Abstract
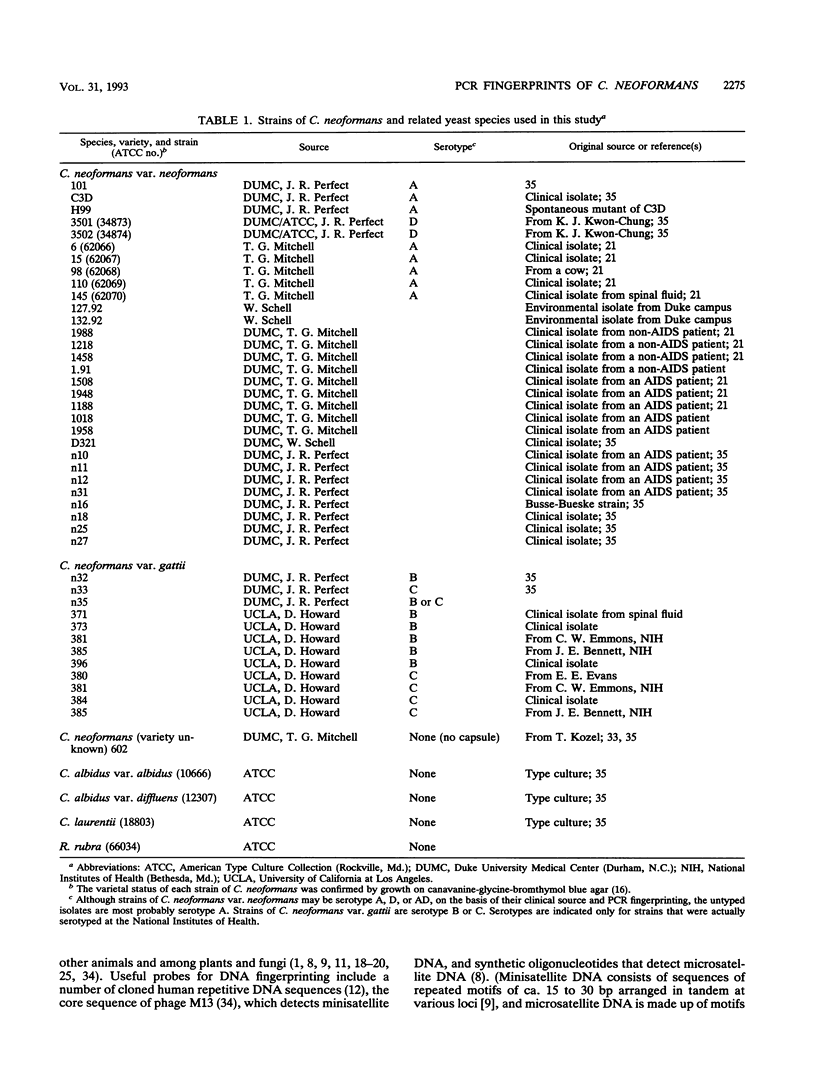
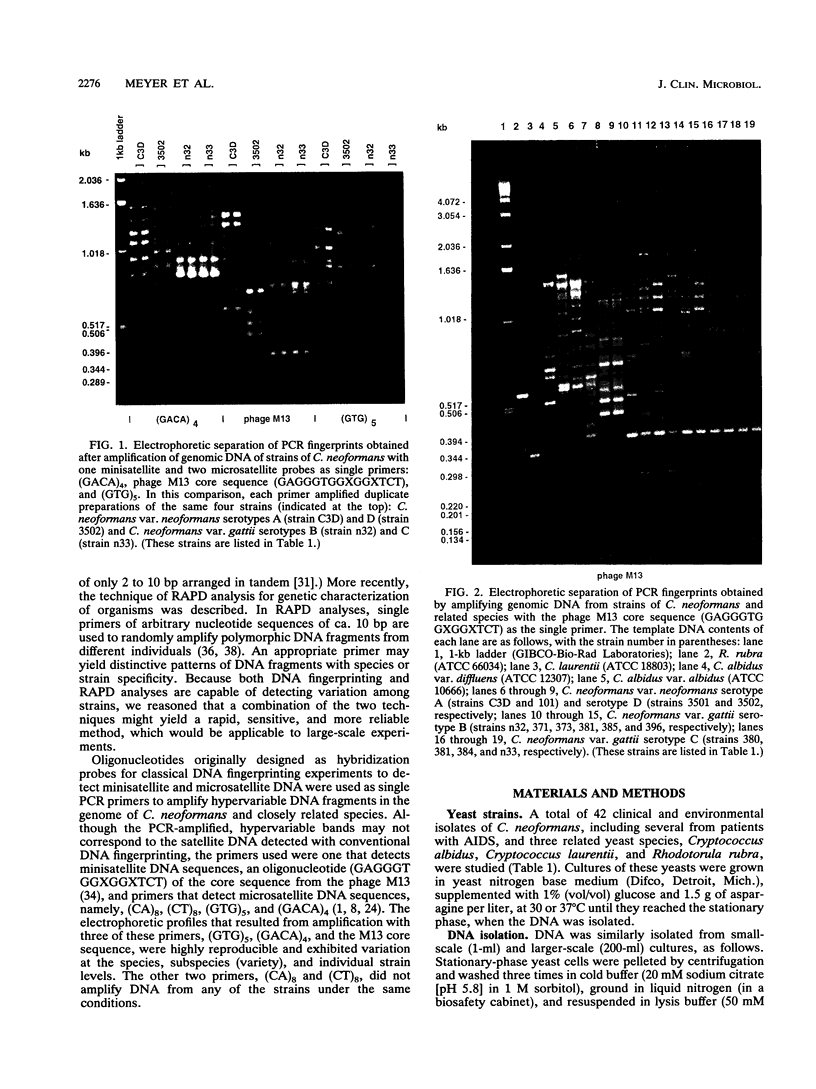
In conventional DNA fingerprinting, hypervariable and repetitive sequences (minisatellite or microsatellite DNA) are detected with hybridization probes. As demonstrated here, these probes can be used as single primers in the polymerase chain reaction (PCR) to generate individual fingerprints. Several conventional DNA fingerprinting probes were used to prime the PCR, yielding distinctive, hypervariable multifragment profiles for different strains of Cryptococcus neoformans. PCR fingerprinting with the oligonucleotide primers (GTG)5, (GACA)4, and the phage M13 core sequence (GAGGGTGGXGGXTCT), but not with (CA)8 or (CT)8, generated DNA polymorphisms with all 42 strains of C. neoformans investigated. PCR fingerprints produced by priming with (GTG)5, (GACA)4, or the M13 core sequence differentiated the two varieties of C. neoformans, C. neoformans var. neoformans (serotypes A and D) and C. neoformans var. gattii (serotypes B and C). Furthermore, strains of serotypes A, D, and B or C could be distinguished from each other by specific PCR fingerprint patterns. These primers, which also successfully amplified hypervariable DNA segments from other species, provide a convenient method of identification at the species or individual level. Amplification of polymorphic DNA patterns by PCR with these primers offers several advantages over classical DNA fingerprinting techniques, appears to be more reliable than other PCR-based methods for detecting polymorphic DNA, such as analysis of random-amplified polymorphic DNA, and should be applicable to many other organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Müller C. R., Epplen J. T. DNA finger printing by oligonucleotide probes specific for simple repeats. Hum Genet. 1986 Nov;74(3):239–243. doi: 10.1007/BF00282541. [DOI] [PubMed] [Google Scholar]
- Bolaños B., Mitchell T. G. Phagocytosis of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27(4):203–217. [PubMed] [Google Scholar]
- Chuck S. L., Sande M. A. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989 Sep 21;321(12):794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- Crowhurst R. N., Hawthorne B. T., Rikkerink E. H., Templeton M. D. Differentiation of Fusarium solani f. sp. cucurbitae races 1 and 2 by random amplification of polymorphic DNA. Curr Genet. 1991 Nov;20(5):391–396. doi: 10.1007/BF00317067. [DOI] [PubMed] [Google Scholar]
- Ellis D. H. Cryptococcus neoformans var. gattii in Australia. J Clin Microbiol. 1987 Feb;25(2):430–431. doi: 10.1128/jcm.25.2.430-431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. H., Pfeiffer T. J. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet. 1990 Oct 13;336(8720):923–925. doi: 10.1016/0140-6736(90)92283-n. [DOI] [PubMed] [Google Scholar]
- Epplen J. T. On simple repeated GATCA sequences in animal genomes: a critical reappraisal. J Hered. 1988 Nov-Dec;79(6):409–417. doi: 10.1093/oxfordjournals.jhered.a110544. [DOI] [PubMed] [Google Scholar]
- Georges M., Lequarré A. S., Castelli M., Hanset R., Vassart G. DNA fingerprinting in domestic animals using four different minisatellite probes. Cytogenet Cell Genet. 1988;47(3):127–131. doi: 10.1159/000132529. [DOI] [PubMed] [Google Scholar]
- Goodwin P. H., Annis S. L. Rapid identification of genetic variation and pathotype of Leptosphaeria maculans by random amplified polymorphic DNA assay. Appl Environ Microbiol. 1991 Sep;57(9):2482–2486. doi: 10.1128/aem.57.9.2482-2486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J. Highly variable minisatellites and DNA fingerprints. Biochem Soc Trans. 1987 Jun;15(3):309–317. doi: 10.1042/bst0150309. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984 Jul;120(1):123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Bennett J. E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol. 1982 Mar;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W., Koch A., Niemann C., Beyermann B., Epplen J. T., Börner T. Differentiation of species and strains among filamentous fungi by DNA fingerprinting. Curr Genet. 1991 Mar;19(3):239–242. doi: 10.1007/BF00336493. [DOI] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., White T. J., Taylor J. W. Comparison of 5.8S ribosomal DNA sequences among the basidiomycetous yeast genera Cystofilobasidium, Filobasidium and Filobasidiella. J Med Vet Mycol. 1992;30(3):207–218. [PubMed] [Google Scholar]
- Nürnberg P., Roewer L., Neitzel H., Sperling K., Pöpperl A., Hundrieser J., Pöche H., Epplen C., Zischler H., Epplen J. T. DNA fingerprinting with the oligonucleotide probe (CAC)5/(GTG)5: somatic stability and germline mutations. Hum Genet. 1989 Dec;84(1):75–78. doi: 10.1007/BF00210676. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Magee B. B., Magee P. T. Separation of chromosomes of Cryptococcus neoformans by pulsed field gel electrophoresis. Infect Immun. 1989 Sep;57(9):2624–2627. doi: 10.1128/iai.57.9.2624-2627.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Lebens G. A. Electrophoretic karyotype of the pathogenic yeast Cryptococcus neoformans. J Gen Microbiol. 1989 Jan;135(1):65–71. doi: 10.1099/00221287-135-1-65. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Lebens G., Hicks J. B. Development of DNA probes for early diagnosis and epidemiological study of cryptococcosis in AIDS patients. J Clin Microbiol. 1992 Apr;30(4):925–930. doi: 10.1128/jcm.30.4.925-930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. M., Mitchell T. G. Strain variation in antiphagocytic activity of capsular polysaccharides from Cryptococcus neoformans serotype A. Infect Immun. 1989 Dec;57(12):3751–3756. doi: 10.1128/iai.57.12.3751-3756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer E. D., Spitzer S. G. Use of a dispersed repetitive DNA element to distinguish clinical isolates of Cryptococcus neoformans. J Clin Microbiol. 1992 May;30(5):1094–1097. doi: 10.1128/jcm.30.5.1094-1097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D. Genomic finger printing goes simple. Bioessays. 1990 Jan;12(1):44–46. doi: 10.1002/bies.950120111. [DOI] [PubMed] [Google Scholar]
- Varma A., Kwon-Chung K. J. DNA probe for strain typing of Cryptococcus neoformans. J Clin Microbiol. 1992 Nov;30(11):2960–2967. doi: 10.1128/jcm.30.11.2960-2967.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A., Kwon-Chung K. J. Restriction fragment polymorphism in mitochondrial DNA of Cryptococcus neoformans. J Gen Microbiol. 1989 Dec;135(12):3353–3362. doi: 10.1099/00221287-135-12-3353. [DOI] [PubMed] [Google Scholar]
- Vassart G., Georges M., Monsieur R., Brocas H., Lequarre A. S., Christophe D. A sequence in M13 phage detects hypervariable minisatellites in human and animal DNA. Science. 1987 Feb 6;235(4789):683–684. doi: 10.1126/science.2880398. [DOI] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990 Aug;172(8):4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetton J. H., Carter R. E., Parkin D. T., Walters D. Demographic study of a wild house sparrow population by DNA fingerprinting. Nature. 1987 May 14;327(6118):147–149. doi: 10.1038/327147a0. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuger A., Louie E., Holzman R. S., Simberkoff M. S., Rahal J. J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986 Feb;104(2):234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]