Abstract
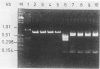
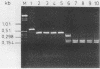
A total of 50 oxacillin-resistant Staphylococcus aureus (ORSA) strains that were clumping factor negative (CFN) and protein A negative by latex agglutination were collected from patients in six different hospitals at different locations in Germany during 1991 and 1992. Antibiograms, bacteriophage typing, and plasmid analysis were performed. The antibiograms showed that, besides oxacillin, all CFN ORSA strains were resistant to gentamicin, clindamycin, erythromycin, ciprofloxacin, and fosfomycin. All these isolates were nontypeable with an international set of phages, and an additional experimental phage set indicated that the strains were phage type 16, 192. Moreover, all isolates possessed a single plasmid of 30 kb, and restriction analysis of those plasmids revealed identical patterns. For genotyping, these 50 isolates were also analyzed by pulsed-field gel electrophoresis (PFGE) and polymerase chain reaction (PCR) of the coagulase and protein A genes and then by restriction enzyme digestion and analysis of restriction fragment length polymorphisms (RFLPs). With 49 strains, electrophoresis of SmaI-digested chromosomal DNA revealed identical PFGE patterns regarding the number and size of the DNA fragments, which could be differentiated from those of clumping factor-positive ORSA strains. Typing for the coagulase gene by PCR revealed PCR products of identical sizes. The AluI restriction digestion patterns of the PCR products were identical. PCR with primers derived from the region of that part of the protein A gene that encodes the immunoglobulin G-binding domains showed a PCR product that was about 170 bp smaller than that of the protein A gene from strains that were positive in the protein A latex agglutination test. Since it is precisely this size that is required in order to encode one immunoglobulin G-binding region, we assume that this is not present in the CFN ORSA strains. The phenotypical and genotypical features identify these very unusual CFN ORSA stains as being of clonal origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bouvet A., Fournier J. M., Audurier A., Branger C., Orsoni A., Girard C. Epidemiological markers for epidemic strain and carrier isolates in an outbreak of nosocomial oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1990 Jun;28(6):1338–1341. doi: 10.1128/jcm.28.6.1338-1341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989 May 4;320(18):1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- Eadon H. J., Pinney R. J. Methicillin-resistant Staphylococcus aureus--an overview. J Clin Pharm Ther. 1991 Dec;16(6):453–462. doi: 10.1111/j.1365-2710.1991.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Finck-Barbançon V., Prevost G., Mazurier I., Piemont Y. A structurally novel staphylococcal protein A from the V8 strain. FEMS Microbiol Lett. 1992 Feb 1;70(1):1–8. doi: 10.1016/0378-1097(92)90554-2. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S. H., Byrne S. K., Zhang J. L., Chow A. W. Molecular typing of Staphylococcus aureus on the basis of coagulase gene polymorphisms. J Clin Microbiol. 1992 Jul;30(7):1642–1645. doi: 10.1128/jcm.30.7.1642-1645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn K., Lenz W., Kayser F. H., Shalit I., Krasemann C. Use of a ribosomal RNA gene probe for the epidemiological study of methicillin and ciprofloxacin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):649–653. doi: 10.1007/BF01964265. [DOI] [PubMed] [Google Scholar]
- Ichiyama S., Ohta M., Shimokata K., Kato N., Takeuchi J. Genomic DNA fingerprinting by pulsed-field gel electrophoresis as an epidemiological marker for study of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1991 Dec;29(12):2690–2695. doi: 10.1128/jcm.29.12.2690-2695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida S., Miyata T., Yoshizawa Y., Igarashi H., Iwanaga S. Nucleotide and deduced amino acid sequences of staphylocoagulase gene from Staphylococcus aureus strain 213. Nucleic Acids Res. 1989 Nov 11;17(21):8871–8871. doi: 10.1093/nar/17.21.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida S., Miyata T., Yoshizawa Y., Kawabata S., Morita T., Igarashi H., Iwanaga S. Nucleotide sequence of the staphylocoagulase gene: its unique COOH-terminal 8 tandem repeats. J Biochem. 1987 Nov;102(5):1177–1186. doi: 10.1093/oxfordjournals.jbchem.a122156. [DOI] [PubMed] [Google Scholar]
- Kreiswirth B., Kornblum J., Arbeit R. D., Eisner W., Maslow J. N., McGeer A., Low D. E., Novick R. P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993 Jan 8;259(5092):227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- Neville L. O., Billington O. J., Kibbler C. C., Gillespie S. H. Methicillin resistant Staphylococcus aureus without clumping factor, protein A, and DNAse. Lancet. 1991 Aug 24;338(8765):518–518. doi: 10.1016/0140-6736(91)90595-g. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., Bialkowska-Hobrzanska H., Romance L., Harry V. S., Parker S. Clonal diversity of methicillin-resistant Staphylococcus aureus in an acute-care institution. Infect Control Hosp Epidemiol. 1992 Jan;13(1):33–37. doi: 10.1086/646420. [DOI] [PubMed] [Google Scholar]
- Phonimdaeng P., O'Reilly M., Nowlan P., Bramley A. J., Foster T. J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990 Mar;4(3):393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Reboli A. C., John J. F., Jr, Platt C. G., Cantey J. R. Methicillin-resistant Staphylococcus aureus outbreak at a Veterans' Affairs Medical Center: importance of carriage of the organism by hospital personnel. Infect Control Hosp Epidemiol. 1990 Jun;11(6):291–296. doi: 10.1086/646174. [DOI] [PubMed] [Google Scholar]
- Schlichting C., Branger C., Fournier J. M., Witte W., Boutonnier A., Wolz C., Goullet P., Döring G. Typing of Staphylococcus aureus by pulsed-field gel electrophoresis, zymotyping, capsular typing, and phage typing: resolution of clonal relationships. J Clin Microbiol. 1993 Feb;31(2):227–232. doi: 10.1128/jcm.31.2.227-232.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf A., Schmidt-Rotte H., Schmidt H., Kunz E., Karch H., Heesemann J. Molecular analysis of nosocomial infection by oxacillin-resistant Staphylococcus aureus lacking protein A and clumping factor. Lancet. 1992 Sep 5;340(8819):621–621. doi: 10.1016/0140-6736(92)92165-c. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Wanger A. R., Morris S. L., Ericsson C., Singh K. V., LaRocco M. T. Latex agglutination-negative methicillin-resistant Staphylococcus aureus recovered from neonates: epidemiologic features and comparison of typing methods. J Clin Microbiol. 1992 Oct;30(10):2583–2588. doi: 10.1128/jcm.30.10.2583-2588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierdt C. H., Hosein I. K., Shively R., MacLowry J. D. Phage pattern-specific oxacillin-resistant and borderline oxacillin-resistant Staphylococcus aureus in U.S. hospitals: epidemiological significance. J Clin Microbiol. 1992 Jan;30(1):252–254. doi: 10.1128/jcm.30.1.252-254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]