Abstract
Little is known about ion channels that regulate the graded, subthreshold properties of nerve terminals. Using the calyx of Held, we demonstrate here a large presynaptic persistent Na+ current with unusually hyperpolarized activation voltage. This feature allowed the current to determine both the resting potential and resting conductance of the nerve terminal. Calyces express presynaptic glycine receptors whose activation depolarizes the synapse. We found that activation of the persistent Na+ current was an essential component in the response to glycine. This Na+ current originated at or very close to the terminal, and was sustained even after trains of large spike-like depolarizations. Because Na+ channels also underlie the presynaptic action potential, we conclude that their action both triggers and modulates exocytosis through control of presynaptic membrane voltage.
Keywords: Na+ channel, calyx of Held, chloride channel, exocytosis, glycine
Introduction
Neurotransmitter release from synapses is triggered by propagated Na+-channel dependent action potentials. Exocytosis can also be modulated by subthreshold changes in membrane potential, either from passive spread of current from the somatodendritic region of neurons or from activation of presynaptic ligand-gated channels (Awatramani et al., 2005; Turecek and Trussell, 2001, 2002). These effects should depend critically on the complement of ion channels that determine how synapses respond to stimuli from these diverse sources. Na+ channels are expressed in synaptic boutons or at the preterminal axonal membrane (Ahern et al., 2000; Engel and Jonas, 2005; Leao et al., 2005), and this distribution may enhance the amplitude or timing of spike-driven transmitter release. By making recordings from the calyx of Held, a giant mammalian nerve terminal, we identified a substantial, steady-state (persistent) Na+ current (Crill, 1996) with novel properties that had profound effects on presynaptic function.
Results
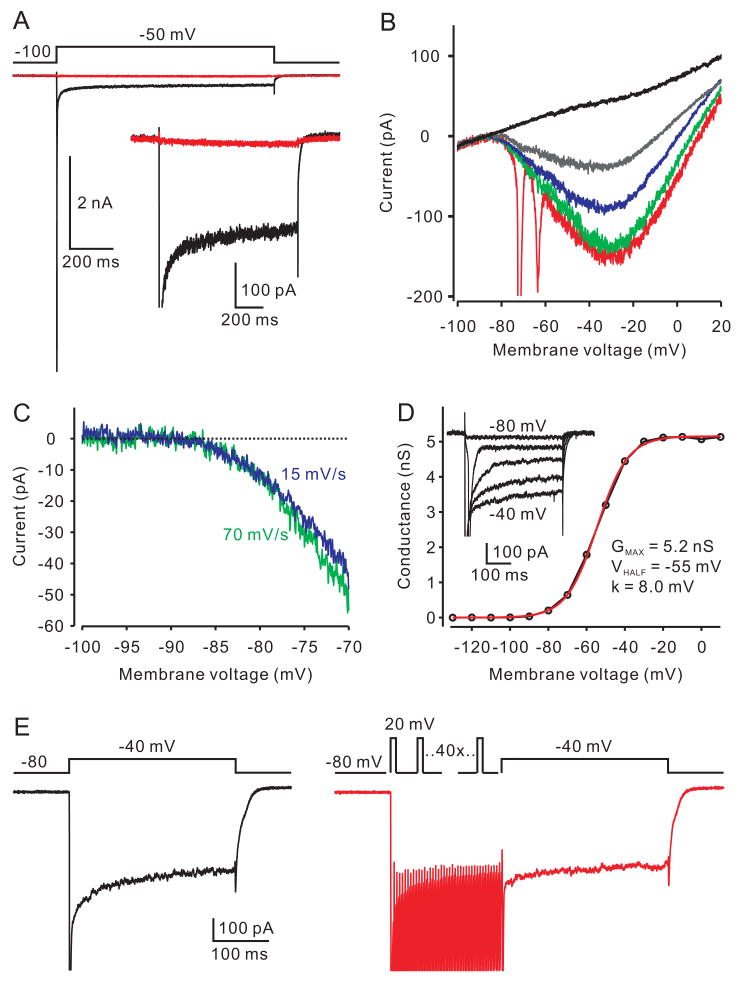
Na+ currents in the calyx of young rats were studied in the presence of K+ and Ca2+ channel blockers (see Methods). A test pulse from −100 mV to −50 mV evoked a large transient inward current followed by a smaller current that was sustained for over 1 second (Fig 1A). Both the transient and persistent currents were blocked by bath application of the Na+-channel blocker tetrodotoxin (TTX, 500 nM), thus identifying the sustained current as persistent Na+ current (INaP). INaP was also examined using a series of voltage ramps of different rates (Fig 1B). Spiking and associated escapes from voltage-clamp appeared in the current trace during the fastest ramp (280 mV/s, red trace), however with slower ramps (70 mV/s, green trace; 16 mV/s, blue trace) escaping spikes were absent while the INaP was only marginally inactivated. INaP evoked with ramps was partially blocked by 10 nM TTX (grey trace) and fully blocked by 500 nM TTX (black trace). By subtraction of a control trace from one with 500 nM TTX, the current voltage relation for INaP could be determined, revealing a remarkably negative detection threshold for activation of −85.2 ± 0.6 mV (Fig 1C; n=8), as compared to previous observations (e.g., Bevan and Wilson, 1999; Enomoto et al., 2006; Taddese and Bean, 2002). Similar properties were seen for synapses recorded close to physiological temperature (34°C) (Supplemental Fig S1).
Fig 1.
Activation of a presynaptic INaP. (A) A depolarizing voltage step evoked a transient Na+ component (arrow) followed by a steady-state (persistent) current (black trace). Both components were fully blocked by 500 nM TTX (red trace). The inset shows the INaP at higher gain. Leak subtraction was applied to the traces. (B) Influence of ramp speed on INaP. The ramp speeds are +280, 70 and 16 mV/s for red, green and blue traces, respectively. The current is partially blocked by 10 nM TTX (grey trace) and fully blocked by 500 nM TTX (black trace). Data in A and B were obtained from one calyx. (C) Expanded view of activation of current in b for two ramps speeds after subtracting trace in TTX. Activation of current is first apparent at about −85 mV. (D) Conductance voltage curve for one calyx, with representative voltage steps shown as inset. Red line is Boltzmann fit with parameters as indicated. (E) Effect of conditioning “spikes” on INaP. Left panel is control response to a voltage step to −40 mV. Peak current is cut off. Right panel shows a step to −40 mV preceded by 40 1-ms steps to +20 mV delivered at 200 Hz. A 4-ms return to −80 mV followed the last brief pulse and before the test pulse to −40 mV. After the pulse train, the mean INaP measured 250–300 ms after pulse onset was 91.2 ±1.8% of control (n=5).
To explore further these activation characteristics, conductance vs voltage plots were constructed using voltage steps in which the INaP was averaged 250–300 ms after pulse onset (Fig. 1D). The ratio of INaP to peak (transient) currents in response to steps was 2.4±0.4% (n=6). Boltzmann fits to these (Fig 1D, red line) revealed a maximal conductance of 4.13 ± 0.88 nS, a VHALF of −50.9± 2.9 mV, and a slope factor of 9.8 ± 0.7 mV (n=6). This slope factor is higher than that of many previous reports of NaP and may account for the more negative activation voltage (Kay et al., 1998; Magistretti and Alonso, 1999; Magistretti et al., 2006; Taddese and Bean, 2002; but see Parri and Crunelli, 1998; Wu et al., 2005). To test if the INaP could be less available (more inactivated) after trains of spikes, we compared INaP before and after a train of 40 1-ms pulses to +20 mV delivered at 200 Hz (Fig. 1 E). These experiments showed that INaP remained at near normal amplitude after the conditioning train (91.2±1.8% of control, n=5).
It is possible that the very negative value for activation is an artifact: if the terminal voltage clamp did not extend far into the axon, and if the axon is depolarized by the K+ channel blockers, then a very negative presynaptic clamp potential would be needed to bring the distal axon to a more normal activation voltage for INaP. Two lines of evidence argue against this possibility. First, we recorded the size and activation voltage for INaPand then estimated the axonal length after loading the terminal with Alexa 594. Fig S2 shows an examples of INaP recorded from a calyx with a >700 μm axon and one with no apparent axon at all, showing that the amplitude and Boltzmann parameters were similar. On average, terminals with axons <50 μm had about 2/3 the INaP of terminals compared with axons > 100 μm (205 ± 54 pA vs 306 ± 9 pA, p=0.11, n= 5 each) and no difference in activation voltage (−84.2 ± 1.0 mV vs −84.4 ± 0.9 mV, p=0.89, n=5 each). Another control was to measure activation voltage in calyces recorded using the same pipette solution used for current clamp, and no K+ channel blockers in the bath. Although this limits how effectively we can control voltage in the positive range, it also prevents the axon resting potential from being very different from the calyx. These experiments gave INaP activation potentials similar to control values (Fig. S3; −84.5 ± 1.3 mV, n=6). Thus, these data show that most INaP arises close to or at the terminal and that its threshold voltage for detection of activation is unusually negative.
It is therefore likely that INaP could be active at, and contribute to, the resting membrane potential. Indeed, in current clamp, application of 2 μM TTX by local pressure ejection hyperpolarized the membrane potential by 2 mV (Fig 2A,B; from −77.3±0.9 mV to −79.3± 1.1 mV, p<0.0001, n=11), and decreased the resting membrane conductance by 27% (Fig 2C; from 4.4 ± 0.4 nS to 3.2 ± 0.5 nS; p < 0.05, n=6). The INaP is unlikely a developmentally transient current since it was larger in older animals (p < 0.01; peak amplitude measured from ramp protocol of 60 ± 19 pA (n=4) for P7–8 rats, 130±18 pA (n=6) for P9 rats, 414 ± 77 pA (n=6) for P10–11 rats).
Fig. 2.
Effects of TTX on resting presynaptic membrane properties. (A–B) Puff application of 2 μM TTX hyperpolarizes the resting potential by about 2 mV (n=9). (C) Bath application of TTX decreased resting conductance (n=5). Conductance was estimated in current clamp around the resting potential.
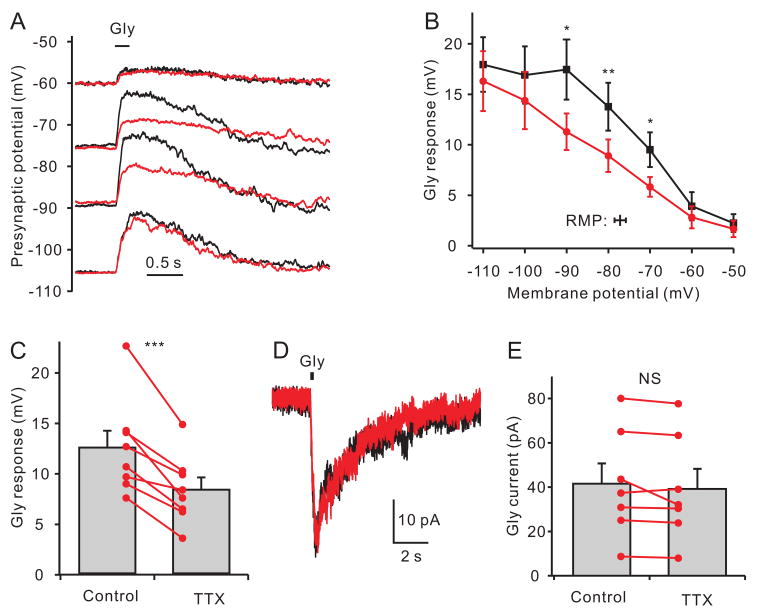
Because the intraterminal concentration of Cl− is high (Price and Trussell, 2006), activation of presynaptic glycine and GABAA receptors depolarize terminals and facilitate exocytosis (Awatramani et al., 2005; Turecek and Trussell, 2001). Given the negative activation range for INaP, we predicted the effects of glycine would be augmented by INaP. Glycine generated a depolarization whose amplitude declined as the membrane potential was shifted towards the Cl− reversal potential of −46 mV (Fig 3A,B). TTX decreased the amplitude of the glycine responses triggered from voltages between −70 ~ −90 mV. At more negative potentials, where INaP was not active, or at potentials closer to the reversal potential for Cl−, TTX had no effect. At −80 mV, close to resting potential (−77 mV), TTX reduced the glycine response by 34% (Fig 3C; from 12.5 ± 1.6 mV to 8.3 ± 1.2 mV, p<0.001, n=8). As expected, the glycine-evoked current under voltage-clamp mode was not altered by TTX (Fig 3D,E; 41.5 ± 9.2 pA in control and 39.2 ± 9.0 pA in TTX, p=0.20, n=7, VHOLD= −80 mV), indicating that TTX did not directly affect glycine receptors.
Fig 3.
Modulation of glycine responses by persistent Na+ current. (A) Calyceal membrane potentials were adjusted by currents injection. Puff application of glycine (1 mM at bar) evoked depolarizing responses (black trace). Bath applied TTX (red trace) decreased the glycine response at voltages between −75 mV and −90 mV, but had no effect at −60 mV and −105 mV. (B) Average data show a maximal effect of TTX on glycine responses (black, control; red, TTX) evoked near the resting potential (symbol at bottom of figure). p < 0.05 for −70 and −90 mV; p < 0.01 for −80 mV, n=5. (C) At −80 mV, close to average resting potential, the glycine response was significantly decreased by TTX (p < 0.001, n=8). (D) Under voltage clamp, the glycine-induced current was not affected by application of TTX in one representative calyx. (E) Data from 7 voltage-clamped calyces showing that TTX does not alter the glycine-evoked current (VHOLD=−80mV).
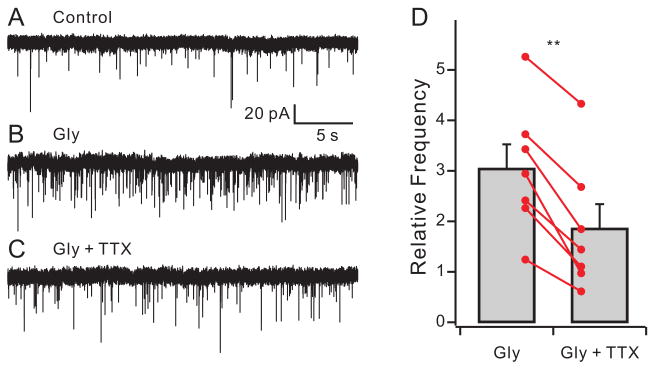
A small change in presynaptic potential produced by glycine or GABA has large effects on transmitter release (Awatramani et al., 2005). It was not possible to test the effectiveness of the glycine or GABA as a modulator of evoked release in the absence of INaP because reducing INaP with TTX also affects the presynaptic spike. However, the depolarizing action of glycine increases the frequency of spontaneous miniature EPSC (mEPSCs) measured in postsynaptic neurons (Turecek and Trussell, 2001), and this provided a means to determine the role of Na+ channels in modulating resting release probability. The presynaptic axon to the calyx does not fire spontaneously in vitro, and when active generates a very large EPSC (Borst et al., 1995). Thus, small spontaneous events are likely due to mEPSCs from the calyx, with a small contribution from non-calyceal inputs (Hamann et al., 2003). Fig 4A–C shows that 200 μM glycine increased the frequency of spontaneous EPSCs 3.04 ± 0.48-fold (p<0.01, n=7, paired t-test; control: 0.42 ± 0.10 Hz; glycine: 1.28 ± 0.37 Hz, p<0.05). Co-application of 500 nM TTX with glycine increased the frequency only 1.86 ± 0.48-fold (p < 0.001, n=7, paired t-test compared with only glycine; 0.78 ± 0.30 Hz, p<0.01). Thus, the effectiveness of the presynaptic modulator was nearly doubled by coactivation of INaP. TTX alone decreased mEPSC rate in only 2 of 7 cells (>20% change in frequency), indicating that the 2 mV contribution of INaP to resting potential alone has little effect on mEPSC rate (0.42±0.11 Hz for control; 0.35±0.06 Hz in TTX, n=7, p=0.24).
Fig 4.
Na+ channels augment the glycine effect on mEPSC frequency. Glutamatergic spontaneous EPSCs are recorded under control condition (A), 200 μM glycine (B), and 200 μM glycine plus 500 nM TTX (C). VHOLD = −77 mV. (D) Relative change of spontaneous frequency by glycine (gly) and glycine plus TTX. The frequency in the presence of glycine is significantly decreased by TTX.
Discussion
INaP is generally associated with the pacemaking or bursting activity of neurons because it is often activated positive to the resting potential but negative to spike threshold (Bevan and Wilson, 1999; Enomoto et al., 2006; Taddese and Bean, 2002). We find that the presynaptic INaP has a more negative activation voltage (~−85 mV); to our knowledge this is unlike other reports of native or recombinant Na+ channels. This biophysical feature enables it to regulate the resting properties of the synapse, an outcome both of the effect of INaP on the resting voltage and conductance, but also the sensitivity of INaP to small depolarizations. Because the probability of vesicle release is sensitive to small changes in presynaptic membrane potential (Awatramani et al., 2005), the INaP can boost the effectiveness of presynaptic modulators that activate ion channels, such as GABA, glycine, and acetylcholine. Recently, a TTX-insensitive non-selective cation channel NALCN, was identified as the major “Na+ leak” channel of neurons (Lu et al., 2007), due to its voltage independent gating. The presynaptic INaP, because of its negative activation voltage, can act both as a resting inward current and as a voltage-sensitive amplifier. Given its TTX sensitivity, it is unlikely to be Ca2+ permeable (unlike NACLN), an important adaptation for an inward resting conductance of nerve terminals.
Since TTX-sensitive Na+ channels can act locally and in the subthreshold voltage range, our results suggest caution in interpreting studies which use Na+ channel blockers to determine whether compounds that regulates synaptic activity act on presynaptic membrane targets or upstream in axonal/somatic/dendritic compartments. Indeed it is possible that receptors previously identified as axonal or somatodendritic could be localized presynaptically (Jeong et al., 2003; Lena et al., 1993; Schwyzer et al., 2002; but see McMahon et al., 1994). Our observations are also relevant to recent studies that show that voltage change in cell bodies may be passively conducted to nerve terminals and there trigger or modulate transmitter release (Alle and Geiger, 2006; Shu et al., 2006). A prediction from the present study is that such ‘analog’ transmission would be controlled by presynaptic INaP, and is consistent with its sensitivity to TTX (Alle and Geiger, 2006).
It remains unclear what is the site of expression of the INaP channels. Our data show that the bulk of the current arises very close to or at the calyx, since changes in axon length had only moderate effects on current amplitude. It is possible that INaP is generated by the same channels that generate the large phasic Na+ current (Taddese and Bean, 2005). A previous study of the calyx showed that inactivating Na+ channels are mainly expressed at pre-terminal membrane, although excised patch experiments suggested some presynaptic Na+ channels (Leao et al., 2005). Engel and Jonas (2005), however, were able to detect Na+ current on mossy fiber boutons of hippocampus. In both studies, inactivation was reported to be complete. Nevertheless one would expect some persistent current near the calyx. A −10 nA peak current, similar to currents reported previously in the calyx (Leao et al, 2005), would produce a −200 pA steady-state current upon 98% inactivation, a level of inactivation similar to published values (Taddese and Bean, 2005); this is indeed consistent with our own estimates of the degree of inactivation (97.6%; mean INaP −229±48pA, n=17). Thus, it is not improbable that INaP is produced by Na+channels near the calyx terminal. If so, this would indicate that terminal or preterminal channels regulate presynaptic membrane properties.
The slope of the Boltzmann curve we obtained was shallower than that reported in many (though not all) previous studies of INaP. There are several possible explanations. A potential artifact is that, as the terminal is clamped to more depolarized regions, more distant regions of axon may be depolarized and their current activated, particularly in the presence of K+ channel blockers. This would spread out the Boltzmann curve and shift the apparent activation voltage negative and the VHALF positive. However, our measured VHALF of −51 mV is similar to published values (Kay et al., 1998; Magistretti et al., 1999;Magistretti et al., 2006; Taddese and Bean, 2002). Moreover, the activation voltage was independent of axon length and was unchanged when K+ channels were active. To explain a shallow Boltzmann slope, it may be that the channel underlying INaP has unusual biophysical features, consistent with its clearly negative initial activation voltage. However, it could be instead that there are two channels underlying the INaP, one having a more negative point of activation and VHALF, and one more conventional. Together, these would spread out the Boltzmann curve. Some cells did indeed seem to show slight evidence of double Boltzmann components but this was inconsistent and measurement error could not be reliably excluded. In either case however, the data argue for a novel and functionally significant component to INaP in the nerve terminal.
Methods
Slice Preparation
The handling and care of animals was approved by OHSU. Coronal slices of brainstem were prepared from 7- to 13-day-old Wistar rats (Awatramani et al., 2005; Borst and Sakmann, 1995; Turecek and Trussell, 2001). Briefly, 180–220 μm thick sections were prepared in ice-cold, low-Ca2+, low-Na+ saline using a vibratome (VT1200S; Leica). Immediately after the slices were cut, they were incubated at 35°C for 30–60 min in normal ACSF and thereafter stored at room temperature. The ACSF for incubation and recording contained (in mM) 125 NaCl, 25 glucose, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 0.4 ascorbic acid, 3 myo-inositol, and 2 Na-pyruvate, pH 7.4 bubbled with 5% CO2/95% O2.
Whole-Cell Recordings
Slices were transferred to a recording chamber and were continually perfused with ACSF (2–3 ml/min) at room temperature, except as noted. Calyces and MNTB neurons were viewed using Dodt contrast optics and a 40× water-immersion objective (Olympus). Pipettes pulled from thick-walled borosilicate glass capillaries (WPI) had open tip resistances of 3–5 MΩ and 2–3 MΩ for the pre- and postsynaptic recordings, respectively. Whole-cell current- and voltage-clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA). Calyx terminals and MNTB neurons were identified visually by their appearance in contrast optics and/or presynaptic fluorescence of Alexa 594 (10–20 μM). For presynaptic current-clamp experiments, pipettes contained (in mM) 110 K-gluconate, 20 KCl, 1 MgCl2, 10 HEPES, 4 MgATP, 0.3 Tris-GTP, and 3 Na2-phosphocreatine and 10 Tris2-phosphocreatine (290 mOsm; pH 7.3 with KOH). For presynaptic voltage-clamp experiments, pipettes contained (in mM) 120 Cs-methane sulphonate, 10 CsCl, 10 TEA-Cl, 1 MgCl2, 10 HEPES, 5 EGTA, 0.4 Tris-GTP, 3 Mg-ATP and 5 Na2-phosphocreatine (293 mOsm, pH 7.3 with CsOH), except as noted. For postsynaptic MNTB principal neuron recording, pipettes contained (in mM) 135 CsF, 4 CsCl, 5 EGTA, 1 MgCl2, 10 HEPES, 2 QX314 and 0.1–1 DIDS (289 mOsm, pH 7.3 with CsOH). Series resistances (6–25 MΩ) were compensated by 60%–80% (bandwidth 3 kHz). Signals were filtered at 10 kHz and sampled at 20 kHz.
To isolate presynaptic Na+ currents in response to voltage steps or ramps, TEA-Cl (10 mM), and 4-AP (2 mM) and CdCl2 (200 μM) were added to ACSF, substituting for NaCl with equal osmolarity. Resting potential was determined in current clamp (zero holding current). Liquid junction potentials were measured for all solutions, and reported voltages are appropriately adjusted. The resting conductance was measured in current clamp mode using current ramps around the resting potential. Drugs were applied by pressure ejection or bath perfusion. TTX (Tocris) and other drugs were stored as aqueous stock solutions at −20 °C.
Analysis
Data were analyzed using Clampfit (Molecular Devices) and Igor (WaveMetrics). mEPSCs were sampled by template matching using a rise time of 0.2 ms and decay of 0.5 ms, threshold of 3X noise SD, using Axograph X. The detection threshold for activation INaP was determined from 2kHz-filtered ramp data by extrapolating a line fitted between −100 and −90 mV; the point of deviation from this line (typically by several pA to be obvious by eye; see Figs. 1, S3) was considered as the point of detectable activation of INaP. Boltzmann functions were used to describe NaP activation: G = GMAX/(1+exp(−(V-VHALF)/k)), where G is conductance in nS, GMAX is the maximal conductance, V is the potential in mV, VHALF is the voltage for half-maximal activation in mV, and k is the slope factor in mV. Statistical significance was established using paired and unpaired t-tests as indicated. Data are expressed as mean±SEM.
Supplementary Material
Acknowledgments
We thank Drs Veeramuthu Balakrishnan, Matt Frerking, Henrique von Gersdorff Craig Jahr, and John Williams for manuscript comments. Supported by NIH grant DC04450.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Hsu SF, Klyachko VA, Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci. 1999;19:7617–7628. doi: 10.1523/JNEUROSCI.19-17-07617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci. 2006;26:3412–3422. doi: 10.1523/JNEUROSCI.5274-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Billups B, Forsythe ID. Non-calyceal excitatory inputs mediate low fidelity synaptic transmission in rat auditory brainstem slices. Eur J Neurosci. 2003;18:2899–2902. doi: 10.1111/j.1460-9568.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Moorhouse AJ, Akaike N. Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J Physiol. 2003;550:373–383. doi: 10.1113/jphysiol.2003.041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Sugimori M, Llinas R. Kinetic and stochastic properties of a persistent sodium current in mature guinea pig cerebellar Purkinje cells. J Neurophysiol. 1998;80:1167–1179. doi: 10.1152/jn.1998.80.3.1167. [DOI] [PubMed] [Google Scholar]
- Leao RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994;59:689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study. J Gen Physiol. 1999;114:491–509. doi: 10.1085/jgp.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Castelli L, Forti L, D’Angelo E. Kinetic and functional analysis of transient, persistent and resurgent sodium currents in rat cerebellar granule cells in situ: an electrophysiological and modelling study. J Physiol. 2006;573:83–106. doi: 10.1113/jphysiol.2006.106682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci. 1998;18:854–867. doi: 10.1523/JNEUROSCI.18-03-00854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Trussell LO. Estimate of the chloride concentration in a central glutamatergic terminal: a gramicidin perforated-patch study on the calyx of Held. J Neurosci. 2006;26:11432–11436. doi: 10.1523/JNEUROSCI.1660-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer L, Mateos JM, Abegg M, Rietschin L, Heeb L, Thompson SM, Luthi A, Gahwiler BH, McKinney RA. Physiological and morphological plasticity induced by chronic treatment with NT-3 or NT-4/5 in hippocampal slice cultures. Eur J Neurosci. 2002;16:1939–1948. doi: 10.1046/j.1460-9568.2002.02259.x. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc Natl Acad Sci USA. 2002;99:13884–13889. doi: 10.1073/pnas.212419699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability. J Neurophysiol. 2005;98:2710–2722. doi: 10.1152/jn.00636.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.