Abstract
Here, we report that Cdk5 activation is stimulated by insulin and plays a key role in the regulation of GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Insulin activation of Cdk5 requires PI3K signaling. Insulin-activated Cdk5 phosphorylates E-Syt1, a 5 C2-domain protein-related to the synaptotagmins that is induced during adipocyte differentiation. Phosphorylated E-Syt1 associates with GLUT4, an event inhibited by the Cdks inhibitor roscovitine. Cdk5 silencing inhibits glucose uptake by 3T3-L1 adipocytes. These studies elucidate a previously unknown activity of Cdk5 and demonstrate the involvement of this kinase in the regulation of insulin-dependent glucose uptake in adipocytes.
The atypical Cdc2-related protein kinase Cdk5 is ubiquitously expressed in mammalian cells and tissues (1). In noncycling cells, Cdk5 phosphorylates multiple substrates to control such diverse phenomena as cell signaling, adhesion and motility, cytoskeletal organization, protein trafficking, and membrane fusion and dynamic organization (2). The membrane-bound Cdk5 effectors, p35/p39, direct and activate the kinase to specific membrane targets via mechanisms that are not yet well understood (3–6). Cdk5 plays a crucial regulatory role in glucose-stimulated insulin secretion in pancreatic cells (7, 8). In addition, CDK5 is involved in the loss of β cell function under glucotoxic conditions, revealing the potential therapeutic value of CDK5 inhibitors in the treatment of type 2 diabetes. Recently, fine mapping and genome-wide association studies have identified SNPs that affect a p35 homolog (9, 10) and calpain 10 (11) as being associated with type 2 diabetes susceptibility. The finding that the specific and strong Cdks inhibitor roscovitine inhibited 2DOG uptake by adipocytes has led us to investigate the involvement of Cdk5 in the regulation of glucose uptake in 3T3-L1 adipocytes.
Here, we report that insulin stimulates Cdk5 activity in 3T3-L1 adipocytes, and knockdown of the kinase inhibits glucose uptake in these cells. Insulin-activated Cdk5 phosphorylates the synaptotagmin homolog E-Syt1 and promotes its association with GLUT4. Both E-Syt1 phosphorylation and GLUT4 association are inhibited by roscovitine. These findings are discussed in the context of the regulation of GLUT4-mediated glucose uptake in adipocytes, Cdk5 deregulation in response to calpains, and the susceptibility of families with the calpain 10 SNP-44 polymorphism to develop type 2 diabetes.
Results
Cdk5 and p35 Are Coexpressed with GLUT4.
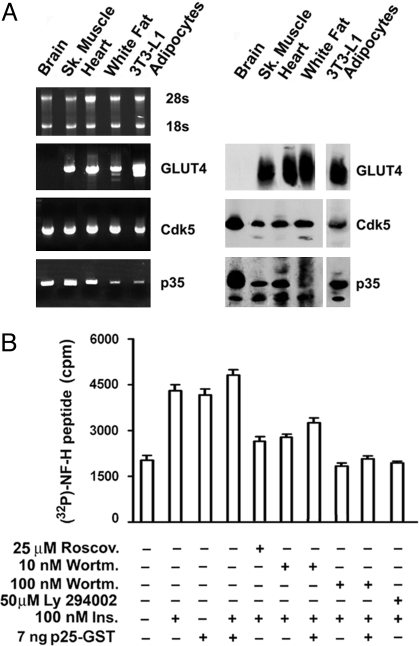
We found comparable levels of Cdk5 transcript and protein in all GLUT4-expressing tissues and cells, including skeletal muscle, cardiac tissue, epidydimal white fat, and 3T3-L1 adipocytes (Fig. 1A). Both p35 transcript and protein were also detected in all samples (Fig. 1A). The 32-kDa Cdk5 protein was abundant and unequally distributed among cytosolic (80%) plasma membrane (9%), microsomal (5%), and nuclear (6%) fractions [supporting information (SI) Fig. S1A]. We identified 5 species of the Cdk5 activator p35, of which the 34–35-kDa and 33–32-kDa doublets were recovered from the plasma membrane and microsomal fractions, respectively, and a small amount of the 32-kDa species was recovered from the nuclear fraction (Fig. S1A). The 5 p35 species were membrane associated, as shown by their near absence from the cytosol. Furthermore, Cdk5 immunoprecipitated from whole membranes of 3T3-L1 adipocytes demonstrated kinase activity comparable with that of recombinant GST-Cdk5, thus confirming the functionality of Cdk5 in adipocytes (Fig. S1B).
Fig. 1.
Cdk5 and p35 mRNA and protein levels in rat tissues and 3T3-L1 adipocytes expressing GLUT4. (A) Levels of GLUT4, Cdk5, and p35 transcripts measured by quantitative RT-PCR in RNA isolates containing comparable amounts of ribosomal 28S and 18S RNA. Levels of GLUT4, Cdk5, and p35 protein as measured by Western blot analysis of postnuclear supernatants. Results are representative of 2 separate experiments. Insulin maximally stimulates Cdk5 kinase activity in 3T3-L1 adipocytes by signaling through the PI3K pathway. (B) Basal and insulin (Ins.)-stimulated 3T3-L1 adipocytes were treated separately for 30 min with the Cdk5 inhibitor roscovitine (Roscov.) and with the PI3K inhibitors wortmannin (Wortm.) and LY294002, as described. Cdk5 was immunoprecipitated (IP) by using the C-8 antibody an identical amounts of protein from whole adipocyte membranes solubilized with detergent. Kinase activity was measured in the immunoprecipitates by using the NF-H peptide as substrate. When required, GST-p25 was included in the kinase reaction mixture to activate maximally Cdk5. Bars ± SEM in the histogram represent the average values of P32 (cpm) incorporated into the NF-H peptide in triplicate samples. The experiment was repeated 3 times.
Insulin Activates Membrane-Associated Cdk5 in 3T3-L1 Adipocytes.
Insulin (100 nM) stimulated Cdk5 kinase activity within 5 min of addition to 3T3-L1 adipocytes, with maximal activity detected after 10 min (Fig. S2A). Cdk5 activation was maximally stimulated by insulin in vivo as p25, the truncated form of p35 that maximally activates Cdk5, failed to further stimulate Cdk5 immunoprecipitated from membranes of insulin-treated 3T3-L1 adipocytes in vitro (Fig. 1B). The stimulatory effect of insulin was strongly inhibited in cells pretreated with the robust Cdk5 inhibitor roscovitine (12) (Fig. 1B and Fig. S2B). The activation by insulin was also strongly inhibited by the phosphatidylinositol 3-kinase (PI3K) inhibitors wortmannin (10–100 nM, 30 min) and LY294002 (50 μM, 30 min) (Fig. 1B). Taken together, these data imply that insulin signals the activation of Cdk5 through the PI3K pathway.
Cdk5 Membrane Protein Substrates in 3T3-L1 Adipocytes.
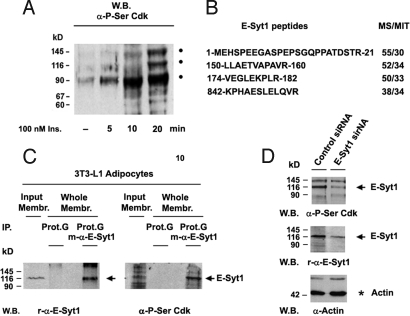
Western blot analysis of membrane protein phosphorylation in 3T3-L1 adipocytes was performed by using an antibody specific for phosphoserine at Cdk-specific phosphorylation sites (P-Ser/Cdk). This study revealed insulin-stimulated phosphorylation at P-Ser/Cdk sites of 3 high-molecular-mass proteins of 90, 116, and 145 kDa (Fig. 2A). Phosphorylation was strongly inhibited both by roscovitine and wortmannin, thus supporting protein phosphorylation by a Cdk activated via insulin signaling and the PI3K pathway (Fig. S3).
Fig. 2.
Cdk5 membrane protein substrates in 3T3-1 adipocytes. Identification of the p116 membrane protein phosphorylated by Cdk5 as E-Syt1. (A) Basal adipocytes kept untreated were stimulated with insulin as indicated and the phosphorylation of identical amounts of detergent-solubilized membrane protein was probed by Western blot analysis using the P-Ser Cdk antibody. The experiment was repeated twice. (B) Identification of the 116-kDa membrane protein extracted from 3T3-L1 adipocytes as E-Syt1 by MS/MS-MS spectra analysis of tryptic peptides (MS/MIT, a Mascot score above MIT is considered as a significant peptide assignment). (C) Immunoprecipitation of the 116-kDa protein obtained from solubilized membranes of 3T3-L1 adipocytes and its reaction with the r-E-Syt1 and P-Ser Cdk antibodies. (D) Decrease in the levels of the 116-kDa E-Syt1 (black arrow) in 3T3-L1 adipocytes targeted with E-Syt1 siRNA.
Characterization of p116 as E-Syt1, a 5-C2 Domain Membrane Protein Related to Synaptotagmins.
Studies using the P-Ser Cdk antibody revealed the presence of phosphorylated p116 in GLUT4 immunoprecipitates (Fig. S4D). Mass spectroscopy analysis of p116-derived peptides identified p116 as E-Syt1 (Fig. 2B). E-Syt1, also known as FAM62A, MBC2 and vp115, is 1 of 3 members of the E-Syt family of C2 domain-rich membrane proteins related to the synaptotagmins (Syt). E-Syt1 is a type I transmembrane protein. Its long C-terminal cytoplasmic region consists of 1 SMP and 5 C2 domains (13). To confirm the identity of p116, we raised antibodies against E-Syt1 in rabbit (α-r-E-Syt1) and mouse (α-m-E-Syt1). Immunoprecipitation and Western blot reaction of a 116-kDa protein from adipocyte membranes with the α-m-E-Syt1 and α-r-E-Syt1 antibodies, and the P-Ser Cdk antibody binding of this protein, confirmed that p116 was indeed E-Syt1 (Fig. 2C and Fig. S4 A and B). This conclusion was further supported by the reaction of the P-Ser Cdk antibody with transfected E-Syt1 in COS7 cells (Fig. S4C) as well as the decrease in levels of phosphorylated p116 after E-Syt1 knockdown by siRNA in 3T3-L1 adipocytes (Fig. 2D).
E-Syt1 Expression Is Induced During Adipocyte Differentiation and Is Phosphorylated by CDK5 at Ser 314 in the First C2 Domain.
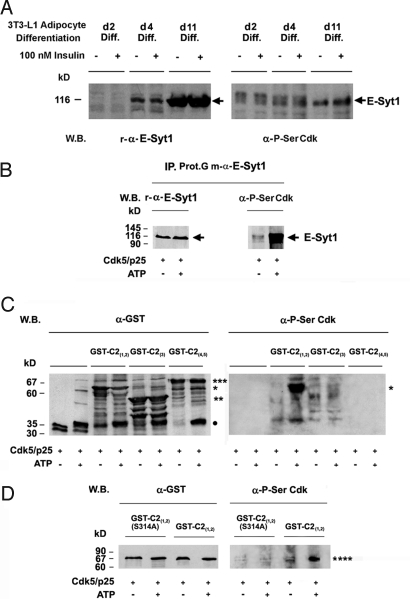
Expression of E-Syt1 was dramatically induced during the differentiation of fibroblasts into adipose cells. E-Syt1 expression coincided with the washout of differentiation factors on day 4 of differentiation, and a strong increase in protein levels was observed between days 4 and 11 of differentiation (Fig. 3A). Although the expression profiles of E-Syt1, p35, and GLUT4 were similar, Cdk5 expression did not change throughout the differentiation process (Fig. 3A and Fig. S5). E-Syt1 was phosphorylated by Cdk5 in vitro, confirming that this protein is a Cdk5 substrate in adipocytes (Fig. 3B). Separate phosphorylation studies of the GST-fusions C2(1-2), C2(3), and C2(4-5) revealed that only the C2(1.2) construct was phosphorylated (Fig. 3C). Furthermore, substitution of Ala for Ser 314 at RSPXXR, the only Cdk5 canonical phosphorylation site in the C2(1.2) construct, abolished its phosphorylation (Fig. 3D), therefore suggesting that Ser-314 is the Cdk5 phosphorylation site in E-Syt1.
Fig. 3.
Induction of E-Syt1 expression via 3T3-L1 fibroblasts differentiation into adipocytes. (A) The levels and phosphorylation status of E-Syt1 in response to Cdk5 were studied in serum-starved and insulin-stimulated (100 nM, 20 min) cells on days 2, 4, and 11 of differentiation (see Materials and Methods), by using r-E-Syt1 and P-Ser Cdk antibodies. Cdk5 targets Ser-314 in the C2(1) domain of E-Syt1. (B) E-Syt1 immunoprecipitated from 3T3-L1 adipocyte membranes and (C) GST (●), GST-C2(1,2) (*), GST-C2(3) (**), GST-C2(4,5) (***), and (D) the GST-C2(1,2)S314A mutant (****) were phosphorylated in vitro by using preactivated Cdk5. Immunoprecipitated E-Syt1 was dephosphorylated by using λ phosphatase (0.4 units) before being subjected to phosphorylation by Cdk5. Controls for the kinase reactions were performed in the absence of ATP. Protein loading and protein phosphorylation by Cdk5 were monitored by Western blot analysis using antibodies specific to E-Syt1, GST, and P-Ser Cdk sites. Experiments were repeated twice.
Insulin Induces the Association Between E-Syt1 and GLUT4.
Immunoprecipitation of proteins from plasma membranes purified from basal and insulin-stimulated adipocytes (20 min, 100 nM) using the anti-E-Syt1 antibody that we developed in mouse, revealed that insulin treatment increased an average of 75% the association of GLUT4 with E-Syt1 (Fig. 4). Interestingly, roscovitine inhibited by an average of 82% the effects of insulin on E-Syt1 immunoprecipitation, suggesting that Cdk5 is required for insulin-stimulated GLUT4-E-Syt1 association (Fig. 4). Furthermore, in the same experiments, we observed that insulin treatment resulted in increased Cdk5 levels in E-Syt1 immunoprecipitates, and that this was also inhibited by roscovitine (Fig. 4).
Fig. 4.
Insulin promotes and roscovitine inhibits the association between E-Syt1 and GLUT4. E-Syt1 was immunoprecipitated from plasma membranes of untreated and insulin-stimulated adipocytes that were or were not pretreated with roscovitine, and the content of E-Syt1, GLUT4, and Cdk5 (arrows) in the immunoprecipitates were measured by using specific antibodies; light-chain antibody (●). Experiments were repeated 3 times.
Cdk5 Knockdown by siRNA Inhibition and Cdk5 Inhibition by Roscovitine Reduce 2DOG Uptake in 3T3-L1 Adipocytes.
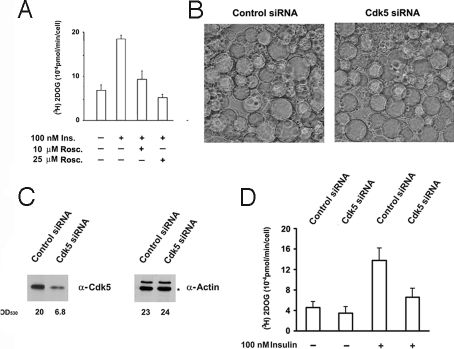
To determine whether Cdk5 regulates glucose uptake in 3T3-L1 adipocytes, we studied the effect of Cdk5 inhibition by roscovitine and Cdk5 silencing by siRNA on glucose transport. Pretreatment of adipocytes with 10 and 25 μM roscovitine before insulin stimulation strongly inhibited glucose uptake by the cells (Fig. 5A). It is noteworthy that glucose uptake was also inhibited, although with less efficiency by the Cdk5 inhibitor kenpaullone, but was not inhibited by EGCG, the inhibitor of the DYRK1A, a kinase inhibited by roscovitine. 3T3-L1 adipocytes transfected with Cdk5 siRNA showed significant decreases in Cdk5 levels and in basal and insulin-stimulated 2-deoxyglucose (2DOG) uptake (Fig. 5 B–D; Fig. S6). This inhibition occurred independently of changes in the insulin-induced activation of Akt or the translocation of GLUT4 from intracellular stores to the plasma membrane (Fig. S7).
Fig. 5.
Cdk5 inhibition by roscovitine and silencing by siRNA decrease glucose uptake in 3T3-L1 adipocytes. (A) 3T3-L1 adipocytes were serum starved for 4 h and then left untreated or stimulated with 100 nM insulin for 20 min before measuring 2DOG uptake. When required, the cells were pretreated for 30 min with 10 or 25 μM roscovitine before insulin stimulation. (B–D) 3T3-L1 adipocytes were transfected on days 8 and 13 of differentiation into adipocytes by using control siRNA or Cdk5 siRNA. On day 14, the adipocytes were observed by microscopy (B), the Cdk5 and actin (*) levels were measured by Western blot analysis (C), and 2DOG uptake was measured under basal and insulin-stimulated conditions (100 nM, 20 min). Bars represent the means of triplicate samples ± SEM in 6 separate experiments (D). See also Fig. S6.
Discussion
In the present study, we show that Cdk5 and its activator, p35, are present in tissues and cells that express GLUT4. We find that insulin stimulates Cdk5 kinase activity in 3T3-L1 adipocytes. The hormone demonstrates maximal stimulation of Cdk5 in situ, as indicated by the failure of p25 to further activate Cdk5 purified from insulin-stimulated adipocytes in vitro. The inhibition of insulin-induced Cdk5 activation by LY294002 and wortmannin is consistent with the essential role of PI3K signaling in this pathway. Inhibition of 2DOG uptake in 3T3-L1 adipocytes stimulated with insulin after Cdk5 inhibition by roscovitine and knockdown by RNAi indicates a crucial role for Cdk5 in the regulation of glucose transport. It is worth noting that the causal relationship between the proteolytic cleavage of p35 by calpains and subsequent deregulation of Cdk5 activity (for review, see ref. 14). Furthermore, the involvement of Cdk5 in the regulation of glucose transport in adipocytes, a cell type known to play a crucial role in glucose homeostasis in the body, and the reported association between calpain 10 gene polymorphisms and type 2 diabetes suggest an interesting line of investigation into the role of Cdk5 in diabetes and its potential use as a therapeutic target.
The findings that in whole membranes, GLUT4 interacts with the Cdk5 substrate E-Syt1, a membrane protein related to synaptotagmin, and that insulin-stimulated GLUT4-mediated glucose transport is attenuated in the absence of functional Cdk5 suggest a mechanism by which Cdk5 regulates glucose uptake in adipocytes. Insulin treatment results in E-Syt1 phosphorylation and GLUT4-E-Syt1 association, Although the association between E-Syt1 and GLUT4 is blocked in cells pretreated with roscovitine, its relationship between the status of E-Syt1 phosphorylation remains unclear. An important remaining question concerns the role of Ser-314 phosphorylation by Cdk5 in the process of association and in the activity of the complex. Preliminary studies indicate the capacity of E-Syt1 to discriminate between different GLUT4 species. Further studies in cellular fractions after different intervals of insulin stimulation should be helpful to learn more on their mechanism of association and how this affects the GLUT4 activity.
C2 domains, such as those present in E-Syt1, often endow proteins with the ability to bind Ca2+ and phospholipids, and, recently, Ca2+ has been shown to mediate the binding of phospholipids to the C2(1) domain of E-Syt2 (13). Because calcium and phosphoinositides are known to regulate insulin-stimulated glucose transport (15–19), it would be interesting to test whether changes in Ca2+ produced by insulin signaling affect the ability of E-Syt1 to bind phospholipids and how this might affect the interaction between E-Syt1 and GLUT4.
Current research in our laboratory aims to uncover the mechanism by which phosphorylated E-Syt1 may mediate Cdk5-stimulated glucose transport. Thus far, studies examining the role of E-Syt1 in glucose transport have been hindered by difficulties associated with silencing E-Syt1 in adipocytes and expressing the Ser314A mutant in these cells. Partial E-Syt1 silencing and possible functional redundancy between members of the E-Syt family are among the factors that may explain why E-Syt1 siRNA experiments have yet to inhibit glucose transport in 3T3-L1 adipocytes. The studies reported herein advance the current knowledge of the mechanisms by which insulin regulates glucose uptake in adipocytes and might provide a molecular framework to study this dysfunction in type 2 diabetes.
Materials and Methods
Antibodies and Reagents.
The antibodies and reagents used in this study are listed in the SI Text.
Measurement of Cdk5 and p35 Expression in Tissues and Cultured Cells.
RT-PCR studies were performed by using the ThermoScript System (Invitrogen) using 2 μg of purified RNA from tissues and 3T3-L1 adipocytes and 10 pmol of specific Cdk5 primers. Cdk5, p35, and GLUT4 levels in 3T3-L1 adipocytes and rat tissues were determined by Western blot analysis of postnuclear supernatants (100 μg of protein).
Cdk5 Kinase Assay.
Whole 3T3-L1 adipocyte membranes from postnuclear supernatants were resuspended in immunoprecipitation (IP) buffer [50 mM Hepes (pH 7.4), 150 mM NaCl, 10% glycerol with protein phosphatase and protease inhibitors] and solubilized for 30 min at 4 °C in 1% Nonidet P-40. Cdk5 protein was immunoprecipitated from clarified solutions made with 0.75% detergent and incubated for 3 h at 4 °C with 5 μl of C-8 anti-Cdk5 antibody bound to protein G-Sepharose. Cdk5 kinase activity was measured in immunoprecipitates from detergent-solubilized postnuclear supernatants of 3T3-L1 adipocytes and recombinant GST-Cdk5 (7–250 ng) activated with GST-p25 (7–250 ng). The kinase assay was performed for 1 h or 10 min at 23 °C in 20 μL of kinase buffer containing either 1 or 100 μM ATP and 2 μCi of γ 32P ATP (3,000 Ci/mmol; Amersham). Histone H1 (4 μg) or synthetic NF-H peptide (0.2 mM) was used as the substrate. Histone H1 and NF-H peptide phosphorylation was determined by autoradiography or by counting the [32P] peptide adsorbed on p81 phosphocellulose discs, respectively.
Cell Culture.
3T3-L1 fibroblasts (ATCC) were cultured in low-bicarbonate DMEM supplemented with 10% calf serum and differentiated into adipocytes as described (20). The PPAR activator troglitazone (7.5 μM) was added to the culture medium on day 3 of differentiation and removed 48 h later. Only homogeneous 3T3-L1 adipocyte populations were used in the experiments. 3T3-L1 adipocytes were cultured for 3 h in serum-free GFG medium [glucose-free/low-bicarbonate DMEM with 0.2% gelatin, 1 mM pyruvate, 5 mM glutamine, essential amino acids, and 20 mM Hepes (pH7.4)] before stimulation with insulin (100 nM, 20 min).
Cellular Fractionation.
3T3-L1 adipocytes were grown for 11 to 15 days in p100 dishes, washed twice with cold PBS, and scraped into cold buffer A [20 mM Hepes (pH 7.4), 0.25 M sucrose, 1 mM EDTA, 50 mM β-glycerophosphate, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonylfluoride, 5 μg/mL of leupeptin, 5 μg/mL of aprotinin, and 1.5 μM pepstatin]. Cell suspensions were then triturated 20 times through a 23-gauge needle. The lysates were centrifuged for 10 min at 800 × g to remove nuclei and cell debris, and the postnuclear supernatants were used to prepare whole membranes and fractions enriched in plasma membrane, microsomes, or cytosol (21).
RNA Interference.
Cdk5 RNA interference was performed in 3T3-L1 adipocytes grown on 10-mm coverslips. The cells were transfected on days 8 and 13 of differentiation by using Cdk5 and control SMART pool siRNA (Dharmacon), according to the manufacturer instructions by using Lipofectamine 2000 (Invitrogen).
Glucose Uptake Assay.
The [H3]2-DOG and [14C]3-O-methyl glucose uptake assays were performed by using 3 × 104 3T3-L1 adipocytes plated on 10-mm coverslips that were incubated in 0.2 mL of GFG medium for 14 min at 37 °C under basal or insulin-stimulated conditions (100 nM, 20 min) (22, 23).
Mass Spectrometry Studies.
p116 protein was resolved by SDS/PAGE and stained with Coomassie blue and P-Ser Cdk antibody. The 116-kDa band was excised and subjected to trypsin digestion (24), and the peptides were separated by using C18 PepMap columns. Peptide analysis was performed by mass spectrometry using a nanoelectrospray ionization source, and MS/MS-MS spectra were acquired in data-dependent mode on an ion-trap mass spectrometer (Esquire HCT; Bruker). MS-MS data were converted into mgf format to search the SWISS PROT and NCBInr databases (release 20060518) by using a MASCOT search engine (25).
Phosphorylation Studies.
E-Syt1 immunoprecipitates were subjected to dephosphorylation overnight at 30 °C by using 1 μL of λ phosphatase (0.4 units) in reaction buffer [50 mM Tris·HCl, 100 mM NaCl, 2 mM DTT, 0,1 mM EGTA, 0,01% Brij 35 (pH 7.5)]. After heat inactivation of the phosphatase (65 °C, 1 h), the samples were phosphorylated by using recombinant GST-Cdk5 preactivated with GST-p25. Phosphorylation was studied by Western blot using the P-Ser Cdk antibody.
Supplementary Material
Acknowledgments.
This work was supported by Instituto de Salud Carlos III (Centro de Investigación Biomédica en Red Área de Enfermedades Hepáticas y Digestivas) and Ministry of Education Grants BFU-2005-07903 and GEN2003-20662-C07-06 (to I.V.S.). G. M. was supported by the Spanish Ministerio de Ciencia e Innovacion.
Note.
Coinciding with the submission of this work for publication, Okada et al. (26) have reported the activation of Cdk5 by insulin and that phosphorylation of TC10α by Cdk5 at the sequence ILTPKKHT197VKKRIGS inhibits GLUT4 translocation to the cell surface. They also reported that transfection of 3T3-L1 adipocytes with the T197A and T197D mutants yielded loss and gain of function, respectively.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900218106/DCSupplemental.
References
- 1.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: A cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. BioEssays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 3.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, et al. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 5.Lim AC, Qu D, Qi RZ. Protein–protein interactions in Cdk5 regulation and function. NeuroSignals. 2003;12:230–238. doi: 10.1159/000074625. [DOI] [PubMed] [Google Scholar]
- 6.Asada A, et al. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cycline-dependent kinase 5 complexes. J Neurochem. 2008;106:1325–1336. doi: 10.1111/j.1471-4159.2008.05500.x. [DOI] [PubMed] [Google Scholar]
- 7.Lilja L, et al. Cyclin-dependent kinase 5 promotes insulin exocytosis. J Biol Chem. 2001;276:34199–34205. doi: 10.1074/jbc.M103776200. [DOI] [PubMed] [Google Scholar]
- 8.Wei FY, et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005;11:1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]
- 9.Steinthorsdottir V, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 10.Pascoe L, et al. Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic beta-cell function. Diabetes. 2007;56:3101–3104. doi: 10.2337/db07-0634. [DOI] [PubMed] [Google Scholar]
- 11.Chen LX, Ji LN, Han XY, Zhu F. Study on Calpain10 gene polymorphism in Chinese type 2 diabetes families. Zhonghua Yi Xue Za Zhi. 2003;83:1856–1859. [PubMed] [Google Scholar]
- 12.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 13.Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci USA. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz JC, Tsai LH. A Jekyll and Hyde kinase: Roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Konstantopoulos N, et al. A purine analog kinase inhibitor, calcium/calmodulin-dependent protein kinase II inhibitor 59, reveals a role for calcium/calmodulin-dependent protein kinase II in insulin-stimulated glucose transport. Endocrinology. 2007;148:374–385. doi: 10.1210/en.2006-0446. [DOI] [PubMed] [Google Scholar]
- 16.Brozinick JT, Jr, Reynolds TH, Dean D, Cartee G, Cushman SW. 1-[N,O-bis-(5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem J. 1999;339:533–540. [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehead JP, et al. The role of Ca2+ in insulin-stimulated glucose transport in 3T3–L1 cells. J Biol Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- 18.Honeyman TW, Strohsnitter W, Scheid CR, Schimmel RJ. Phosphatidic acid and phosphatidylinositol labelling in adipose tissue. Relationship to the metabolic effects of insulin and insulin-like agents. Biochem J. 1983;212:489–498. doi: 10.1042/bj2120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou MM, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 20.Tafuri SR. Troglitazone enhances differentiation, basal glucose uptake, and Glut1 protein levels in 3T3–L1 adipocytes. Endocrinology. 1996;137:4706–4712. doi: 10.1210/endo.137.11.8895337. [DOI] [PubMed] [Google Scholar]
- 21.Karnieli E, et al. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981;256:4772–4777. [PubMed] [Google Scholar]
- 22.Harrison SA, Buxton JM, Clancy BM, Czech MP. Insulin regulation of hexose transport in mouse 3T3–L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990;265:20106–20116. [PubMed] [Google Scholar]
- 23.Whitesell RR, Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979;254:5276–5283. [PubMed] [Google Scholar]
- 24.Gevaert K, Vandekerckhove J. In-Gel Digestion of Protein Spots for Mass Spectrometry. Amsterdam: Elsevier Academic; 2006. pp. 379–382. [Google Scholar]
- 25.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Okada S, et al. CDK5-dependent phosphorylation of the Rho family GTPase TC10{alpha} regulates insulin-stimulated GLUT4 translocation. J Biol Chem. 2008;283:35455–35463. doi: 10.1074/jbc.M806531200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.