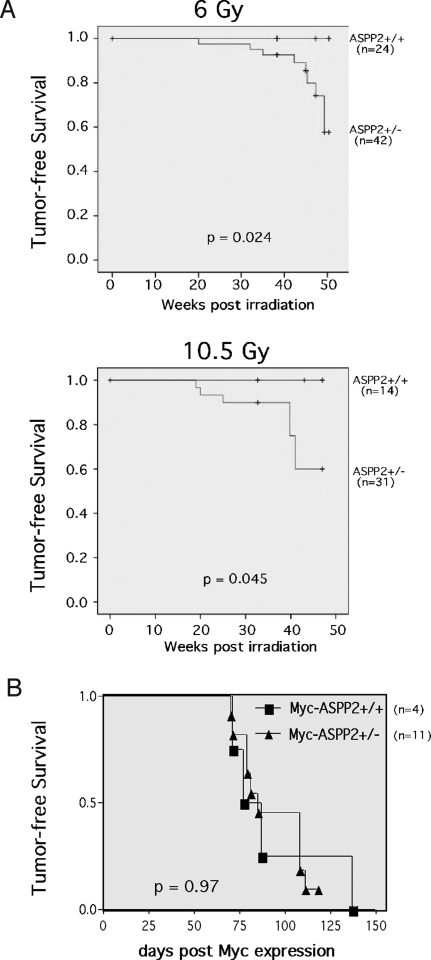
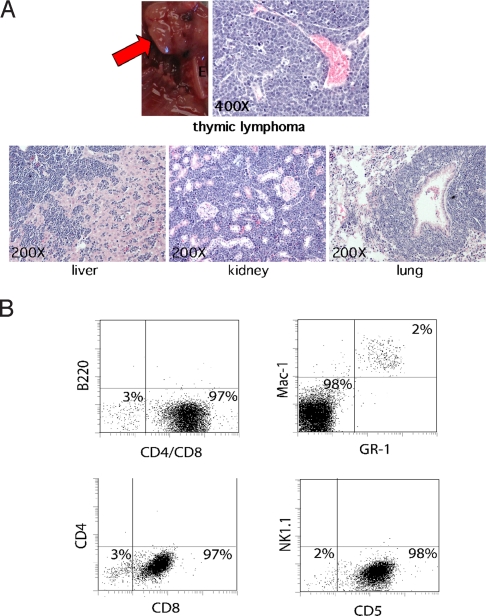
Because ASPP2 is damage-inducible (2, 4, 6) and has a tumor-suppression function (Fig. 3), we reasoned that ASPP2+/− mice would demonstrate an increased incidence of γ-irradiation-induced tumors. To examine this, we γ-irradiated 6-week-old ASPP2+/+ and ASPP2+/− mice with a total of 6.0 Gy or 10.5 Gy (in divided weekly fractions) and measured the tumor-free survival (Fig. 4A). ASPP2+/+ mice did not develop tumors during the observed time. However, ASPP2+/− mice developed tumors after 6.0-Gy or 10.5-Gy irradiation (P = 0.024 and P = 0.045 respectively, log-rank test). In the ASPP2+/− mice that developed tumors, histopathology demonstrated aggressive high-grade lymphomas, along with circulating lymphoma cells in the peripheral blood and bone marrow (Fig. 5). Immunophenotyping of bone marrow typically demonstrated near-complete replacement by tumor cells that had a light scatter profile characteristic of large lymphocytes and expressed the panhematopoietic marker CD45. As shown in Fig. 5B, tumor cells also coexpressed T cell markers, including CD5 and CD8, but did not express CD4 or Nk1.1 (natural killer cell), B cell (B220), and myeloid (Mac-1/Gr-1) markers, nor did they express progenitors (c-kit) (Fig. S4). These results are consistent with a high-grade T cell lymphoma of thymic origin.
Fig. 4.
ASPP2+/− mice have an increased incidence of γ-irradiation-induced lymphomas. (A) Kaplan–Meier lymphoma-free survival curves for ASPP2+/+ and ASPP2+/− mice after 6.0-Gy or 10.5-Gy (P = 0.024 or P = 0.045, log-rank test) total γ-irradiation delivered in divided weekly fractions starting at 6 weeks old. (B) Kaplan–Meier lymphoma-free survival curves for tet-o-MYC;EμSR-tTA mice (29) after conditional c-Myc expression in an ASPP2+/+ or ASPP2+/− background (P = 0.97, log-rank test).
Fig. 5.
High-grade thymic T cell lymphomas induced in ASPP2+/− mice after γ-irradiation. (A) Thymic lymphoma (red arrow) in an ASPP2+/− mouse. H&E-stained microscopic sections of lymphoma-infiltrated liver, kidney, and lung from this mouse. (B) Immunophenotyping of tumor cells in unfractionated bone marrow from this mouse. A total of 2% of normal myeloid progenitors coexpressed Mac-1 and Gr-1 (Upper Right). A total of 98% of the bone marrow was replaced by tumor cells expressing CD5 and CD8 (Lower), but not B220 (Upper Left), CD4, and Nk1.1 markers (Lower).
To explore whether oncogene-induced T cell lymphomas would be accelerated in ASPP2 haploinsufficient mice, we crossed our ASPP2+/− mouse with a transgenic mouse that develops T cell lymphomas after conditional expression of the MYC protooncogene in hematopoietic cells (29). These mice contain both a MYC cDNA under the control of a tetracycline-responsive minimal promotor (tet-o-MYC), and a tetracycline-transactivating protein (tTA) under control of the Ig heavy-chain enhancer and the SRα promoter (EμSR-tTA). MYC transgene expression was induced by doxycycline withdrawal, and tumor-free survival was determined. No significant difference was observed in T cell lymphoma development between the ASPP2+/+ and ASPP2+/− backgrounds (Fig. 4B).
ASPP2+/− Thymocytes Have an Attenuated Apoptotic Response to γ-Irradiation.
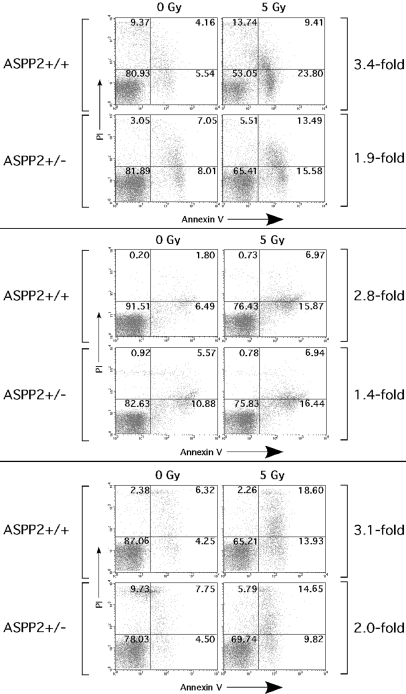
Because ASPP2+/− mice are predisposed to γ-irradiation-induced thymic lymphomas (Figs. 4 and 5), we wanted to determine whether ASPP2+/− thymocytes had an attenuated apoptotic response to γ-irradiation. Primary thymocytes were isolated from sibling ASPP2+/+ and ASPP2+/− mice, maintained in short-term culture for 24 h, subjected to 5-Gy γ-irradiation, and then assayed 4 h later (a dose and time point that induces <50% apoptosis). The fold-change in the percentage of apoptotic cells was determined by flow cytometry and Annexin-propidium iodide (PI) staining on irradiated thymocytes or on nonirradiated control thymocytes cultured in parallel (Fig. 6). Dotplots from 3 separate sets of paired sibling ASPP2+/+ and ASPP2+/− thymocytes are shown with (Fig. 6, right column) and without (Fig. 6, left column) γ-irradiation. In the ASPP2+/+ thymocytes (Fig. 6, top rows of paired sets), there were 3.4-, 2.8-, and 3.1-fold increases in the percentage of Annexin V-staining cells (Fig. 6, lower right and upper right quadrants) after γ-irradiation. In contrast, in the matched sibling ASPP2+/− thymocytes (Fig. 6, bottom rows of paired sets), there were only 1.9-, 1.4-, and 2.0-fold increases in the percentage of Annexin V-staining cells after γ-irradiation.
Fig. 6.
ASPP2+/− thymocytes have an attenuated apoptotic response to γ-irradiation. Flow cytometry on ASPP2+/+ or ASPP2+/− primary thymocytes 4 h after 5-Gy γ-irradiation (right column) or 0-Gy γ-irradiation (left column). The percentage of cells in each quadrant (relative to total cells) is indicated. The fold-increase in apoptosis between 0-Gy and 5-Gy γ-irradiated thymocytes was determined by the increase in percentage of Annexin V-stained cells (lower right plus upper right quadrants).
ASPP2+/− MEFs Have an Attenuated G0/G1 Cell Cycle Checkpoint After γ-Irradiation.
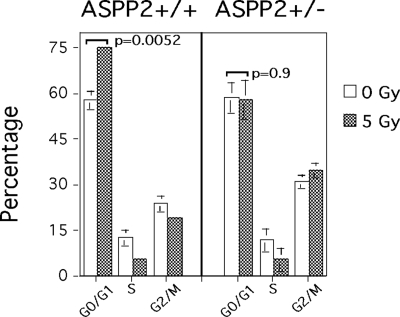
To explore how reduced ASPP2 expression might affect nonapoptotic cellular stress response pathways, we used primary MEFs (which normally undergo a γ-irradiation-induced G0/G1 arrest) to measure changes in cell cycle checkpoints 24 h after 5 Gy of γ-irradiation (Fig. 7). As expected, ASPP2+/+ MEFs had a significant increase in the percentage of cells in G0/G1 (from 57% to 75%; Fig. 7 Left). In contrast, γ-irradiated ASPP2+/− MEFs did not have a significant increase in the percentage of cells in G0/G1 (Fig. 7 Right).
Fig. 7.
ASPP2+/− MEFs have an attenuated G0/G1 checkpoint. Cell cycle distribution shows an increase in G0/G1-arrested ASPP2+/+ MEFs but not ASPP2+/− MEFs 24 h after 5-Gy γ-irradiation (P = 0.0052 and P = 0.9, unpaired 2-tailed Student's t test, respectively). Mean values of triplicate experiments are shown with SDs.
Discussion
We found that aging ASPP2+/− mice have accelerated spontaneous tumor development compared with ASPP2+/+ mice (Fig. 3A). The large number of mice in this experiment strengthens our finding that ASPP2 is a haploinsufficient tumor suppressor. Consistent with other reports (27, 30), we did not find ASPP2 loss of heterozygosity in tumors arising in ASPP2+/− mice (Figs. S1 and S2).
Because ASPP2 promotes damage-induced apoptosis and inhibits survival in cell culture (2, 4–6), our findings that ASPP2+/− mice have a significant increase in γ-irradiation-induced T cell lymphomas provides in vivo evidence that ASPP2 plays a role in damage-induced tumor suppression in susceptible organs (Fig. 4A). However, at higher doses (12 Gy), this was not statistically significant, as might be expected if the genotoxic damage exceeded a threshold in which reduced ASPP2 levels could not accelerate lymphomagenesis. Interestingly, reduced ASPP2 levels did not accelerate development of Myc-induced T cell lymphomas (Fig. 4B), even though defects in p53 can accelerate lymphomas in this model (31). Perhaps ASPP2 has a more important role in tumor suppression after genotoxic damage-induced (as opposed to oncogene-induced) tumorigenesis, suggesting a role in DNA repair and maintenance of genomic stability. However, the oncogenic context driving tumor formation—along with defective tumor suppressor pathways—is incompletely understood (refs. 32–34 and references within), and ASPP2 function in these different contexts remains to be clarified. Moreover, high levels of MYC transgene expression in this mouse (29) may overwhelm modest tumor suppression defects in ASPP2+/− mice; thus, we cannot rule out that ASPP2 could suppress MYC-induced lymphomas in other model systems.
ASPP2−/− mice die from an unknown early embryonic lethal defect (Figs. 1 and 2) that could not be compensated for by the homologous proapoptotic family member ASPP1 (2). Although in vitro evidence has not shown major functional differences between ASPP2 and ASPP1 (2, 5, 35), the nonredundant in vivo findings suggest that ASPP2 has distinct biologic functions. Conversely, the defective lymphatic vessel formation of ASPP1−/− mice suggests ASPP1 has distinct in vivo functions that cannot be compensated by ASPP2 (36). In contrast to our findings, another ASPP2-deficient mouse exhibits early postnatal lethality (27). Although background strain may contribute to differences in developmental phenotypes, it is likely that the different targeting strategies to disrupt exon 3 (27) or exons 10–17 (Fig. 1) may also contribute. Indeed, the complexity of the ASPP2 locus suggests there might be multiple gene products, and we are investigating this hypothesis.
We did not observe genetic cooperation between ASPP2 and p53 to suppress tumor development (Fig. S3). This is partially consistent with the recent report on another ASPP2+/−;p53+/− mouse showing ASPP2 does not cooperate with p53 to suppress sarcoma or lymphoma development at 72 weeks, although at 42 weeks there is an increase in lymphomas (27, 30). Given that ASPP2 and p53 cooperation may be tumor type-specific (27), subtle strain-specific modifiers and/or the different targeting strategies could account for the differences. It is also possible that our ASPP2+/−;p53+/− mouse experiment was underpowered to detect modest alterations in tumor latency. Thus, we cannot firmly conclude that ASPP2 tumor suppression is p53-independent, although this raises the intriguing possibility that p53-independent mechanisms are involved (5, 8–16). Likewise, ASPP1 does not cooperate with p53 in vivo, because impaired lymphatic development of ASPP1−/− mice is not altered in a p53+/− or p53−/− background (36).
The attenuated apoptotic response of primary ASPP2+/− thymocytes (Fig. 6) suggests a potential mechanism for lymphomagensis in γ-irradiated mice (Figs. 4 and 5), because apoptotic defects may result in the persistence of thymocytes that have acquired tumorigenic mutations. Interestingly, we found a G0/G1 checkpoint defect in ASPP2+/− MEFs (Fig. 7). Because tumor suppression involves a variety of cellular functions in addition to apoptosis (34, 37), this tantalizingly suggests that ASPP2 function is far more complex than just simply enhancing proapoptotic p53 transcriptional programs (2). Previous reports using tumor cell culture have also hinted that ASPP2 (and/or isoforms) might modulate expression of cell cycle-regulating genes (25), perturb cell cycle progression (9), bind and modulate other proteins (8–13, 38, 39), or be regulated by pathways involved in diverse functions (6, 14–16). The net result of these G0/G1 checkpoint (or other) defects in ASPP2 haploinsufficient cells could manifest as a tumor-prone phenotype because of the accumulation of cell populations that will ultimately evolve into frank neoplastic growth. The molecular mechanisms underlying the apoptotic and cell cycle defects in ASPP2+/− cells remain unknown, and we are currently exploring how reduced ASPP2 expression can modulate global transcriptional and posttranscriptional networks.
Our findings provide in vivo evidence that reduction of ASPP2 expression results in acceleration of spontaneous and γ-irradiation-induced tumors, and that apoptotic and cell cycle arrest damage checkpoints are attenuated. These results rigorously confirm the mounting evidence for the role of ASPP2 as a tumor suppressor (18–24, 27), and they expand upon potential mechanisms of how ASPP2 functions.
Methods
ASPP2 Allele-Targeted Disruption.
ASPP2+/− mice were generated by using the Oregon Health and Science University (OHSU) Transgenic Core. Details are in SI Materials and Methods.
Quantitative RT-PCR.
Reverse transcription was performed on total RNA and quantitative PCR was performed with TaqMan technology (Invitrogen) using standard conditions described in ref. 19. Details are in SI Materials and Methods.
Western Blotting.
Western blot analysis was performed as described in ref. 4 and in SI Materials and Methods. Rabbit anti-ASPP2 Ab1 was raised against an expressed GST-amino terminus ASPP2 fusion protein (gift from Rachael Neve, Harvard University, Boston, MA) (40); rabbit anti-ASPP2 Ab2 was as described in ref. 9.
Cell Culture.
Details are in SI Materials and Methods. Day 11.5 MEFs were prepared using standard techniques (41). Thymocytes were isolated from 5- to 6-week-old mice and cultured for <24 h before use.
Flow Cytometry.
Flow cytometry was performed by using standard techniques with details in SI Materials and Methods. Apoptosis by Annexin V staining (6), cell cycle analysis by PI staining (14), and tumor cell imunophenotyping (42) were all performed as described previously.
Mouse Colonies and Tumor Monitoring.
IUCAC-compliant protocols were approved by the OHSU Department of Comparative Medicine. Details are in SI Materials and Methods. Newly weaned pups were irradiated with weekly 3-Gy fractions (6 Gy total dose) or weekly 2.62-Gy fractions (10.5 Gy total dose). Starting at 10 weeks after irradiation, mice were monitored weekly for tumors and automated complete cell counts. Moribund mice, or mice with circulating leukemia/lymphoma cells, were killed for necropsy. Expert hematopathologic review was provided by the OHSU Pathology Core. Survival curves were calculated with SPSS v15.0 software.
Supplementary Material
Acknowledgments.
This work was supported in part by U.S. Public Health Service Grants CA104997 and CA85773 (to C.D.L.), CA076316 (to L.N.), HL069133 and HL077818 (to W.H.F.), CA89305 (to D.W.F.), 5-P30-CA69533 (to the OHSU Knight Cancer Institute), P01 CA034233 (to the Stanford University Lymphoma Program Project Grant); the Burroughs Welcome Fund, the Damon Runyon Foundation (to D.W.F.), the Leukemia and Lymphoma Society (to A.C.F.), and gifts in the memory of Dr. Donald Peterson (to C.D.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809080106/DCSupplemental.
References
- 1.Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S. Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci USA. 1994;91:6098–6102. doi: 10.1073/pnas.91.13.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuels-Lev Y, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, et al. Expression of 53BP2 and ASPP2 proteins from TP53BP2 gene by alternative splicing. Biochem Biophys Res Commun. 2004;315:434–438. doi: 10.1016/j.bbrc.2004.01.079. [DOI] [PubMed] [Google Scholar]
- 4.Lopez CD, et al. Proapoptotic p53-interacting protein 53BP2 is induced by UV irradiation but suppressed by p53. Mol Cell Biol. 2000;20:8018–8025. doi: 10.1128/mcb.20.21.8018-8025.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergamaschi D, et al. ASPP1 and ASPP2: Common activators of p53 family members. Mol Cell Biol. 2004;24:1341–1350. doi: 10.1128/MCB.24.3.1341-1350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, et al. Control of ASPP2/(53BP2) protein levels by proteasomal degradation modulates p53 apoptotic function. J Biol Chem. 2005;280:34473–34480. doi: 10.1074/jbc.M503736200. [DOI] [PubMed] [Google Scholar]
- 7.Gorina S, Pavletich N. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science. 1996;274:1001–1005. doi: 10.1126/science.274.5289.1001. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa H, et al. APCL, a central nervous system-specific homologue of adenomatous polyposis coli tumor suppressor, binds to p53-binding protein 2 and translocates it to the perinucleus. Cancer Res. 2000;60:101–105. [PubMed] [Google Scholar]
- 9.Naumovski L, Cleary ML. The p53-binding protein 53BP2 also interacts with Bcl2 and impedes cell cycle progression at G2/M. Mol Cell Biol. 1996;16:3884–3892. doi: 10.1128/mcb.16.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, et al. NF-kB subunit p65 binds to 53BP2 and inhibits cell death induced by 53BP2. Oncogene. 1999;18:5177–5186. doi: 10.1038/sj.onc.1202904. [DOI] [PubMed] [Google Scholar]
- 11.Espanel X, Sudol M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J Biol Chem. 2001;276:14514–14523. doi: 10.1074/jbc.M008568200. [DOI] [PubMed] [Google Scholar]
- 12.Helps N, Barker H, Elledge SJ, Cohen P. Protein phosphatase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS Lett. 1995;377:295–300. doi: 10.1016/0014-5793(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Hamada T, Matsui T, Date T, Iwabuchi K. Hepatitis C virus core protein interacts with p53-binding protein, 53BP2/Bbp/ASPP2, and inhibits p53-mediated apoptosis. Biochem Biophys Res Commun. 2004;315:788–795. doi: 10.1016/j.bbrc.2004.01.124. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Padiernos E, Ding F, Lossos I, Lopez CD. Apoptosis stimulating protein of p53–2 (ASPP2/53BP2L) is an E2F target gene. Cell Death Diff. 2004;12:358–368. doi: 10.1038/sj.cdd.4401536. [DOI] [PubMed] [Google Scholar]
- 15.Fogal V, et al. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Diff. 2005;12:369–376. doi: 10.1038/sj.cdd.4401562. [DOI] [PubMed] [Google Scholar]
- 16.Hershko T, Chaussepied M, Oren M, Ginsberg D. Novel link between E2F and p53: Proapoptotic cofactors of p53 are transcriptionally upregulated by E2F. Cell Death Diff. 2005;12:377–383. doi: 10.1038/sj.cdd.4401575. [DOI] [PubMed] [Google Scholar]
- 17.Mori T, Okamoto H, Takahashi N, Ueda R, Okamoto T. Aberrant overexpression of 53BP2 mRNA in lung cancer cell lines. FEBS Lett. 2000;465:124–128. doi: 10.1016/s0014-5793(99)01726-3. [DOI] [PubMed] [Google Scholar]
- 18.Sgroi DC, et al. In vivo expression profile analysis of human breast cancer progression. Cancer Res. 1999;59:5656–5661. [PubMed] [Google Scholar]
- 19.Lossos I, Natkunam Y, Levy R, Lopez CD. Apoptosis stimulating protein of p53 (ASPP2) expression differs in diffuse large B-cell and follicular center lymphoma: Correlation with clinical outcome. Leuk Lymph. 2002;43:2309–2317. doi: 10.1080/1042819021000040017. [DOI] [PubMed] [Google Scholar]
- 20.Cobleigh M, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res. 2005;11:8623–8631. doi: 10.1158/1078-0432.CCR-05-0735. [DOI] [PubMed] [Google Scholar]
- 21.Pomeroy S, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 22.Hedenfalk M, et al. Gene-expression profiles in hereditary breast cancer. N Eng J Med. 2001;344:539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, et al. Downregulated mRNA expression of ASPP and the hypermethylation of the 5′-untranslated region in cancer cell lines retaining wild-type p53. FEBS Lett. 2005;579:1587–1590. doi: 10.1016/j.febslet.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 24.Sarraf A, Stancheva I. Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol Cell. 2004;15:595–605. doi: 10.1016/j.molcel.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Iwabuchi K, et al. Stimulation of p53-mediated transcriptional activation by the p53-binding proteins, 53BP1 and 53BP2. J Biol Chem. 1998;273:26061–26068. doi: 10.1074/jbc.273.40.26061. [DOI] [PubMed] [Google Scholar]
- 26.Bergamaschi D, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167. doi: 10.1038/ng1070. [DOI] [PubMed] [Google Scholar]
- 27.Vives V, et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev. 2006;20:1262–1267. doi: 10.1101/gad.374006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatachalam S, et al. Retention of wild-type p53 in tumors from p53 heterozygous mice: Reduction of p53 dosage can promote cancer formation. EMBO J. 1998;17:4657–4667. doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 30.Vives V, Slee E, Lu X. ASPP2: A gene that controls life and death in vivo. Cell Cycle. 2006;5:2187–2190. doi: 10.4161/cc.5.19.3266. [DOI] [PubMed] [Google Scholar]
- 31.Giuriato S, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci USA. 2006;103:16266–16271. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochownik E, Li Y. The ever expanding role for c-Myc in promoting genomic instability. Cell Cycle. 2007;6:1024–1029. doi: 10.4161/cc.6.9.4161. [DOI] [PubMed] [Google Scholar]
- 33.Efeyan A, Serrano M. p53: Guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 34.Evan G, et al. Oncogene-dependent tumor suppression: Using the dark side of the force for cancer therapy. Cold Spring Harbor Symposia on Quantitative Biology. 2005;LXX:263–273. doi: 10.1101/sqb.2005.70.054. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan A, Lu X. ASPP: A new family of oncogenes and tumor suppressor genes. Br J Cancer. 2007;96:196–200. doi: 10.1038/sj.bjc.6603525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirashima M, et al. Lymphatic vessel assembly is impaired in ASPP1-deficient mouse embryos. Dev Biol. 2008;316:149–159. doi: 10.1016/j.ydbio.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Langton P, Colombani J, Aerne B, Tapon N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell. 2007;13:773–782. doi: 10.1016/j.devcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Meek S, Lane W, Piwnica-Worms H. Comprehensive proteomic analysis of interphase and mitotic 14–3-3-binding proteins. J Biol Chem. 2004;279:32046–32054. doi: 10.1074/jbc.M403044200. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Liu W, Naumovski L, Neve RL. ASPP2 inhibits APP-BP1-mediated NEDD8 conjugation to cullin-1 and decreases APP-BP1-induced cell proliferation and neuronal apoptosis. J Neurochem. 2003;85:801–809. doi: 10.1046/j.1471-4159.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 41.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 42.Montfort M, Olivares C, Mulcahy J, Fleming W. Adult blood vessels restore host hematopoiesis following lethal irradiation. Exp Hematol. 2002;30:950–956. doi: 10.1016/s0301-472x(02)00813-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.