Abstract
Retinoic acid (RA) receptors (RARs) α, β, and γ heterodimerized with rexinoid receptors (RXRs) α, β, and γ mediate the RA signal. To analyze the contribution of the transcriptional activity of RXRα, the main RXR during embryogenesis, we have engineered a mouse line harboring a transcriptionally silent RXRα mutant that lacks the activation functions AF1 and AF2. All homozygous mutants (Rxraafo) display the ocular defects previously observed in compound Rar-null and Rxra/Rar-null mutants, thus demonstrating that a transcriptionally active RXRα is required during eye development. In contrast, the vast majority of Rxraafo fetuses do not display the Rxra-null mutant hypoplasia of the myocardium, thus demonstrating that RXRα can act as a transcriptionally silent heterodimerization partner. Similarly, a transcriptionally silent RXRα mutant can support early embryogenesis, as Rxraafo/Rxrb-null embryos display a normal morphology, contrasting with the severe malformations exhibited by compound Rxra/Rxrb-null embryos. Along the same line, we show that a silent RXRα mutant is sufficient to allow the initial formation of the placental labyrinth, whereas later steps of trophoblast cell differentiation critically requires the AF2, but not the AF1, function of RXRα.
Keywords: activation function, gene knockout, nuclear receptor, retinoic acid, transcriptional activity
The retinoic acid (RA) receptors (RARs) α, β, and γ isotypes (that bind all-trans and 9-cis RA) and the rexinoid receptors (RXRs) α, β, and γ isotypes (that bind 9-cis RA only) are ligand-dependent transcriptional regulators acting in the form of RXR/RAR heterodimers to control expression of target genes (1, 2). Based on structural and functional similarities within the nuclear receptor (NR) superfamily, 6 distinct regions (A to F) are defined in RARs and RXRs (1, 3). The highly variable N-terminal A/B region contains a ligand-independent transcriptional activation function (AF1) and displays serine residues whose phosphorylation modulate the AF1-mediated transcriptional activity (4). Region C bears the highly conserved DNA-binding domain (DBD), whereas region D functions as a flexible hinge between the DBD and the C-terminal E/F region. Region E is functionally complex, as it contains the ligand-binding domain (LBD), a dimerization interface and a ligand-inducible transcriptional AF (AF2), which involves a highly conserved amphipathic α-helix containing the AF2-activating domain (AD) core (1, 2). Binding of an agonistic ligand induces a transconformation of the LBD, involving notably helix 12, and results in the generation of a surface that allows the binding of coactivators, while corepressors are concomitantly released (5–7). Both AF1 and AF2 display distinct properties, which depend on promoter context and cell type, and cooperatively contribute in cultured cells to transcription of target genes (1, 8–11).
The synergism observed between liganded RAR and RXR on the transcriptional activation of target genes in vitro, either in cell-free systems or cultured transfected cells, revealed that RXRs are not a priori silent partners (refs. 1 and 2 and references therein). However, in RXR/RAR heterodimers, the ligand-dependent transcriptional activity of RXR appears “subordinated” to the binding of an agonistic ligand to its RAR partner (refs. 1, 3, and 12 and references therein). In the case of several other NRs that heterodimerize with RXR, such as peroxisome proliferator-activated receptors (PPARs) (reviewed in refs. 13 and 14), RXR agonists can activate transcription on their own (15). A molecular mechanism accounting for RXR subordination and permissivity in heterodimers has been proposed (7). Such permissivity might integrate retinoid signaling into other NR/RXR signaling pathways, but would also raise the question as to whether 9-cis RA could be a physiological ligand for RXRs. This would indeed create a problem of promiscuity, because it would result in concomitant activation of the RXR/RAR-mediated retinoid signaling pathway (1, 2, 16).
To investigate the in vivo relevance of the RA signal transduction mechanisms characterized in vitro and determine the actual functions of RARs and RXRs, we have generated several mouse lines carrying loss-of-function mutations at Rar and Rxr loci (reviewed in ref. 17). Compound mutants, in which a null mutation of a given RAR isotype is associated either with a Rxra-null, a Rxraaf1o (deletion of RXRα AF1) or a Rxraaf2o (deletion of RXRα AF2) mutation, altogether recapitulate the abnormalities exhibited by Rar-null mutants (18–21). This synergism between Rar and Rxra loss-of-function mutations supports the conclusion that RXRα/RARα, RXRα/RARβ and RXRα/RARγ heterodimers are the functional units transducing RA signals required for embryonic development, notably body shaping, hindbrain patterning, placentation, and heart and eye morphogenesis (reviewed in ref. 17). To gain further insights into the role of RXRα during development, we have generated a mouse line expressing a transcriptionally silent RXRα lacking both AF1 and AF2. The phenotypic analysis of these Rxraafo mutants demonstrates that a silent RXRα can support embryonic shaping, early steps of placentation, and heart development, whereas a transcriptionally active RXRα is critically required for eye morphogenesis and late steps of placentation.
Results and Discussion
Mutant Mice Bearing Targeted Deletions of RXRα AF1 and AF2.
Generating mice expressing an RXRα lacking both AF1 and AF2 was not possible by crossing mice bearing the Rxraaf1o mutation (21) with those bearing the Rxraaf2o mutation (20), because these mutations are too close to expect a crossing-over event associating them on the same allele. Therefore, we introduced the Rxraaf1o mutation (deletion of amino acids 11–132, AF1o) through homologous recombination into the VG30 embryonic stem (ES) cell line bearing the Rxraaf2o mutation (deletion of LBD helix 12, amino acids 450–467, AF2o), and a loxP-flanked neomycin-resistance cassette (neo) into intron 9 (Rxraaf2oN allele; see Fig. 1A) (20). We modified the A/B region-targeting vector (pB48) (21) by replacing the loxP-flanked neo cassette by a loxP-flanked hygromycin-resistance cassette (hygro; Fig. 1A). The resulting vector (pB67-hygroR) was used to obtain VG30.82 ES cells, bearing the RxraafoHN allele (Fig. 1 A and B), which were injected into blastocysts to generate a mouse line. Rxra+/afoHN mice were crossed with transgenic mice expressing the Cre recombinase at the 1-cell stage (see SI Materials and Methods), yielding Rxra+/afo mice (Fig. 1A, line CB4), which were identified by Southern blot analysis (Fig. 1B, lane 4) and PCR (Fig. 1 A and C). It is worth noting that the RXRα protein lacking AF1 and AF2 (RXRαAFo) did not exert a dominant negative effect as Rxra+/afo heterozygous mice never displayed phenotypical abnormalities.
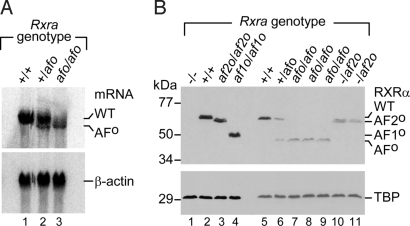
Fig. 1.
Targeted deletion of the AF1-containing A/B region and AF2 AD core (helix 12 of LBD) of RXRα. (A) Schematic representation of the Rxra wild-type (+, WT) and Rxraaf2oN (VG30) alleles, the targeting vector (pB67-HygroR), the homologous recombinant RxraafoHN (VG30.82), and the Cre-rearranged Rxraafo (CB4) alleles. Exons 2–10 are indicated by numbered black boxes. Exons 2 and 3 encode for the AF1, and E10 for the AF2 AD core. The Rxraaf1o (AF1o) and Rxraaf2o (AF2o) mutations are indicated by asterisks under exons 2 and 10, respectively. The positions of probes A and B, and PCR primers 1–4 are indicated. Black arrowhead flags represent loxP sites. Restriction sites: N, NcoI; S, SpeI. (B) Southern blot analysis of genomic DNA (Rxra genotype as indicated) by using probe A (Upper) and probe B (Lower). The sizes of the restriction fragments are indicated in kilobases (kb). The genomic DNA was from WT ES cells (lane 1, +/+), VG30 ES cells (lane 2, Rxra+/af2oN), VG30.82 ES cells (lane 3, Rxra+/afoHN), an adult mouse from line CB4 (lane 4, Rxra+/afo), and an E12.5 embryo (lane 5, Rxraafo/afo). The open arrowheads indicate the position of the fragments that would have been detected in case of Cre-mediated inversion of the genomic region containing E4 to E9 (see SI Materials and Methods). (C) PCR analysis of embryos (Rxra genotype as indicated) by using either primers 1 and 2 to detect the loxP site indicative of the Rxraaf1o mutation (Upper), or primers 3 and 4 to detect the loxP site indicative of the Rxraaf2o mutation (Lower).
Rxra+/afo mice were intercrossed to generate Rxraafo/afo mutants (referred to as Rxraafo mutants), expressing only the truncated RXRα mRNA (AFo; Fig. 2A, lane 3). This truncated mRNA was expressed at lower levels than its wild-type (WT) counterpart (Fig. 2A, lane 1). Analysis of nuclear protein extracts from fetuses revealed that the expression level of the RXRα protein lacking AF1 and AF2 (RXRαAFo; Fig. 2B) was ≈2-fold lower than that of the WT protein (Fig. 2B, lane 6). Therefore, the Rxraafo mutation alters the steady-state level of the RXRα truncated mRNA and protein. Because the amount of RXRαAFo protein in Rxraafo fetuses was similar to that of the RXRαAF2o protein in fetuses harboring 1 Rxra-null (18) and 1 Rxraaf2o allele (hereafter referred to as Rxra−/af2o; Fig. 2B, compare lanes 7–9 with lanes 10 and 11), the phenotype of the Rxraafo mutants was compared with those of Rxra−/af2o and Rxra+/− fetuses (Fig. 2B, lanes 10 and 11).
Fig. 2.
RXRα expression in Rxraafo embryos. (A) Northern blot analysis of total RNA (10 μg per lane) extracted from E12.5 embryos (Rxra genotype as indicated), by using a RXRα cDNA (Upper). The identities of the wild-type (WT) and mutant (AFo) RXRα mRNA are indicated on the right. A mouse β-actin probe was used to quantify the RNA samples (Lower). (B) Western blot analysis of nuclear extracts (15 μg) prepared from E12.5 embryos (Rxra genotype as indicated) resolves on 10% SDS/PAGE by using an 1/500 dilution of the anti-RXRα antibody (Upper). A Rxra-null embryo is used as negative control (−/−, lane 1), whereas WT (+/+, lane 2), Rxraaf2o/af2o (lane 3), and Rxraaf1o/af1o (lane 4) embryos were used to visualize the migration of the intact protein, or of RXRα lacking its AF2 AD core (AF2o) or its AF1 (AF1o). Note that the RXRαAFo protein (Rxraafo/afo, lanes 6–9) expresses at a lower level than the WT protein (Rxra+/+, lanes 2 and 5). For comparative purposes, samples from E12.5 embryos harboring 1 Rxra-null and 1 Rxraaf2o allele (Rxra−/af2o) are illustrated (lanes 10 and 11). The amount of protein in each lane was assessed by using an anti-TBP antibody (Lower).
Among 446 mice born from Rxra+/afo intercrosses, no Rxraafo mutant was recovered at postnatal day 8 (P8). Caesarean delivery of 183 pups at embryonic day 18.5 (E18.5) yielded 30 Rxraafo mutants, instead of the 46 expected, of which 16 were stillborn. Living E18.5 Rxraafo mutants did not survive for more than a few hours, and their weight was reduced by about one-third [0.81 g for Rxraafo mutants (n = 12) versus 1.24 g for WT littermates (n = 23)]. Living Rxraafo mutants were recovered at E14.5 with a Mendelian distribution. Thus, the Rxraafo mutation is lethal between E14.5 and E18.5, similarly to the Rxraaf2o mutation (20), whereas the Rxra-null mutation is lethal earlier (18, 22).
A Transcriptionally Active RXRα Is Essential for Eye Morphogenesis.
RXRα, acting in heterodimers with RARβ and RARγ, is instrumental to eye morphogenesis (18, 19, 23). We found here that all E14.5 Rxraafo mutants (n = 10) displayed a persistent and hyperplasic primary vitreous body (PHPV, R in Fig. 3 B and C), closer eyelid folds (arrowhead), a thickened ventral portion of the corneal stroma (C), a shorter ventral retina (V), and a ventral rotation of the lens (L) (compare Fig. 3 A with B and C; Table 1). Additionally, a small optic disk coloboma (i.e., an abnormal opening of the retina at the optic nerve exit point [OD and asterisk, compare Fig. 3 A with C)] was observed in 90% of these mutants. All Rxraafo mutants (n = 3) displayed an agenesis of the sclera at E18.5 (SC; Fig. 3 D and E). Thus, Rxraafo mutants recapitulated with the same penetrance (i.e., 90–100%) all defects of the Rxra-null ocular syndrome (18), which represents an aspect of the fetal vitamin A-deficiency (VAD) syndrome (24). Because Rxraafo mutants expressed approximately half the normal amount of RXRα protein (see Fig. 2B), they were further compared with Rxra+/− and Rxra−/af2o fetuses (see above). At E14.5, Rxra+/− fetuses had normal eyes, whereas 1 out of 4 Rxra−/af2o fetuses displayed the characteristic Rxra-null ocular syndrome and the 3 others had only a bilateral PHPV. Altogether these data show that the reduced expression of RXRαAFo cannot account for the severe ocular malformations of Rxraafo mutants, thus indicating that a transcriptionally silent RXRα is unable to support ocular morphogenesis. Note, however, that we cannot rule out the unlikely possibility that expression of RXRαAFo could be selectively and severely reduced in some tissues, as a consequence of manipulation of the RARα locus during mutagenesis.
Fig. 3.
Rxraafo mutants consistently display severe ocular defects, and only occasionally a hypoplasic myocardium. Shown are frontal histological sections through eyes or hearts of E14.5 and E18.5 fetuses. (B, C, and E) Ocular abnormalities of Rxraafo fetuses are similar to those previously observed in Rxra-null mutants (see Results and Discussion and ref. 18). (F and H) The ventricular myocardium of Rxraafo mutants appears normal in the vast majority of the cases. (G and I) The “spongy” mutant's myocardium illustrated here represents an exception. Note that H and I are 5-fold higher magnifications of the boxes in F and G, respectively. A, aorta; C, cornea; CL, compact layer of the ventricular myocardium; D, dorsal retina; fAT, left atrium; L, lens; M, muscle; MB, membranous portion of the interventricular septum; NR, neural retina; OD, optic disk; P, pulmonary trunk; PR, pericardium; R, retrolenticular mesenchyme (persistent hyperplasic primary vitreous body); RPE, retinal pigment epithelium; SC, sclera; V, ventral retina; fVT and rVT, left and right ventricles. The asterisk in C indicates a coloboma of the optic disk, and the arrowheads in A–C point to the upper eyelids. [Scale bar in I: 110 μm (A–C), 40 μm (D and E), 440 μm (F and G), and 90 μm (H and I).]
Table 1.
Developmental abnormalities in Rxraafo mutants
| Abnormalities | Age and no. of Rxraafo fetuses |
|
|---|---|---|
| E14.5(n = 10) | E18.5 (n = 3) | |
| Ocular defects | ||
| Ventral rotation of the lens (Rxra#) | # | ND |
| Closer eyelid folds (Rxra#) | # | NA2 |
| Thickened corneal stroma (Rxra#) | # | ND |
| Agenesis of the sclera (Rxra#) | NA1 | # |
| Retrolenticular membrane (PHPV) (Rxra#) | # | # |
| Shortening of ventral retina (Rxra#) | # | # |
| Coloboma of the optic disc (Rxra) | B:6/10; U:2/10 | 0 |
| Cardiovascular defects | ||
| Ventricular myocardium hypoplasia (Rxra#) | 2/10 | 1/3 |
| Agenesis of the conotruncal septum (Rxra) | 1/10 | 0 |
| Abnormal arteries derived from aortic arches | 1/10 | 0 |
| Glandular defects | ||
| Agenesis of Harderian glands | NA1 | # |
| Shortening of sublingual duct | NA1 | # |
Rxra, These abnormalities are observed in Rxra-null fetuses. #, This abnormality is completely penetrant (and bilateral). U, unilateral; B, bilateral; NA1, not applicable as the corresponding structures are not yet formed at E14.5; NA2, not applicable, as the eyelids are fused together at E18.5; ND, not determined, as it is difficult to evaluate small changes in the size or position of the corresponding structures at E18.5. Note that all E18.5 Rxraafo fetuses displayed a bilateral agenesis of the Harderian glands and shortening of the sublingual gland ducts, which both could not be observed in Rxra-null fetuses because of their early death. Note also that absence of the optic disc coloboma in E18.5 Rxraafo fetuses indicates that this defect at E14.5 corresponds to a delayed closure of the optic fissure, rather than a developmental arrest. For further details concerning these abnormalities, see refs. 18, 19, 32, and 34.
The proposed explanation for the occurrence of the Rxra-null ocular syndrome in only 15% of Rxraaf2o mutants was a functional compensation of the mutation by RXRβ whose ablation, on its own, does not yield developmental defects (20, 25). The increase of eye defects from 15–100% in Rxraaf2o/Rxrbaf2o mutants (in which functional compensation by RXRβ AF2 is abrogated) further demonstrates that RXRα AF2 is indispensable for eye morphogenesis (Table S1). It is noteworthy that the ligand-dependent transactivation functions of RXR may not exclusively depend on the AF2, because binding of an agonistic ligand does not always trigger the positioning of RXRα helix H12 in the active conformation (26). Therefore, the abnormal ocular phenotype of Rxraaf2o and Rxraafo mutants reflects a key role of RXRα AF2 in regulating the transcription of RA-target genes involved in eye development, but does not provide information on RXR ligands.
RXRα AF1 can also be instrumental to eye development, as assessed from the presence of a PHPV in some Rxraaf1o mutants and, most importantly, from the complete penetrance of the Rxra-null ocular syndrome in Rxraaf1o mutants additionally lacking either the Rarb or Rarg genes (21). Our present data showing that the eye defects of Rxraafo mutants are absent in most Rxra−/af2o fetuses (differing from Rxraafo mutants only by the presence of the RXRα AF1) confirm that the RXRα AF1-containing A/B region is actually involved in ocular development.
A Transcriptionally Silent RXRα Can Support Myocardial Growth.
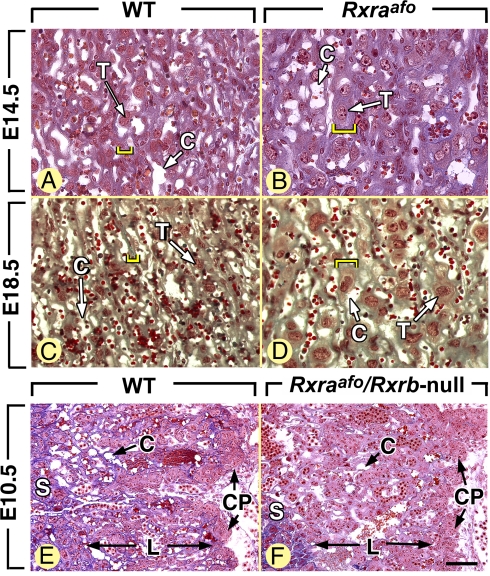
RXRα plays a crucial role in transducing RA signals necessary for myocardial growth, as its absence consistently induces a hypoplasia of the compact layer of the ventricular myocardium (HVM) that (i) causes cardiac failure and death around E14.5 and (ii) represents a hallmark of the fetal VAD syndrome (18, 22, 24). Only 20% (2 of 10) of E14.5 Rxraafo mutants displayed an HVM undistinguishable from that of Rxra-null and Rxraaf2o fetuses (Table 1). Similarly, only 1 of 3 Rxraafo fetuses analyzed at E18.5 displayed an HVM manifested by a “spongy” appearance of the ventricular wall (compare Fig. 3 F and H with G and I). That a RXRα lacking both AF1 and AF2 can support myocardial growth in a vast majority of fetuses (i.e., ≈80%) provides evidence that RXRα can act as a transcriptionally silent heterodimeric partner in vivo. However, HMV, that is very rare in Rxraaf1o/Rxrb/g-null mutants (21) and affects only 5% of Rxraaf2o mutants (20) and only 20% of Rxraafo mutants, is increased to ≈50% upon further inactivation of the Rxrb gene (Rxraaf2o/Rxrb-null mutants) (20), or deletion of the RXRβ AF2 (Rxraaf2o/Rxrbaf2o mutants) (Table S1). Therefore, in “unfavorable” genetic backgrounds that are borderline for RA signaling, both AF1 and AF2 of RXRα are instrumental to activating the RA target genes involved in myocardial growth.
Agenesis of the conotruncal septum, another defect frequently induced by dietary VAD (24), was found in only 1 of 10 E14.5 Rxraafo mutants (Table 1), and absence of the membranous portion of the ventricular septum (MB, Fig. 3 F and G), which is the late, inevitable outcome of conotruncal septum agenesis, was not detected in E18.5 Rxraafo mutants (Table 1). Our findings that agenesis of the conotruncal septum is much less penetrant in Rxraafo than in Rxra-null mutants (i.e., ≈8% versus 30%) (18), together with the fact that it occurs only occasionally in (i) Rxraaf1o/Rxrb/g-null mutants (21), (ii) Rxraaf2o, Rxraaf2o/Rxrb-null and Rxraaf2o/Rxrb/g-null (20) and (iii) Rxraaf2o/Rxrbaf2o (Table S1), indicate that atranscriptionally silent RXRα can support the fusion of the conotruncal ridges, but that its AF1 and AF2 are instrumental in genetic backgrounds where the RA signaling is deficient. Interestingly, Rxra−/afo fetuses (n = 2), expressing only one-fourth of the normal amount of RXRα protein (see previous discussion), did not display HVM or conotruncal septum defects, showing that even a limited amount of RXRαAFo can be sufficient to support heart development.
That similar defects of cardiomyocyte proliferation and differentiation are caused by Rxra- and Rara-null mutations has suggested that RXRα/RARα are the preferential heterodimers involved in myocardial growth (19). The present study further indicates that RXRα does not necessarily participate in the activity of these heterodimers, but instead can merely allow their binding to response elements located in target genes whose transcription is promoted through the RA-liganded RARα.
A Transcriptionally Silent RXRα Can Support Early Embryogenesis.
The occurrence of severe embryonic defects (e.g., abnormal body turning, absence of the second and third branchial arches, supernumerary otocysts and enlargement of the fifth rhombomere) in all compound Rxra/b-null mutants (27), but not in Rxra-null (18, 22) or Rxrb-null (28) mutants indicate that some functional compensation between RXRα and RXRβ can occur, but does not imply that RXRα and RXRβ are functionally equivalent during early development.
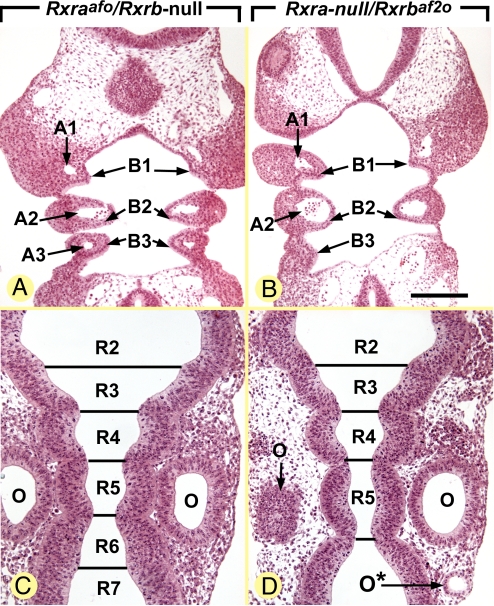
We have compared the outcome of the Rxraafo mutation in the Rxrb-null genetic background with that of the Rxrbaf2o mutation in the Rara-null background. In contrast to Rxra/b-null mutants (27), E9.5 Rxraafo/Rxrb-null mutants (n = 3) appeared externally and histologically normal (Fig. 4 A and C). In particular, their branchial arches (Fig. 4A) and rhombomeres (Fig. 4C) were normally patterned. Among 5 Rxraafo/Rxrb-null mutants analyzed at E10.5, 1 was alive and externally similar to a WT E10.5 embryo, whereas the 4 other were growth-retarded, displaying the external appearance of normal E9.5 embryos. In contrast, all Rxra-null/Rxrbaf2o mutants (n = 4) analyzed at E9.5 displayed a set of defects reflecting a block in RA signaling (29, 30), namely: (i) a bilateral hypoplasia of the third branchial arches, which lacked the corresponding artery (A3 and B3; compare Fig. 4 A and B); (ii) an enlargement of the fifth rhombomere (R5; compare Fig. 4 C and D); and (iii) supernumerary otic vesicles (O*; Fig. 4D). Altogether these data indicate that, on its own, a transcriptionally silent RXRα expressed at a level twice lower than normal (the condition observed in compound Rxraafo/Rxrb-null embryos; see previous discussion) is more efficient in supporting early morphogenesis than a normal amount of RXRβ lacking its AF2 (the condition observed in compound Rxra-null/Rxrbaf2o embryos). This observation supports the view that RXRα and RXRβ are not actually equivalent, RXRα being the main RXR involved in the transduction of RA signals required for proper embryonic shaping and patterning of the branchial arches and hindbrain. Because mutant embryos lacking Rara and Rarg (30), or lacking the RA-synthesizing enzyme RALDH2 (31), both display an Rxra/b-null mutant-like phenotype, early development most probably depends on RXRα/RARα and RXRα/RARγ heterodimers that are RA-liganded. The present study further indicates that RXRα does not participate in the transcriptional activity of these heterodimers, but instead plays a “passive” role in their binding to DNA response elements.
Fig. 4.
Rxra-null/Rxrbaf2o embryos display several congenital abnormalities, whereas Rxraafo/Rxrb-null embryos appear normal. Frontal histological sections at comparable levels of the branchial arches and hindbrain of E9.5 embryos. A1–A3, branchial arch arteries 1 to 3; B1–B3, branchial arches 1 to 3; O and O*, orthotopic and ectopic otocysts, respectively; R2–R7, rhombomeres 2 to 7. [Scale bar in B: 160 μm (A and B) and 110 μm (C and D).]
Placentation Alternately Requires a Transcriptionally Silent RXR, Then an Active RXRα.
The placentas of embryos carrying null mutations of both Rxra and Rxrb (Rxra/b-null placentas) display an early and severe developmental defect, already obvious at E9.5 and characterized by the absence of formation of the labyrinthine zone of the chorioallantoic placenta (27). In contrast, placentas of Rxraafo/Rxrb-null embryos (n = 3) appeared histologically normal at E10.5 (Fig. 5 E and F), and placentas of Rxra-null/Rxrbaf2o embryos analyzed at E9.5 (n = 3) were also unaffected. Along these lines, formation of the labyrinth normally takes place in placentas lacking all RXR AF1 activities (Rxraaf1o/Rxrb/g-null placentas) or all AF2 activities (Rxraaf2o/Rxrb/g-null placentas) (21). Thus, at E8.5, a transcriptionally silent RXRαAFo can, on its own, support the initial formation of the placental labyrinth from the chorionic plate. Labyrinthine agenesis is never associated with compound Rar/Rar- and Rxr/Rar-null mutant embryos (19, 32–34), but a similar, although less severe, abnormality (i.e., a labyrinthine hypoplasia) is associated with embryos lacking either Pparb or Pparg (35, 36). Altogether, these data indicate that the functional units involved in the initial stages of placentation, presumably RXRα/PPARβ and RXRα/PPARγ heterodimers, do not require a transcriptionally active RXRα.
Fig. 5.
Late (i.e., E14.5–E18.5), but not early (i.e., E8.5–E10.5), placentation steps are altered in Rxraafo mutants. Cross-sections through the thickness of placenta from WT and mutant embryos (genotypes as indicated). C, capillaries; CP, chorionic plate; L, labyrinth; S, spongiotrophoblast; T, labyrinthine trophoblast cell. The yellow brackets in A–D indicate the mean thickness of labyrinthine trabeculae. [Scale bar in F: 40 μm (A–D) and 80 μm (E and F).]
At later fetal stages of gestation, the placenta of Rxra-null and Rxraaf2o mutant displays a thickening of the labyrinthine trabeculae, which are interposed between maternal blood sinuses and fetal capillaries, and represent the placental barrier permitting nutrient and gas exchanges between the maternal and fetal circulations. This defect is compatible with fetal development to parturition, but probably results in a reduction of the placental efficiency accounting for the small weight of the Rxraaf2o mutants at birth (21, 37). Similarly to the above-mentioned mutants, Rxraafo fetuses (n = 6) showed abnormally thick labyrinthine trabeculae (compare yellow brackets in Fig. 5 A and C to B and D, respectively), and an ill-defined frontier between the spongiotrophoblast and the labyrinth. In contrast, Rxraaf1o/Rxrb/g-null mutants have normal placentas (21). Therefore, between E14.5 and the term of pregnancy (E19.0), a transcriptionally active RXRα AF2, but not RXRα AF1, is required for the proper differentiation of the trophoblast of the labyrinthine trabeculae. The nature of the RXRα dimerization partner involved in late placentation is still a matter of debate. On the one hand, Rxraaf2o/Rar (a, b, or g)-null mutants are obtained at the expected Mendelian ratio at E18.5 and are not more growth-deficient than Rxraaf2o mutants, therefore strongly suggesting that inactivating a Rar in the Rxraaf2o background does not worsen the placental defects (21). However, placental defects are responsible for the fetal deaths occurring at late stages of pregnancy in VAD rabbits and rats (38–40), and a thickening of the labyrinthine trabeculae is a hallmark of retinoid deficiency in the rat (41). These latter data suggest that, contrary to our previous thoughts (21), RA-liganded RXRα/RAR heterodimers may be involved in the late stages of the labyrinthine trophoblast cell differentiation. Rarg is apparently the main Rar coexpressed with Rxra in the mouse placenta (37), and among the 3 types of compound Rar/Rar-null mutants, only those lacking both Rara and Rarg are markedly growth retarded (32). It is therefore probable that RA-liganded RXRα/RARγ heterodimers, and possibly RXRα/RARα heterodimers, in which RXRα is transcriptionally active, play an important role in the establishment of functional maternal-fetal exchanges.
Conclusion
The role played by RXRs as either “active” or “silent” heterodimerization partners in the transcription of target genes, inferred from in vitro studies, has been a controversial issue (2). The present genetic study clearly shows that RXRα can be either transcriptionally active (thus acting in synergy with its RAR partner) or inactive within RXR/RAR heterodimers, depending on the developmental event under consideration. For instance, both RXRα AF1 and AF2 can be dispensable for heart development, whereas an active RXRα is required for ocular morphogenesis and the late steps in trophoblast differentiation. Assuming that a requirement of RXRα AF2 actually reflects the binding of an agonistic ligand (20), such a requirement raises the question of the possible existence and of the nature of a physiological RXR ligand(s) in vivo. However, because 9-cis RA is undetectable in rodent embryos (42, 43) and binds to both RARs and RXRs, it is doubtful that the RXR physiological ligand could be 9-cis RA (refs. 16 and 44, and references therein).
Experimental Procedures
Mutant Mice.
Experimental procedures to generate and genotype the Rxraafo mutant line are described in SI Materials and Methods. All mice, with a mixed (50%) C57BL/6–129/Sv (50%) genetic background, were housed in an animal facility licensed by the French Ministry of Agriculture (agreement B67–218-5) and all animal experiments were supervised by N.B.G. (agreement 67–205) in compliance with the European legislation on care and use of laboratory animals. Detection of the Rxra- and Rxrb-null mutations and deletion of RXRα AF2-AD core (corresponding to helix 12 amino acids 503–521; referred to as Rxrbaf2o) were as described (18, 28, 45).
RNA and Protein Analysis.
Total RNA preparation, nuclear protein extracts, and Northern and Western blots were according to standard procedures. RXRα protein was detected by using the 4RX3A2 monoclonal antibody (46) and peroxidase-conjugated goat anti-mouse IgG that was revealed by chemoluminescence according to the manufacturer's instructions (Amersham Biosciences, GE Healthcare Life Sciences). The membranes were washed with a 0.5 M glycine buffer (pH = 3), and subsequently probed with an anti-TBP monoclonal antibody (47).
Histology.
Embryos and fetuses were fixed in Bouin's fluid, embedded in paraffin, serially sectioned, and stained with Groat's Hematoxylin and Mallory's trichrome (48).
Supplementary Material
Acknowledgments.
We thank C. Rochette-Egly (Institut de Génétique et de Biologie Moléculaire) for the gift of antibodies, C. Birling-Ziegler, and the staff of Institut de Génétique et de Biologie Moléculaire and Institut Clinique de la Souris (ICS) common services. This work was supported by funds from Centre National de la Recherche Scientique, Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg, and the Association pour la Recherche sur le Cancer. B.M. was the recipient of fellowships from the French Ministry of Research, the Ligue Nationale contre le Cancer, Association pour la Recherche sur le cancer, and the Université Louis Pasteur de Strasbourg.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813143106/DCSupplemental.
References
- 1.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 2.Chambon P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 3.Laudet V, Gronemeyer H. The Nuclear Receptor Facts Book. London: Academic; 2002. [Google Scholar]
- 4.Rochette-Egly C, Chambon P. F9 embryocarcinoma cells: A cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol Histopathol. 2001;16:909–922. doi: 10.14670/HH-16.909. [DOI] [PubMed] [Google Scholar]
- 5.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: Structure and function. Curr Opin Cell Biol. 1998;10:384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 6.Perissi V, Rosenfeld MG. Controlling nuclear receptors: The circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 7.Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;15:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 8.Nagpal S, et al. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992;70:1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- 9.Nagpal S, Friant S, Nakshatri H, Chambon P. RARs and RXRs: Evidence for two autonomous transactivation functions (AF-1 and AF-2) and heterodimerization in vivo. EMBO J. 1993;12:2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanguedolce MV, Leblanc BP, Betz JL, Stunnenberg HG. The promoter context is a decisive factor in establishing selective responsiveness to nuclear class II receptors. EMBO J. 1997;16:2861–2873. doi: 10.1093/emboj/16.10.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bommer M, Benecke A, Gronemeyer H, Rochette-Egly C. TIF2 mediates the synergy between RARalpha 1 activation functions AF-1 and AF-2. J Biol Chem. 2002;277:37961–37966. doi: 10.1074/jbc.M206001200. [DOI] [PubMed] [Google Scholar]
- 12.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discovery. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 14.Rastinejad F. Retinoid X receptor and its partners in the nuclear receptor family. Curr Opin Struct Biol. 2001;11:33–38. doi: 10.1016/s0959-440x(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 15.DiRenzo J, et al. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol. 1997;17:2166–2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calléja C, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2005;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 18.Kastner P, et al. Genetic analysis of RXR alpha developmental function: Convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 19.Kastner P, et al. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Mascrez B, et al. The RXRalpha ligand-dependent activation function 2 (AF-2) is important for mouse development. Development. 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- 21.Mascrez B, et al. Differential contributions of AF-1 and AF-2 activities to the developmental functions of RXR alpha. Development. 2001;128:2049–2062. doi: 10.1242/dev.128.11.2049. [DOI] [PubMed] [Google Scholar]
- 22.Sucov HM, et al. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 23.Matt N, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 25.Krezel W, et al. RXRγ null mice are apparently normal and compound RXRα+/−/RXRβ−/−/RXRγ−/− mutant mice are viable. Proc Natl Acad Sci USA. 1996;93:9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love JD, et al. The structural basis for the specificity of retinoid-X receptor-selective agonists: New insights into the role of helix H12. J Biol Chem. 2002;277:11385–11391. doi: 10.1074/jbc.M110869200. [DOI] [PubMed] [Google Scholar]
- 27.Wendling O, Chambon P, Mark M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc Natl Acad Sci USA. 1999;96:547–551. doi: 10.1073/pnas.96.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastner P, et al. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 29.Dupé V, Ghyselinck NB, Wendling O, Chambon P, Mark M. Key roles of retinoic acid receptors alpha and beta in the patterning of the caudal hindbrain, pharyngeal arches and otocyst in the mouse. Development. 1999;126:5051–5059. doi: 10.1242/dev.126.22.5051. [DOI] [PubMed] [Google Scholar]
- 30.Wendling O, Ghyselinck NB, Chambon P, Mark M. Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development. 2001;128:2031–2038. doi: 10.1242/dev.128.11.2031. [DOI] [PubMed] [Google Scholar]
- 31.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 32.Lohnes D, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn C, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 34.Ghyselinck NB, et al. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int J Dev Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- 35.Barak Y, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 36.Barak Y, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapin V, Dollé P, Hindelang C, Kastner P, Chambon P. Defects of the chorioallantoic placenta in mouse RXRalpha null fetuses. Dev Biol. 1997;191:29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- 38.Mason KE. Foetal death, prolonged gestation, and difficult parturition in the rat as a result of vitamin A deficiency. Am J Anat. 1935;57:303–349. [Google Scholar]
- 39.Lamming GE, Salisbury GW, Hays RL, Kendall KA. The effect of incipient vitamin A deficiency on reproduction in the rabbit. II. Embryonic and fetal development. J Nutr. 1954;52:227–236. doi: 10.1093/jn/52.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Howell JM, Thompson JN, Pitt GA. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. II. The female rat. J Reprod Fertil. 1964;7:251–258. doi: 10.1530/jrf.0.0070251. [DOI] [PubMed] [Google Scholar]
- 41.Noback CR, Takahashi YI. Micromorphology of the placenta of rats reared on marginal vitamin-A-deficient diet. Acta Anat. 1978;102:195–202. doi: 10.1159/000145637. [DOI] [PubMed] [Google Scholar]
- 42.Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev Dyn. 1995;202:312–323. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- 43.Matt N, et al. Contribution of cellular retinol-binding protein type 1 to retinol metabolism during mouse development. Dev Dyn. 2005;233:167–176. doi: 10.1002/dvdy.20313. [DOI] [PubMed] [Google Scholar]
- 44.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev. 2006;64:532–538. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 45.Mascrez B, et al. Ligand-dependent contribution of RXRbeta to cholesterol homeostasis in Sertoli cells. EMBO Rep. 2004;5:285–290. doi: 10.1038/sj.embor.7400094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rochette-Egly C, et al. Detection of retinoid X receptors using specific monoclonal and polyclonal antibodies. Biochem Biophys Res Commun. 1994;204:525–536. doi: 10.1006/bbrc.1994.2491. [DOI] [PubMed] [Google Scholar]
- 47.Brou C, et al. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21:5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mark M, et al. Two rhombomeres are altered in Hoxa-1 mutant mice. Development. 1993;113:319–338. doi: 10.1242/dev.119.2.319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.