Abstract
Anxiety disorders are the most prevalent mental disorders in developed societies. Although roles for the prefrontal cortex, amygdala, hippocampus and mediodorsal thalamus in anxiety disorders are well documented, molecular mechanisms contributing to the functions of these structures are poorly understood. Here we report that deletion of Lynx2, a mammalian prototoxin gene that is expressed at high levels in anxiety associated brain areas, results in elevated anxiety-like behaviors. We show that LYNX2 can bind to and modulate neuronal nicotinic receptors, and that loss of Lynx2 alters the actions of nicotine on glutamatergic signaling in the prefrontal cortex. Our data identify Lynx2 as an important component of the molecular mechanisms that control anxiety, and suggest that altered glutamatergic signaling in the prefrontal cortex of Lynx2 mutant mice contributes to increased anxiety-related behaviors.
Keywords: anxiety, LYNX2, nicotinic, prefrontal cortex
Fear and anxiety are adaptive functions that help us survive, which when prolonged or intense can lead to anxiety disorders. Anxiety disorders are the most prevalent psychiatric disorders, costing more than $40 billion annually in the United States (1, 2). Our knowledge of the biological basis of anxiety disorders is based largely on studies of animal models of fear and anxiety (3–6), and human brain imaging studies (7). That work has implicated several brain structures in the regulation of anxiety, including the amygdala, medial prefrontal cortex (mPFC), mediodorsal thalamus (MDT), CA1 region of hippocampus and the bed nucleus of stria terminalis (8). Lynx2 is a member of a family of prototoxin genes that encode small proteins expressed in distinct patterns in the brain that can modulate neuronal nicotinic acetylcholine receptor (nAChR) activity in vitro (9–11), and contribute to neuronal function and viability in vivo (12). Interestingly, Lynx2 is expressed specifically in many structures involved in the control of anxiety, including the prefrontal cortex, the amygdala, the hippocampus and the mediodorsal thalamus as well as in other regions such as dentate gyrus and specific brainstem nuclei (www.stjudebgem.org). Given the expression of Lynx2 in anxiety associated brain areas, the ability of the LYNX family of proteins to modulate neurotransmitter receptors, and the fact that mood and anxiety disorders are thought to be due to altered neurotransmission (13, 14), we sought to test the involvement of the Lynx2 gene in fear and anxiety through studies of Lynx2 knockout (KO) mice.
Results
Loss of Lynx2 Has No Effect on Gross Motor Behavior or Sensory Processing.
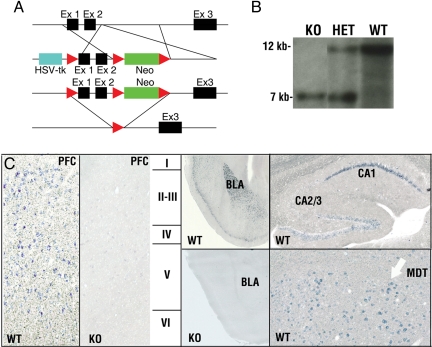
Lynx2−/− mice were produced using standard gene targeting procedures in C57BL/6 embryonic stem (ES) cells (Fig. 1A). After crossing to a germline Cre recombinase expressing line (15), we generated inbred C57BL/6 mice in which two exons of Lynx2 gene were deleted. Deletion of these exons in genomic DNA was demonstrated by Southern blot analysis (Fig. 1B). Loss of Lynx2 mRNA expression in the brains of the knockout mice was verified by in situ hybridization, as illustrated in Fig. 1C for the prefrontal cortex and amygdala.
Fig. 1.
Map of Lynx2−/− targeting construct, Southern Blot analysis confirming the deletion of Lynx2 gene and in situ hybridization on Lynx2−/− and WT brain sections with Lynx2 probe. (A) Map of the targeting construct used to generate Lynx2-conditional KO mice. These mice were bred to EIIa::cre mice to generate the Lynx2-null-mutant mice, in which exons 1 and 2 and the neo cassette were deleted. (B) Southern Blot analysis of the genomic DNAs of KO, heterozygous and WT mice. (C) In situ hybridization on Lynx2−/− and WT brain sections with Dig-labeled Lynx2 probe. Prefrontal cortex, hippocampus, amygdala, and mediodorsal thalamus are shown. BLA, basolateral amygdala; MDT, mediodorsal thalamus.
The growth rate and longevity of Lynx2−/− mice were normal compared with those in WT mice, and they did not display any obvious anatomical abnormalities. Given the high levels of Lynx2 expression in the brain, a battery of tests was conducted to assess whether loss of Lynx2 leads to alterations in behavior. To determine whether there were any major motor abnormalities in Lynx2−/− mice, we first used the open-field exploration test. No significant differences were noted between Lynx2−/− and wild-type (WT) mice in terms of distance moved, percentage of time spent moving, the mean velocity of movement, or the time spent in the center of the open field (thigmotaxis) [supporting information (SI) Fig. S1]. Lynx2−/− mice did not display any ataxia or tremor, and normal sensory responses were observed in ear-twitch and eye-blink reflexes (16), in the hotplate test, and buried food assay (Fig. S1). These results establish that the Lynx2−/− animals display grossly normal motor and sensory functions.
Lynx2−/− Display Elevated Fear and Anxiety-Like Behaviors.
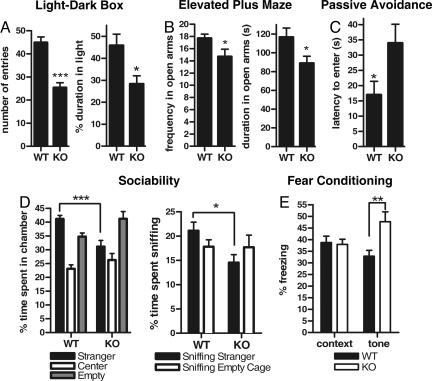
The failure to observe changes in thigmotaxis in Lynx2−/− mice is noteworthy, since thigmotaxis has been used as a test for anxiety-like behaviors. However, given the high levels of Lynx2 expression in brain regions involved in anxiety, further tests were conducted to assess whether loss of Lynx2 leads to alterations in other anxiety-related behaviors. The light-dark exploration test is based on the natural aversion of rodents to brightly illuminated spaces, and it is thought to measure aspects of generalized anxiety (17). Using this test, we observed that Lynx2−/− (n = 10) animals spent less time in the light compared to WT (n = 10) mice (P < 0.01) (Fig. 2A) and made fewer transitions to light compartment (P < 0.0001), suggesting an increased level of generalized anxiety. As a second test for anxiety-like behaviors, we used the social interaction test, which is based on the tendency of mice to spend time with a novel mouse rather than an empty cage. Highly anxious mice avoid this type of social interaction, and prefer to remain alone (18). We observed that Lynx2−/− (n = 8) mice, in contrast to WT (n = 10) controls, spend significantly less time (P < 0.001) with the novel mouse and less time sniffing and interacting with the novel mouse (P < 0.05), preferring to spend time in the empty compartment (Fig. 2D). Rodents also have an aversion to elevated open spaces that is reflected in their behavior when placed on an elevated plus-maze (19). In this test, Lynx2−/− mice (n = 10) made fewer transitions to (P < 0.05) and spent less time in the open arms than WT mice (n = 10) (P < 0.05) (Fig. 2B). Finally, to measure baseline anxiety levels and learning in Lynx2−/− and WT mice, we used a 3-day passive avoidance task (20). On Day 1, we placed the animals inside the light compartment to habituate the mice to the apparatus and measured the latency to go into the dark compartment upon elevation of the guillotine door that separates the two compartments. We observed that Lynx2−/− mice (n = 10) spent significantly more time than WT mice (n = 10) before they entered the mysterious dark compartment (P < 0.05) (Fig. 2C). On Day 2, mice were trained to associate the dark compartment with a mild electric shock, and their learning behavior was tested on Day 3. When we compared their latencies to enter dark chamber on Day 2 and Day 3, we observed that both Lynx2−/− and WT mice displayed similar learning behavior (Fig. S1). We conclude from these data that Lynx2−/− animals exhibit clear anxiety-like behaviors that are not prominent in WT mice.
Fig. 2.
Lynx2−/− mice have elevated fear and anxiety-like behavior. (A) In light-dark box test, Lynx2−/− mice spent less time in light compartment (P < 0.01) and made less number of transitions to the light than WT mice (P < 0.0001), showing elevated anxiety-like behavior. (B) In elevated plus-maze test, Lynx2−/− mice spent less time in open arms (P < 0.05) and made less transitions to the open arms than WT mice (P < 0.05). (C) On Day 1 of passive avoidance test, Lynx2−/− mice spent significantly more time than WT mice before they entered the dark compartment (P < 0.05). (D) In social interaction test, KO mice spent less time in a compartment with a stranger mouse (P < 0.001) and spent less time sniffing and interacting with the stranger mouse than WT mice (P < 0.05). (E) In fear conditioning test, during audio-conditioned learning task, Lynx2−/− mice froze more times than WT mice (P < 0.01).
We next tested Lynx2−/− mice for Pavlovian fear conditioning. The mice were trained on Day 1 to associate a mild electric shock to a tone, the conditioned stimulus (CS). The following day, the mice were placed in the training environment without any auditory cue, and their behavior observed as a measure of contextual fear. In this test, Lynx2−/− mice “froze” upon entry into the chamber as often as WT mice (Fig. 2E). The mice were then placed in a novel environment and the number of freezes recorded both in the absence of the CS, and in response to the CS. Lynx2−/− mice (n = 12) exhibited significantly more freezing behavior to the tone than WT mice (n = 12) during the CS (F (1, 22) = 11.6403, P < 0.005), suggesting either an enhanced fear response in Lynx2−/− animals, or an enhancement of auditory-cued-associative learning due to loss of LYNX2 (Fig. 2E).
LYNX2 Forms Stable Associations with nAChRs and Modulates Their Function.
It has been well documented that both nicotine and nAChRs can influence anxiety-related behaviors (21, 22). The Lynx family of prototoxin genes encode small Ly6 like molecules related to snake venom alpha neurotoxins that are expressed in the nervous system as glycophosphoinositol-linked cell surface proteins (23). LYNX1 can modulate both the desensitization kinetics and single channel conductance of nAChRs (10), and its deletion in vivo leads to altered responses to nicotine (12). To find out whether the effect of Lynx2 on anxiety-related behaviors might be through an effect on nAChRs, we tested whether LYNX2 can modulate nAChR activity.
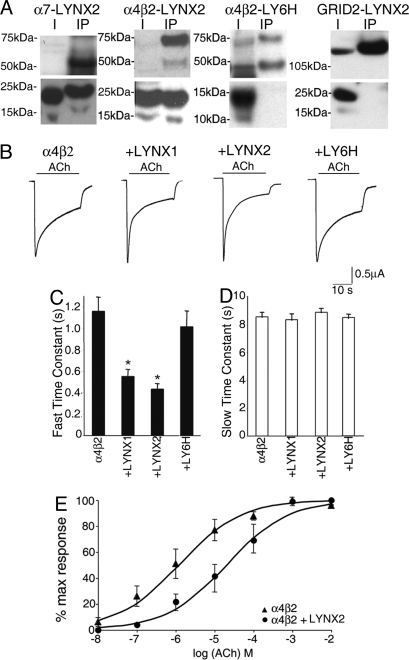
As an initial test for the direct action of LYNX2 on nAChRs, two types of experiments were conducted. First, we assayed the nAChR binding activity of LYNX2 by co-immunoprecipitation studies in HEK293 cells. As shown in Fig. 3A, LYNX2 can bind to the widely expressed nAChR subtypes, α7, α4β2. The specificity of these interactions is demonstrated by the failure of LYNX2 to form complexes with the co-expressed GRID2 glutamate receptor, and by the failure of the closely related LY6H protein to form complexes with any of the receptors assayed. In separate experiments, we have observed that LYNX2 can also bind to α4β4 and muscle nAChRs, but that it does not bind to α2β2 receptors (data not shown). From these data it is evident that LYNX2 and LYNX1 binding to nAChRs is specific and similar, although these two proteins are expressed in distinct neuronal populations.
Fig. 3.
LYNX2 stably associates with nAChRs and enhances the desensitization and decreases the ACh sensitivity of nAChRs. (A) Membrane extracts from HEK293 cells expressing α7-LYNX2, α4β2-LYNX2, α4β2-LY6H, and GRID2- LYNX2 were analyzed. The first lane in each blot shows a sample of the membrane extract input and is indicated with letter I. The second lane in each blot contains the immunoprecipitation fractions incubated with an antibody. The upper blots show that α7 (≈52 kDa), α4 (≈69 kDa) and β2 (≈45 kDa) subunits immunoprecipitated with α7 and α4 antibodies respectively. LYNX2 was also detected with anti-flag antibody in the IP fraction (≈15–20 kDa), demonstrating that LYNX2 form stable complexes with both α7 and α4β2 nAChRs, whereas LY6H did not IP with α4β2 nAChRs (lower blots). LYNX2 did not form stable complexes with GRID2 (≈114 kDa) receptor when immunoprecipitated with GRID2 antibody. (B) Representative recordings of voltage-clamped oocytes expressing α4β2 nAChRs alone, or in combination with LYNX1, LYNX2, and LY6H. The inward currents were evoked by 20-second periods of superfusion (horizontal calibration bar) with external saline containing 1 mmol/l ACh. Oocytes coexpressing α4β2 nAChRs with either LYNX1 or LYNX2 showed a significantly faster initial desensitization immediately after the peak response upon agonist application. Co-expression of LY6H had no effect on the desensitization kinetics. (C and D) Bar graphs representing the values of the fast and slow time constants obtained as previously described (10). In oocytes coexpressing α4β2 nAChRs with either LYNX1 or LYNX2, the fast time constant (C) is significantly faster, whereas the slow time constant during the second phase remained the same (D). Both constants are unaffected in oocytes coexpressing ly6h. (E) Dose-response curves for ACh in oocytes expressing α4β2 (triangles) and α4β2 nAChRs co-expressing LYNX2 (circles). Each plotted value is the mean ± SEM of 5 oocytes. The normalized peak amplitudes (I/Imax) at each indicated ACh concentration were fitted to the Hill equation: I/Imax[ACh]nH/([ACh]nH+EC50)nH (drawn lines). The fitted parameters were EC50 = 1.2 μmol/l for α4β2, 21 μmol/l for α4β2 +LYNX2, nH = 0.55 in both cases.
To determine whether LYNX2 interactions with AChRs could alter their properties, we measured macroscopic currents elicited by acetylcholine (ACh) using two-electrode voltage clamp recordings in Xenopus oocytes expressing α4β2 nAChRs alone, or together with LYNX1, LYNX2 and LY6H. In all cases, upon application of ACh, inward currents peaked within 1 second and then decayed with a biphasic profile to a steady plateau. Desensitization was measured by fitting two exponential equations to the desensitization currents during ACh application and calculating the fast (Fig. 3C) and slow (Fig. 3D) time constants as previously described (10). In oocytes co-expressing either LYNX1 or LYNX2, the ACh evoked-responses showed a faster desensitization compared to oocytes expressing either α4β2 alone or co-injected with LY6H. As shown in Fig. 3C, the average value for the fast time constant is significantly less in oocytes co-expressing LYNX1 and LYNX2, and is not affected in oocytes co-expressing LY6H. No difference was observed in the slow time constant. Faster desensitization kinetics may reflect differences in ligand sensitivity, as we have observed when LYNX1 associates with α4β2 receptors (10), and conversely in Lynx1−/− neurons (12). To address this point, we performed dose-response experiments with ACh in α4β2 oocytes with or without LYNX2. Dose-response curves, obtained by normalizing the peak amplitudes at each ACh concentration to the maximal peak amplitude at 1 mmol/l ACh, revealed a shift of ≈20-fold toward higher ACh concentrations in the presence of LYNX2 (α4β2 EC50 = 1.2 μmol/l, α4β2+LYNX2 EC50 = 21 μmol/l) (Fig. 3E). We conclude that LYNX2 can directly interact and modulate nAChRs in vitro, and suggest that this interaction may provide a mechanistic underpinning for the behavioral data discussed above.
Altered Responses to Nicotine in Lynx2−/− Mice.
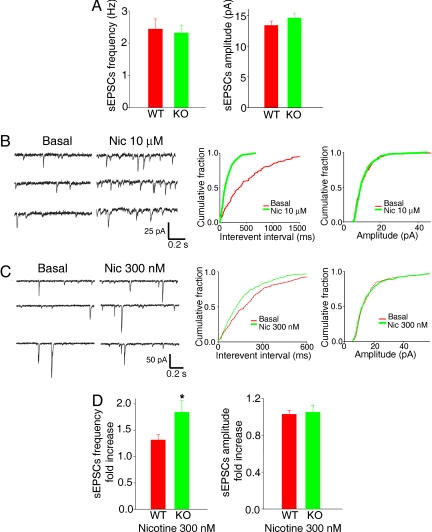
Taken together, the behavioral and biochemical studies we report above suggest that loss of LYNX2 could result in increased fear and anxiety due to enhanced nAChR activity in cells that participate in circuits controlling these behaviors. It has been proposed that enhanced activation of medial prefrontal cortex (mPFC) by inputs from the mediodorsal thalamus (MDT) might be involved in the control of fear and anxiety (24). Furthermore, activation of nicotinic receptors in preterminals projected from MDT to mPFC can enhance glutamate release to layer V neurons (25). Since Lynx2 is expressed at high levels in the MDT, we reasoned that measurements of sEPSCs in layer V pyramidal neurons might reveal altered glutamate release in response to enhanced nAChR activity in Lynx2−/− mice. To test this idea, we performed whole cell recordings in the layer V pyramidal neurons in mPFC brain slices. When we compared the frequencies and amplitudes of sEPSCs, we observed no significant difference between WT and Lynx2−/− mice (Fig. 4A).
Fig. 4.
Lynx2 null mutation enhances the ability of nAChR stimulation to increase sEPSCs frequency compared to WT mice in layer V pyramidal neurons in mPFC. (A) Bar graphs show the mean sEPSC frequency (left) and amplitude (right) in WT and Lynx2−/− mice layer V pyramidal neurons (WT: 6 mice, 12 cells; Lynx2−/−: 6 mice, 14 cells, P > 0.05). (B) Representative traces showing the effects of 10 μmol/l nicotine application on the sEPSCs (Left). Holding potential was −70 mV in voltage clamp mode. Right shows the cumulative curves of frequency and amplitude before and after application of 10 μmol/l nicotine. Nicotine increased the frequency of sEPSCs significantly assessed by Kolmogorov-Smimov analysis (P < 0.0001). (C). Representative traces and cumulative curves show that 300 nmol/l nicotine application increased the frequency of sEPSCs. (D). Bar graphs show the mean sEPSC frequency and amplitude fold increase after 300 nmol/l nicotine application in WT and Lynx2−/− mice. The response to nicotine was significantly more enhanced in Lynx2−/− mice than in WT controls (WT: 5 mice, 11 cells; Lynx2−/−: 5 mice, 11 cells). (*, P < 0.05.)
We then examined the responses to nicotine in WT and Lynx2−/− mice. Consistent with previous reports (25), the frequency of sEPSCs in layer V pyramidal neurons in mPFC was significantly increased by application of 10 μmol/l nicotine (Fig. 4B). This increase was blocked by TTX, suggesting that activation of sodium channels in presynaptic terminals is required for this effect. The effects of 10 μmol/l nicotine application were also blocked by the nAChR antagonist Dihydro-β-erythroidine hydrobromide (10 μmol/l); implicating the activation of high affinity nAChRs in this effect. These results are consistent with previous reports demonstrating that high affinity α4β2nAChRs located in the thalamocortical projections are involved in nicotine induced increases in sEPSCs in layer V pyramidal cells in mPFC (25). Since our results demonstrate that LYNX2 can modulate the sensitivity of nAChRs to nicotine in vitro, we decided to test whether there are any differences in sEPSCs between WT and Lynx2−/− mice at lower concentration of nicotine. We found that 300 nmol/l nicotine application increased sEPSC frequency in both WT and Lynx2−/− mice (Fig. 4 C and D). Interestingly, the response to nicotine was significantly more enhanced in Lynx2−/− mice than in WT controls: nicotine (300 nmol/l) caused a 1.84 ± 0.22 fold increase of sEPSC frequency in Lynx2−/−mice (Fig. 4D, n = 11 cells, 5 mice) compared to 1.31 ± 0.10 fold increase of sEPSC frequency in WT mice (Fig. 4D, n = 11 cells, 5 mice) (P < 0.05). Given the in vitro actions of LYNX2 on nAChRs, and the high levels of expression of Lynx2 in MDT, our data suggest that the increased sensitivity of mPFC layer V neurons to nicotine in Lynx2−/− mice may result from release of inhibition by LYNX2 on nAChRs due to the null mutation in these mice. As a consequence of the enhanced nAChR activation we have observed in layer V neurons of the mPFC, one might expect supranormal activation of the basolateral amygdala by the mPFC, contributing to the increased fear and anxiety-like behaviors documented in the Lynx2−/− mice.
Discussion
Our data establish several important findings regarding the role of the Lynx2 gene in vivo. First, Lynx2−/− mice demonstrate increased anxiety-like behaviors as measured by three independent rodent assays of anxiety and increased conditioned fear. Second, binding of LYNX2 to nAChRs in vitro results in increased receptor desensitization and decreased affinity for acetylcholine. Consequently, loss of LYNX2 is predicted to cause an increase in nAChR activity in Lynx2 expressing neurons. Third, deletion of the Lynx2 gene results in increased glutamatergic activity in response to nicotine in layer V neurons of the mPFC. Taken together, these data suggest that Lynx2 has an important and specific role in setting cholinergic tone in cells known to participate in the control of anxiety. Furthermore, when compared with previous studies of LYNX1, these data also establish that the LYNX family of small modulator proteins, while biochemically similar, subserve quite different and important biological functions in vivo.
The behavioral consequences of Lynx2 deletion are interesting with respect to previous studies of fear and anxiety. Results from the light-dark box assay, the social interaction test, and the elevated plus-maze all indicate an increase in generalized anxiety levels in the mutant mice. Although the basolateral amygdala (BLA) has been implicated in both anxiety and fear, the neuroanatomical relationships between these emotions are not completely understood. Because a wide variety of studies have shown that the BLA is required for auditory fear conditioning responses and Lynx2 is heavily expressed in BLA, we also tested Lynx2−/− mice for fear conditioning paradigm. Although enhanced freezing to tone in fear conditioning tests in Lynx2−/− mice might also be related to an increase in generalized anxiety, enhanced associative learning during the fear conditioning task could also explain this phenotype. Given the ability of LYNX2 to bind to and modulate nAChRs, we believe that the behavioral effects of LYNX2 deletion may result from alterations in nAChR function in cells participating in anxiety-related circuits. For example, the glutamatergic circuitry from MDT to mPFC to BLA has been implicated in the regulation of anxiety-related behaviors (24). We have demonstrated here that nicotine acts through high affinity nAChRs to increase glutamate release from presynaptic terminals in the mPFC, and that loss of LYNX2 leads to increased glutamatergic activity. Given previous studies demonstrating that high affinity nAChRs are present in presynaptic terminals from the MDT to the mPFC (25), and the high expression of Lynx2 in the MDT, the most parsimonious interpretation of our results is that loss of LYNX2 in the MDT results in elevated nAChR activity in thalamocortical neurons, resulting in enhanced release of glutamate from these terminals. We propose that the consequent increased activity of layer V neurons in mPFC of Lynx2−/− mice contributes to the elevation in anxiety-related behaviors in these mice.
Direct comparisons between our results and previous studies implicating nAChR activity in the control of anxiety are difficult because of the large numbers of receptor subtypes and their complex expression patterns in the brain. Nevertheless, it is well established that nicotine and nAChRs can impact anxiety levels in both humans and rodents (14, 26). In humans, although smokers generally accept nicotine as an anxiolytic (27), nicotine was shown to have an anxiogenic effect in several studies (28, 29). Furthermore, both genetic background and genetic perturbations of nicotinic receptors in rodents result in variations in anxiety-related phenotypes (21, 30, 31).
Although the effects of LYNX2 loss in layer V pyramidal neurons in mPFC are likely to be mediated by high affinity-α4β2 receptors, our in vitro data suggest that LYNX2 may modulate the activities of several nAChR types. We expect, therefore, that the actions of LYNX2 may be mediated by several different nAChRs, so long as they are co-expressed with LYNX2. Thus, at each site of anxiety-related circuitry where Lynx2 is expressed, the precise mechanisms by which LYNX2 contributes to the regulation of anxiety may differ. Given these complexities, further cell specific genetic studies of LYNX2 and its impact on anxiety-related behaviors may help in the identification of specific cell types controlling anxiety.
Materials and Methods
Cloning.
Lynx2 cDNA was subcloned into pBluscript-SK for in situ hybridization probe. Ly6 domains and GPI anchorage sites and signals of Lynx1, Lynx2, and Ly6h were subcloned into pFlag-CMV-1. cDNAs encoding nAChR α4, β2, α7, β4, α1, β1, γ, and δ subunits were kind gifts from Jerry A. Stitzel, Marc Ballivet and José Ramirez-LaTorre. They were subcloned into pCS2+ in fusion with flag tags and used for IP and in vitro transcription experiments. cDNAs encoding Lynx1, Lynx2, and Ly6h were also subcloned into pCS2+ and used for in vitro transcription experiments. The expression plasmid for Grid2 was provided by Zhenyu Yue.
In Situ Hybridization.
Adult brain sections were prepared as described (32). Digoxigenin (Dig-) labeled riboprobe was transcribed using 2 μmol/l Dig-NTP (Boehringer-Mannheim) in the transcription reaction. (Please see SI Materials and Methods for details.)
Expression and IP from HEK293T Cells.
HEK 293T cells were transfected by calcium phosphate precipitation with the expression vectors containing the cDNAs of Lynx1, Lynx2, Ly6h, α7 nAChR, Flag-tagged α1, α4, β2, β4, γ and δ nAChR subunits and Flag-tagged Grid2 receptor. Two days after transfection, cells were washed, harvested, and used for IP as described (10).
Oocyte Electrophysiology.
The cRNAs for Lynx1, Lynx2, Ly6h and nAChR subunits (α4, β2, β4, α1, β2, γ and δ) were synthesized with T7 or SP6 RNA polymerases (mMESSAGE mMACHINE, Ambion, Austin, TX) through in vitro transcription. To quantify the yield of the synthesized transcripts, they were analyzed by agarose gel electrophoresis. Xenopus oocytes were injected with 0.5 ng of the cRNA encoding each nAChR subunit and with 3 ng of LYNX1, LYNX2, or LY6H (20 nl per oocyte). The electrophysiological recordings were done as described (10) .
Open-Field Exploration Test.
Experimenter was blind as to the genotype of the mice in the behavior experiments. Procedures for the behavior tests were approved by the Institutional Animal Care and Use Committee of the Rockefeller University. Animals were group housed in a 12 hour dark, 12 hour light cycle, and tests were performed during the light phase. For all behavior studies the controls were age matched C57BL/6 WT mice. The behavior tests were performed on mice between 13 and 16 weeks of age. Four mice were tested every session in separate arenas. The containers were cleaned with isopropanol before each session. The test room was illuminated with a 60-W bulb in the middle of the room. Ethovision software and equipment (Noldus) were used for open field exploration test. The camera and software distinguished the black test mice from the white background. The computer recorded the movement patterns of the test mice for 20 minutes and then calculated the reported behaviors.
Light-Dark Box Test.
The test apparatus (45 × 25 × 30 cm) consisted of light and dark compartments separated by an open door. The dark compartment was one third of the total size and had a lid on top. The light compartment was illuminated by a 60-W bulb. Mice behavior was recorded for 10 minutes, and the time spent in each compartment and number of transitions between compartments was analyzed by Ethovision software.
Social Interaction Test.
The apparatus for social interaction test was built based on the design described previously (18). (See SI Materials and Methods for details.)
Passive Avoidance Test.
PACS-30 Passive/Active avoidance box from Columbus Instruments (USA) was used for these studies. (See SI Materials and Methods for details.)
Fear Conditioning Test.
A conditioning chamber from Med Associates was used for fear conditioning tests as described (12). On training day, the mice were presented with two tones (30 seconds, 80 db) paired with foot shocks (2 seconds, 0.5 mA). (See SI Materials and Methods for details.)
Statistical Analysis of Behavior Tests.
GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA) was used for statistical analysis of the data. Two-tailed Student t tests were used for analyzing the open field exploration behavior, hot-plate test, olfaction test, elevated plus-maze test, light-dark box test, social interaction test and baseline anxiety measurements of passive avoidance test. To analyze data from the passive avoidance learning and fear conditioning tests, two-way analysis of variance with Bonferroni's post tests was used.
Whole Cell Recordings in Medial Prefrontal Cortex Slices.
Medial prefrontal cortex brain slices were prepared from 4–5-week-old C57BL/6 and Lynx2−/− mice in adherence with protocols approved by the Rockefeller University Animal Care and Use Committee. (See SI Materials and Methods for details.)
Supplementary Material
Acknowledgments.
We thank Thomas Jessell at Columbia University for generously providing Lynx2−/− ES cells used for generating Lynx2−/− mice; Turgay Tekinay and Tanya Stevens for critically reading the manuscript; and Aylin Aslantas, Wendy Lee, Marta Slimak, Mahmut Bekin, and Daryll Wilson for their technical help. This work was supported by the Howard Hughes Medical Institute, Irma L. and Abram S. Croll Charitable Trust, and by grants from the National Institutes of Health (DA-17279 and DA 10044) and Deutsche Forschungsgemeinschaft (SFB 665).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813109106/DCSupplemental.
References
- 1.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PE, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 3.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Heisler LK, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GW, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 7.Rauch SL, Shin LM. Kenneth L, Davis D. C., Joseph T., Coyle, Nemeroff C., editors. Neuropsychopharmacology: The Fifth Generation of Progress. 2002:953–966. (American College of Neuropsychopharmacology) [Google Scholar]
- 8.Davis M. In: Neuropsychopharmacology: The Fifth Generation of Progress. Davis KL, Coyle JT, Nemeroff C, editors. Washington, DC: American College of Neuropsychopharmacology; 2002. pp. 931–951. [Google Scholar]
- 9.Miwa JM, et al. lynx1, an Endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 10.Ibanez-Tallon I, et al. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 11.Chimienti F, et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 12.Miwa JM, et al. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51:587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Neumeister A, Daher RJ, Charney DS. Anxiety disorders: Noradrenergic neurotransmission. Handb Exp Pharmacol. 2005:205–223. doi: 10.1007/3-540-28082-0_8. [DOI] [PubMed] [Google Scholar]
- 14.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 17.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 18.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 19.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 20.Zorrilla EP, et al. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): Mediation by CRF1 receptors. Brain Res. 2002;952:200–210. doi: 10.1016/s0006-8993(02)03345-0. [DOI] [PubMed] [Google Scholar]
- 21.Salas R, et al. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booker TK, et al. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Bamezai A. Mouse Ly-6 proteins and their extended family: Markers of cell differentiation and regulators of cell signaling. Arch Immunol Ther Exp (Warsz) 2004;52:255–266. [PubMed] [Google Scholar]
- 24.Swanson CJ, et al. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 25.Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- 26.Morissette SB, et al. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychol Bull. 2007;133:245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- 27.Pomerleau OF. Nicotine as a psychoactive drug: Anxiety and pain reduction. Psychopharmacol Bull. 1986;22:865–869. [PubMed] [Google Scholar]
- 28.Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- 29.Breslau N, Klein DF. Smoking and panic attacks: An epidemiologic investigation. Arch Gen Psychiatry. 1999;56:1141–1147. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- 30.Labarca C, et al. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci USA. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross SA, et al. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.