Abstract
In insects, farnesyl pyrophosphate (FPP) is converted to juvenile hormone (JH) via a conserved pathway consisting of isoprenoid derived metabolites. The first step of this pathway is presumed to be hydrolysis of FPP to farnesol in the ring gland. Based on alignment of putative phosphatases from D. melanogaster with the phosphatase domain of soluble epoxide hydrolase, Phos2680 and Phos15739 with conserved phosphatase motifs were identified, cloned and purified. Both D. melanogaster phosphatases hydrolyzed para-nitrophenyl phosphate, however, Phos15739 also hydrolyzed FPP with a Kcat/Km of 2.1 X 105 M−1s−1. RT-PCR analysis revealed that Phos15739 was expressed in the ring gland and its expression was correlated with JHIII titer during development of D. melanogaster. N-acetyl-S-geranylgeranyl-L-cysteine was found to be a potent inhibitor of Phos15739 with an IC50 value of 4.4 μM. Thus, our data identify Phos15739 as a FPP phosphatase that likely catalyzes the hydrolysis of FPP to farnesol in D. melanogaster.
Keywords: farnesyl pyrophosphate, juvenile hormone, phosphatase
INTRODUCTION
Juvenile hormones (JHs), the unique sesquiterprenoid hormones synthesized and secreted by the corpora allata (CA) region of ring glands play important roles in insect embryonic development, metamorphosis, and reproduction [1, 2]. JH is critical for insect maturation and the concentration of JH is regulated by tissue specific rates of biosynthesis, release, and degradation [3]. Because FPP hydrolysis to farnesol is generally considered to be the first committed step in JH biosynthesis [2], this step may be critical in controlling endogenous JH levels and/or rates of JH production. Insects synthesize FPP, the 15-carbon precursor of JH, dolichol, ubiquinon and prenylated proteins, from acetyl-CoA via the classical mevalonate pathway. Thus, insect enzymes involved in FPP synthesis have been identified based on conservation of the mevalonate pathway between vertebrates and insects. However, due to the apparent lack of vertebrate orthologs of enzymes involved in the synthesis of JH from FPP, no structural data for the insect FPP pyrophosphatase, farnesol oxidase or farnesal dehydrogenase are available [2, 5]. JH acid methyltransferase and JH epoxidase, the last two enzymes involved in JH biosynthesis, have been previously identified [4, 6].
Recently, the N-terminal phosphatase domain of mammalian soluble epoxide hydrolase was reported to be in the haloacid dehalogenase superfamily [7]. Structure and sequence alignment of mammalian sEH with phosphonoacetaldehyde hydrolase (PhAH) from Bacillus cereus, haloacid dehalogenase (HAD) from Xanthobacter autotrophicus, and phosphoserine phosphatase (PSP) from Methanococcus jannaschii revealed three common motifs with Asp-9 as the catalytic nucleophile [7]. Previously, the effect of hsEH on isoprenoid phosphate hydrolysis was evaluated in our lab. FPP was found to be a substrate of the N-terminal domain of hsEH with a turnover number of 0.25 ± 0.07 s−1, and a series of isoprenoid-derived compounds were identified as hsEH phosphatase inhibitors [8]. Based on the hypothesis that mammalian soluble epoxide hydrolases evolved by fusion of the primitive haloacid dehydrogenase N-terminal domain and the primitive haloalkane dehalogenase C-terminal domain [9], we reasoned that a distinct and separate N-terminal phosphatase domain might exist in insects. Furthermore, due to the uniqueness of JH biosynthesis in insects, inhibitors disrupting FPP hydrolysis to farnesol might be of potential use as insect growth regulators.
Here, we report the identification of phosphatase candidates from the D. melanogaster genome by comparing conserved regions of phosphatases from hsEH, PhAH, HAD and PSP. Based on analysis of substrate specificity, localization and developmental expression, our data suggest that Phos15739 catalyzes the hydrolysis of FPP to farnesol in D. melanogaster.
MATERIALS AND METHODS
Chemicals and heterologous expression
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. Geranylgeranyl pyrophosphate was purchased from Biomol International (Plymouth Meeting, PA). Plasmids pFLC-1 containing the cDNA of D. melanogaster CG2680 and CG15739 were obtained from the Drosophila Genomics Resource Center. The Bac-to-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA) was used for expressing recombinant proteins. Sf21 insect cells were grown at 27°C in EX-CELL™420 insect serum-free medium (JRH Biosecience, Levexa, KS) supplemented with 3% fetal bovine serum (JRH Biosecience, Levexa, KS) and penicillin-streptomycin.
Reverse transcription
Total RNA from ring glands of early 3rd instar larva was isolated using Trizol (Invitrogen, Carlsbad, CA, USA). The concentration and quality of the RNA samples were estimated by measuring optical density at 260nm, 260nm/280 nm ratio and agarose gel electrophoresis. After isolation, RNA was stored at −80°C until analyzed. RNAs were reverse transcribed with random primers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Primers used for amplifying genes encoding putative phosphatases from D. melanogaster are listed in Supplemental Table 1. PCR conditions were 95°C for 2 min followed by 30 cycle of 95°C for 30 s; 55°C, 30 s, 72°C, 1 min; with a final extension at 72°C for 10 min.
Construction of baculoviruses expressing Phos2680 and Phos15739
The cDNAs of CG2680 and CG15739 were subcloned into pFastBac-HTa by PCR amplification using primers CG2680-v-forward: 5′-ACGAACCAACGAATTCATGTCGCCACCACGAC-3′, CG2680-v-reverse: 5′-CGGCCCGCGGAA GCTTACCTATTTTTGAATATTTTC-3′; CG15739-v-forward: 5′-ACCAGAACGGGAATTCATGGC CAAACCCCAGCAC-3′, and CG15739-v-reverse: 5′-TCCTCCGCCGAAGCTTGTCTAGACACG CGACTTG -3′ respectively. Following an initial denaturation at 95°C for 1 min, an amplification profile of 30 cycles with denaturation at 95°C for 30 sec, annealing at 52°C for 30 sec, and extension at 72°C for 1 min, and then a final extension at 72°C for 7 min was used. The PCR products were double digested with EcoR1 and HindIII and ligated into plasmid pFactBac-HTa to form pFastBac-2680 and pFastBac-15739. The generation of baculoviruses expressing Phos2680 and Phos15739 was performed according to manufacturer’s instructions.
To construct the Phos15739 variant Asp28Ala (D28A), primers DM15739D28A-forward: 5′-ACCGGGTGGTCAGCGCGATCGATGGGCTTCCTG-3′ and DM15739D28A-reverse: 5′-ACGCCATCGATCGCGCTGACCACCCGGTCGAAAG-3′ were used for site-direct mutagenesis. The same PCR program and method listed above were used.
Protein purification
Phos2680, Phos15739 and Phos15739-D28A with N-terminal His tags were purified on HIS-Select Cartridges (Sigma-Aldrich; St. Louis, MO) following the manufacturer’s instructions. His-tagged proteins were concentrated by Amicon ultra centrifugal filter devices (Millipore; Billerica, MA). Protein concentration and purity were determined by BCA assay reagent from Pierce (Rockford, IL) and SDS-PAGE respectively.
Enzyme assays
The specific activity of Phos2680 and Phos15739 toward p-NPP was measured in 96-well plates. Phos2680 (50 ng) or Phos15739 (50 ng) were added in 50 μL reaction buffer (100 mM MES, pH 6.0, 2 mM Mg 2+) containing substrate with final concentrations from 0 mM to 20 mM at room temperature for various times, followed by adding 100 μL NaOH (0.5N) to stop the reaction. The nitrophenol product was detected at OD405 with a SpectraMax-190 plate reader (Molecular Devices, Sunnyvale, CA).
The analysis of Phos2680 and Phos15739 activity using isoprenoid monophosphate and pyrophosphate substrates was determined using PiPer Phosphate Assay Kit (Molecular Probes; Eugene, OR) as previously described [8]. Reaction mixtures of isoprenoid mono- or pyrophosphate substrates and Phos2680 or Phos15739 were incubated in 100 μL of reaction buffer at room temperature for various times, followed by adding 50 μL reagent containing EDTA, maltose, maltose phosphorylase, glucose oxidase, horseradish peroxidase, and Amplex® Red reagent. The same assay was also used to determine whether Phos15739 was a pyrophosphatase or monophosphatase by adding inorganic pyrophosphatase to the reaction mixture before reagent mixture.
Inhibitors of Phos2680 and Phos15739
Inhibition assays were performed using p-NPP as substrate as described [8] with slight modification. N-acetyl-S-geranyl-L-cysteine, N-acetyl-S-farnesyl-L-cysteine, N-acetyl-S-geranylgeranyl-L-cysteine and S-Farnesyl-thioacetic acid were pre-incubated with Phos2680 (50 ng) or Phos15739 (150 ng) for 5 min, and then reacted with 640 μM or 240 μM p-NPP in reaction buffer (MES, 100 mM, pH 6.0, Mg2+, 2 mM). The reaction was stopped by adding 200 μL of 0.5 N NaOH and absorbance was measured at 405 nm.
TLC identification of products from hydrolysis of FPP
Production of farnesol from FPP by Phos15739 was monitored on TLC using hexane/ethyl-acetate (4:1) as the mobile phase [10]. A ten microliter reaction mixture of 1 mM FPP and 400 ng Phos15739 was quenched with an equal volume of methanol at 1 min and 30 min, and loaded on the TLC plate. Pure FPP, FMP, FOH and Phos15739 were utilized as controls.
Quantitative RT-PCR (Q-RT-PCR)
The CG15739 transcript in different development stages of Drosophila was quantified using a 7500 Fast Real-time PCR system (Applied Biosystems, Foster City, CA). Total RNAs were extracted from different stages of D. melanogaster and RNAs were converted to cDNAs as described above. Q-RT-PCR was carried out in 20 μl reaction volume containing SYBR Green Master Mix (Applied Biosystems), total RNAs, and the specific primer pairs (Supplemental Table 1). Cycling conditions were 1 cycle at 95°C for 20 s, followed by 40 cycles of 3 s at 95°C and 30 s at 60°C. Melting analysis was performed at 60°C to 95°C. Each sample was run 3 or more times for Q-RT-PCR analyses. The mRNA expression of CG15739 gene was normalized using actin as the endogenous control gene.
RESULTS
Homology analysis
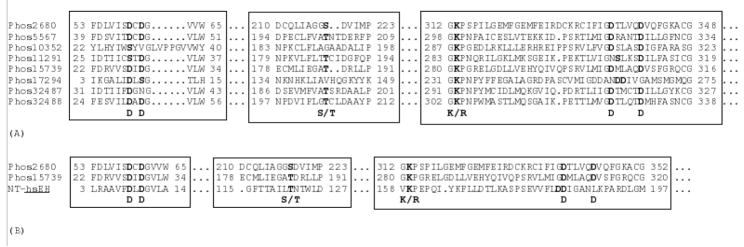
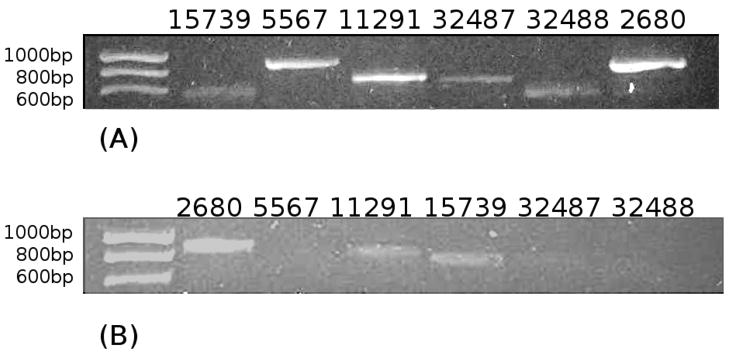
Following release of the D. melanogaster genome sequence, eight gene encoded products, Phos2680, Phos5567, Phos10352, Phos11291, Phos15739, Phos17294, Phos32487 and Phos32488 were identified as putative phosphatase paralogues. Alignment of these (Fig. 1A) showed that they had one or more conserved motifs typical of phosphatases including; 1) an aspartic acid residue as the catalytic nucleophile, 2) a serine or threonine for binding the phosphate group, and, 3) two aspartic acid residues thought to be important for Mg2+ binding [7, 11]. Two phosphatases (Phos17294 and Phos10352) were eliminated from further analysis because they lacked the second serine or threonine conserved motif. CG2680 and CG15739 encoded gene products containing a DXDGV motif, the conserved sequence found in the N-terminal phosphatase domain of hsEH (Fig. 1B), while CG11291 had a less well conserved SXDGV motif. Reverse transcription analysis suggested that 6 phosphatase genes were expressed in early 3rd instar larva (Fig. 2A), however, only CG2680, CG11291 and CG15739 were expressed in ring glands of early 3rd instar larva (Fig. 2B). Based on these results, Phos2680 and Phos15739 were selected as the most likely farnesyl phosphatase candidates.
Figure 1.
Sequence alignment of eight putative phosphatases from D. melanogaster (A) and sequence alignment of Phos15739, Phos2680 with N-terminal domain of hsEH (B). Boxes indicate the three phosphatase motifs as described by Cronin (7), and bold letters indicate the potential conserved residues in each motif.
Figure 2.
RT-PCR analysis of putative phosphatase gene expression in 3rd instar Drosophila melanogaster larvae. (A) RT-PCR using total RNA isolated from 3rd early instar larvae as template, primers are listed in supplemental table 1. From left to right: CG15739 (703 bp), CG5567 (933 bp), CG11291 (795 bp), CG32487 (697 bp), CG32488 (612 bp), and CG2680 (962 bp). (B) Expression of putative phosphatases in the ring glands of 3rd instar larvae by RT-PCR. From left to right: CG2680, CG5567, CG11291, CG15739, CG32487, and CG32488.
Enzyme activity
p-NPP was initially used as a substrate to measure phosphatase activity of purified recombinant Phos2680 and Phos15739. Both Phos2680 and Phos15739 had p-NPPphosphatase activity, with Vmax of 36 μmol/min/mg and 6.1 μmol/min/mg, with corresponding Km values of 640 μM and 240 μM respectively (Supplemental Table 2). Phos2680 and Phos15739 were Mg2+-dependent and had maximum p–NPP activity at pH 6.0 (data not shown).
Since the N-terminal domain of hsEH was reported to be a phosphatase with isoprenoid mono- and pyrophosphate hydrolysis activity [8], different isoprenoid mono- and pyrophosphates were also utilized as substrates for Phos2680 and Phos15739. The kinetics of Phos2680 toward most of the isoprenoid mono- and pyrophosphates substrates except IMP could not be determined with the concentration ranges used. IMP was a good substrate for Phos2680 with Km of 35 μM and Vmax of 59 μmol/min/mg. Unlike Phos2680, Phos15739 catalyzed hydrolysis of most of the isoprenoid mono- and pyrophosphates substrates and had a high turnover rate and Vmax. The 20-carbon substrates GGMP and GGPP, however, were not good substrates (Table 1). GMP was the best Phos15739 substrate with Kcat/Km of 18 X 105 M−1 s−1. IMP, FMP and FPP were also good substrates with Kcat/Km of 1.2 X 105, 3.1 X 105, 2.1 X 105 M−1 s−1 respectively. IPP and GPP were considered to be weak substrates compared with the other mono- and pyrophosphates analyzed.
Table 1.
Phosphatase activity of Phos15739 with isoprenoid mono- and pyrophosphate substrates
| Substrate | Km (μM±S.E.) | Vmax (μmol.min−1mg−1±S.E.) | Kcat (s−1±S.E.) | Kcat/Km (M−1 s−1) |
|---|---|---|---|---|
| IMP | 84 ± 27 | 18 ± 2.7 | 10 ± 1.5 | 1.2 × 105 |
| GMP | 81 ± 6.7 | 250 ± 9.3 | 140 ± 5.3 | 18 × 105 |
| FMP | 160 ± 20 | 85 ± 4.2 | 49 ± 2.4 | 3.1 × 105 |
| GGMPa | >1000a | NDa | NDa | NDa |
| IPPb | 430 ± 150 | 2.1 ± 0.39 | 1.2 ± 0.22 | 0.027 × 105 |
| GPPb | 20 ± 8. 3 | 1.4 ± 1.5 | 0.78 ±0.09 | 250.39 × 105 |
| FPPb | 48 ± 13 | 18 ± 1.8 | 10 ± 1.04 | 2. 1 × 105 |
| GGPPa | >1000a | NDa | NDa | NDa |
GGMP and GGPP are poor substrates of Phos15739, kinetics could not be determined (ND)
Values are reported as apparent kinetics.
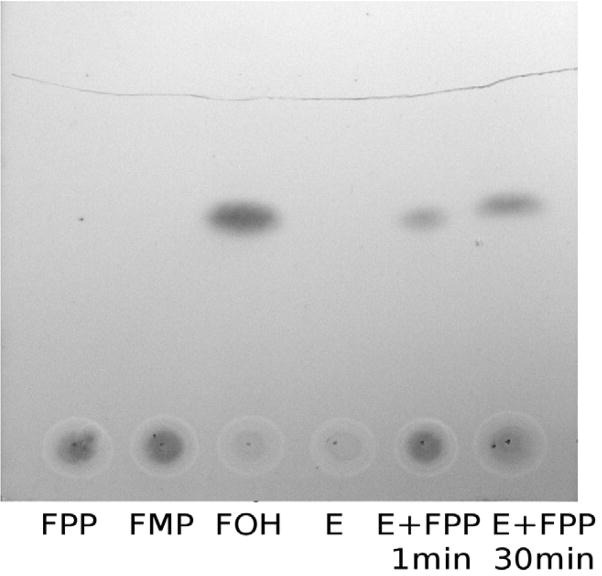
For all the isoprenoid pyrophosphates substrates used, we found no evidence of pyrophosphate release from Phos15739 catalyzed reactions (data not shown). Thus we conclude that Phos15739 is a phosphatase rather than a pyrophosphatase. The final product of FPP hydrolysis by Phos15739 was confirmed to be farnesol using thin layer chromatography (Fig. 3).
Figure 3.
Thin-layer chromatography showing the production of farnesol from FPP by Phos15739. FPP (1 mM, 10 μL), FMP (1 mM, 10 μL), Farnesol (FOH, 1 mM, 10 uL), and Phos15739 (E, 400 ng in 10 μL) were used as standards. FPP and Phos15739 (E + FPP, 400ng and 10 μL of 1 mM respectively) were incubated at room temperature for 1 min or 30 min before loading. The mobile phase was hexane/ethyl acetate (4:1).
To identify the catalytic nucleophile of Phos15739, the Asp-28 of Phos15739 was mutated to Ala-28 to generate variant Phos15739D28A. The enzymatic activity of purified Phos15739D28A was then measured using p-NPP and FPP as substrates. No activity was detected with either substrate under the same reaction conditions as the positive control Phos15739 (data not shown).
Inhibitors of Phos2680 and Phos15739
Because of similarities of Phos2680 and Phos15739 with the N-terminal domain of hsEH, five previously used inhibitors of the N- terminal phosphatase domain of hsEH [8, 12] were evaluated with both Phos2680 and Phos15739. S-Farnesyl-thioacetic acid and N-acetyl-S-geranylgeranyl-L-cysteine were good inhibitors of Phos2680 with IC50 values of 5.1 ± 1.4 and 14 ± 1.9 μM respectively (Table 2). For Phos15739, N-acetyl-S-geranylgeranyl-L-cysteine (IC50 of 4.4 ± 2.0 μM) was the best inhibitor of the compounds tested (Table 2), followed by N-acetyl-S-geranyl-L-cysteine and N-acetyl-S-farnesyl-L-cysteine (IC50 = 6.3 ± 1.7 and 28 ± 6.8 μM respectively). However, S-Farnesyl-thioacetic acid and taurolithocholic acid 3-sulfate were poor inhibitors of Drosophila Phos15739. N-acetyl-S-farnesyl-L-cysteine was previously found to be the best inhibitor of hsEH, indicating inhibitor selectively among Phos2680, Phos15739 and hsEH.
Table 2.
In vitro inhibition of Phos2680 and Phos15739
| Inhibitor | IC50 (μM±S.E.) of Phos2680 | IC50 (μM±S.E.) of Phos15739 |
|---|---|---|
| N-acetyls-geranyl-L-cysteine | >100 | 6.3 ± 1.7 |
| N-acetyl-S-farnesyl-L-cysteine | >100 | 28 ± 6.8 |
| N-acetyl-S-geranylgeranyl-L-cysteine | 14 ± 1.9 | 4.4 ± 2.0 |
| S-Farnesyl-thioacetic acid | 5.1 ± 1.4 | >100 |
| Taurolithocholic acid 3-sulfate | >100 | >100 |
p-NPP (640 μM and 240 μM) was used as the substrate for Phos2680 and Phos15739 respectively.
Stage-Specific Expression of CG15739
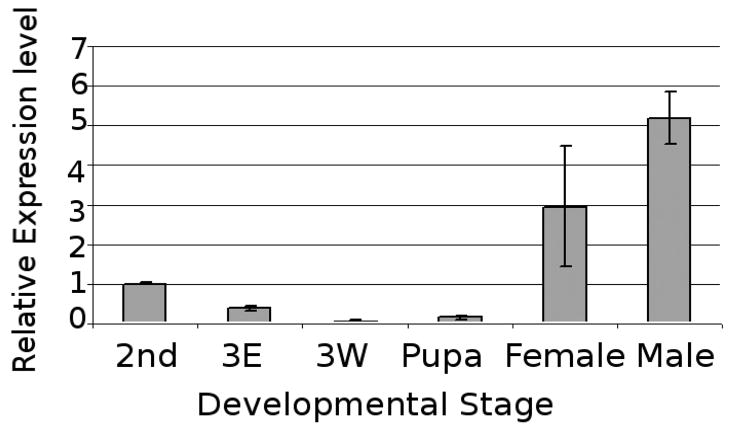
To analyze the developmental regulation of CG15739 expression, CG15739 transcript levels were monitored using Q-RT-PCR. In the developmental stages we evaluated, CG15739 transcript level changes were correlated with previously published JH titer levels (Fig. 4). Setting expression levels of second instar larvae as 1.0, transcript expression levels of 3rd early larvae decreased to 0.4, and were barely detectable in 3rd instar wondering larvae and pupae (0.1 for both) corresponding to low JH titer. On the other hand, the highest CG15739 transcript levels were detected in adult (male 5.2, female 3.0), at the time of maximal JHIII titer [13].
Figure 4.
Comparative expression of CG15739 in different developmental stages of Drosophila melanogaster by Q-RT-PCR. 2nd, 2nd instar larvae; 3E, 3rd instar early stage; 3W, 3rd instar wondering stage; pupa, pupae collected with first 4 hr; female and male adult, adult samples collected after eclosion.
DISCUSSION
Identifying enzymes involved in the conversion of farnesyl pyrophosphate to JH has been difficult due to the apparent lack of this pathway in vertebrates. Recently, an ortholog of the Bombyx mori methyltransferase which converts farnesoic acid to methyl farnesonate was identified in the Aedes expressed sequence tags (ESTs) collection [4], and a cytochrome P450 (CYP15A1) catalyzing stereoselective expoxidation of methyl farnesonate to JHIII in the CA of the cockroach was found using ESTs from the CA of Diploptera punctata [6]. ESTs from D. punctata, Aedes aegypti and Anopheles albimanus were compared to find potential genes encoding enzymes involved in juvenile hormone biosynthesis. Only one enzyme, Anopheles orthology EAA01914, was predicted to have activity capable of converting FPP to farnesol [14]. However, sequences from D. melanogaster producing significant alignments with Anopheles EAA01914 show homology to trans-isoprenyl diphosphate synthases including farnesyl diphosphate synthases and geranylgeranyl diphosphate synthases, enzymes catalyzing the elongation of isoprenoid substrates to produce prenyl diphosphates.
The genome of D. melanogaster harbors several genes with protein products predicted to be phosphatases. In our study, Phos2680 and Phos15739 were selected as the two most likely candidates for catalyzing FPP hydrolysis based on amino acid sequence analysis of these proteins with subclass I of the haloacid dehalogenase (HAD) superfamily including mammalian sEH, HAD, PSP, and PhAH [7, 15]. Genes CG2680 and CG15739 encode phosphatases displaying the three typical conserved motifs (motif I: DXDX[T/V][L/V]; motif II: [S/T]XX; and motif III: K-[G/S][D/S]XXX[D/N]) for nucleophile attack, substrate binding, and Mg2+ binding respectively [16] (Fig. 1B). Both CG2680 and CG15739 were also observed in the ring gland of D. melanogaster using reverse transcription PCR. Six other putative phosphatases from D. melanogaster were eliminated from further analysis because of a lack of structural motifs or non-detectable expression in ring glands.
Variant Phos15739D28A was generated by substituting residue Asp-28, the equivalent of hsEH Asp-9, with Ala. The purified mutant Phos15739D28A exhibited no p-NPP or FPP activities (data not shown), consistent with our hypothesis that Asp-28 located in motif I is the catalytic nucleophile of the enzyme.
Although both Phos2680 and Phos15739 showed high catalytic activity using p-NPP, only Phos15739 efficiently hydrolyzed FPP and released monophosphate from the reaction. Previously, the enzyme thought to catalyze hydrolysis of FPP to farnesol was thought to be a pyrophosphatase, which releases pyrophosphate directly from FPP [2]. Our data show that Phos15739 is a mono-phosphatase which hydrolyzes FPP to FMP then to farnesol.
Since FPP is a key intermediate for JH biosynthesis, a balance between synthesis and hydrolysis of FPP seems critical. In insects, IPP is converted to dimethylallyl diphosphate (DMAPP) by isopentenyl-diphosphate isomerase, followed by the condensation of IPP (5-carbon) into DMAPP and sequentially to GPP (10-carbon) and FPP (15-carbon) by farnesyl diphosphate synthetase [14]. Although IPP and GPP were substrates of Phos15739, the lower Km/Kcat of Phos15739 with IPP (0.027 X105 M−1 s−1) and GPP (0.39 X105 M−1 s−1) suggest these compounds are rather poor substrates. On the other hand, both FMP and FPP are approximately 10-fold better substrates for Phos15739 with Km/Kcat of 3.1X105 and 2.1 X105 M−1 s−1 (Table 1).
Six forms of JH sharing a similar synthesis pathway have been identified and characterized in insects, and the relative level of JHs in insects is one of the major factors regulating insect development and metamorphosis [17]. The regulation of JH titer is therefore critical for insect maturation and thus this pathway is an attractive target for insecticide development [5]. We identified Phos15739 as an insect FPPase and therefore insecticide target by aligning Drosophila phosphatases with the N-terminus of hsEH, however, the effect of various inhibitors on the hsEH phosphatase and Phos15739 were surprisingly different. In our study, N-acetyl-S-geranylgeranyl-L-cysteine with an IC50 value of 4.4 μM was the best inhibitor of Phos15739. N-acetyl-S-farnesyl-L- cysteine, the best isoprenoid-derived inhibitor (IC50 as 0.84 μM) [8], and taurolithocholic acid 3-sulfate the best sulfate inhibitor for the hsEH N-terminal phosphatase (IC50 equal to 5 μM) [12], however, had IC50 values of 28 and over 100 μM for Phos15739. Optimizing inhibitors of Phos15739 using N-acetyl-S-geranylgeranyl-L-cysteine as a lead compound may provide a means of disrupting JH biosynthesis and consequentially inducing premature metamorphosis or preventing reproduction in insects.
The ring gland, the endocrine/neurohaemal organ of insects, includes two lateral prothoracic glands, corpus cardiacum and corpus allatum (CA). The CA is considered the likely site of synthesis and secretion of JH in D. melanogaster [18]. The expression of Phos15739 in ring glands was confirmed by RT-PCR using cDNA synthesis from ring glands as template. Therefore, to the best of our knowledge, Phos15739 is the first FPP mono-phosphatase that has been confirmed to be expressed in ring glands.
In D. melanogaster, the titre of JHIII decreases from a high level in 2nd instar larvae to relative lower levels in 3rd instar larvae, and is undetectable in early pupa [13]. In adult females, JH is again synthesized as it is required to activate ovary maturation during reproduction [19, 20]. In males, JH has been shown to play an important role for protein synthesis in accessory glands [21]. Mated males have 3-fold more JH and released twice as much sex pheromone as virgin males, suggesting that JH is involved in the development of sexual signaling, promoting male mating behavior and reproductive maturity [22]. In our study, CG15739 expression was detected in ring glands, and high levels of transcript were detected in 2nd instar larvae and adults, whereas relatively lower levels of transcript were detected in 3rd instar wondering larvae and pupae of D. melanogaster (Fig. 4). Our study also showed that expression levels of Phos15739 protein were correlated with previously reported JHIII levels. A peak of Phos15739 appeared in 2nd instar larvae, and decreased in the wondering stage, followed by very low expression in the pupal stage. Because JH titer is determined by its rate of biosynthesis, release, and degradation [3] our results suggest that Phos15739 might be important in regulating JH levels in vivo.
In summary, we show that Phos15739 from D. melanogaster is localized in the ring gland and hydrolyzes FPP to farnesol. The coordinated expression of CG15739 with JH titer during development suggests a potential regulatory role in JH biosynthesis. Inhibition of this enzyme in vivo will be important for understanding its role in regulating JH levels and, potentially for insect control.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants ES011630 and a grant from the University of Connecticut Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Truman JW, Riddiford LM. Endocrine Insights into the Evolution of Metamorphosis in Insects. Annu Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- 2.Belles X, Martin D, Piulachs MD. The Mevalonate Pathway and the Synthesis of Juvenile Hormone in Insects. Annu Rev Entomol. 2005;50:181–199. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- 3.Kort CADd, Granger NA. Regulation of JH titers: the relevance of degradative enzymes and binding proteins. Arch Insect Biochem Physiol. 1996;33:1–26. [Google Scholar]
- 4.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minakuchi C, Riddiford LM. Insect juvenile hormone action as a potential target of pest management. J Pestic Sci. 2006;31:77–84. [Google Scholar]
- 6.Helvig C, Koener JF, Unnithan GC, Feyereisen R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA. 2004;101:4024–4029. doi: 10.1073/pnas.0306980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronin A, Mowbray S, Durk H, Homburg S, Fleming I, Fisslthaler B, Oesch F, Arand M. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci. 2003;100:1552–1557. doi: 10.1073/pnas.0437829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enayetallah AE, Grant DF. Effects of human soluble epoxide hydrolase polymorphisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun. 2006;341:254–260. doi: 10.1016/j.bbrc.2005.12.180. [DOI] [PubMed] [Google Scholar]
- 9.Argiriadi MA, Morisseau C, Hammock BD, Christianson DW. Detoxification of Environmental Mutagens and Carcinogens: Structure, Mehcanism, and Evolution of Liver Epoxide Hydrolase. Proc Natl Acad Sci. 1999;96:10637–10642. doi: 10.1073/pnas.96.19.10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornby JM, Kebaara BW, Nickerson KW. Farnesol Biosynthesis in Candida albicans: Cellular Responseto Sterol Inhibition by Zaragozic Acid B. Antimicrob Agents Chemother. 2003;47:2366–2369. doi: 10.1128/AAC.47.7.2366-2369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronin A, Homburg S, Durk H, Richter I, Adamska M, Frere F, Arand M. Insights into the catalytic mechanism of human sEH phosphatase by site-directed mutagenesis and LC-MS/MS analysis. J Mol Biol. 2008;383:627–640. doi: 10.1016/j.jmb.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Tran KL, Aronov PA, Tanaka H, Newman JW, Hammock BD, Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry. 2005;44:12179–12187. doi: 10.1021/bi050842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bownes M, Rembold H. The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur J Biochem. 1987;164:709–712. doi: 10.1111/j.1432-1033.1987.tb11184.x. [DOI] [PubMed] [Google Scholar]
- 14.Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem Mol Biol. 2006;36:366–374. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen KN, Dunaway-Mariano D. Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem Sci. 2004;29:495–503. doi: 10.1016/j.tibs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Kim R, Jancarik J, Yokota H, Kim S. Crystal structure of phosphoserine phosphatase from Methanococcus jannaschii, a hyperthermophile, at 1.8 Å resolution. Structure. 2001;9:65–71. doi: 10.1016/s0969-2126(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert LI, Granger NA, Roe RM. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem Mol Biol. 2000;30:617–644. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 18.Richard DS, Applebaum SW, Sliter TJ, Baker FC, Schooley DA, Reuter CC, Henrich VC, Gilbert LI. Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila melanogaster: a putative juvenile hormone in the higher Diptera. Proc Natl Acad Sci. 1989;86:1421–1425. doi: 10.1073/pnas.86.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochem Mol Biol. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Caroci AS, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. J Exp Biol. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TG, DeMoor S, Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant27 mutant phenotype. Insect Biochem Mol Biol. 2003;33:1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Teal PEA, Gomez-Simuta Y, Proveaux AT. Mating experience and juvenile hormone enhance sexual signaling and mating in male Caribbean fruit flies. Proc Natl Acad Sci. 2000;97:3708–3712. doi: 10.1073/pnas.060034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.