Abstract
Since flavonoids scavenge reactive oxygen species, they may potentially protect against ischemia/reperfusion injury. This study compared the scavenging capacity of specific flavonoids towards different reactive oxygen species. Whether the differential oxidant scavenging capacity correlated with their protective efficacy in ischemia/reperfusion injury of cardiomyocytes was determined. The free radical scavenging capacity of five flavonoids (wogonin, baicalin, baicalein, catechin and procyanidin B2) was analyzed using electron spin resonance spectrometry for 3 radicals: 1,1-diphenyl-2picrylhydrazyl (DPPH), superoxide and hydroxyl radical. A well-established chick cardiomyocyte model of ischemia (1 h)/reperfusion (3 h) was used to evaluate flavonoid-induced protection against ischemia/reperfusion injury in chronic treatment (pretreated 72 h and treated through ischemia/reperfusion) and acute treatment protocols (during ischemia/reperfusion or only at reperfusion). The cell viability was assessed by propidium iodide. The DPPH scavenging was most significant with catechin, followed by procyanidin B2, baicalein, baicalin, and wogonin. The superoxide scavenging was, similarly, most significant with catechin, followed by baicalein, procyanidin B2, and baicalin. For hydroxyl radical, only baicalein showed a significant scavenging capacity (> 50% reduction in ESR signal). For the cardiomyocyte studies, all flavonoids but wogonin showed protection against ischemia/reperfusion injury in the chronic treatment protocol. When flavonoids were administered only during ischemia/reperfusion, baicalein, procyanidin B2, and catechin significantly reduced cell death. If flavonoids were administered just at reperfusion, only baicalein and procyanidin B2 had protective effects, and the efficacy was less. Flavonoids possess specific but differential radical scavenging capacity, which, in conjunction with the timing of treatment, affects their protective efficacy in cardiomyocytes exposed to ischemia/reperfusion.
Keywords: Flavonoids, Reperfusion injury, Scavenging capacity, Superoxide, Hydroxyl radical
1. Introduction
Flavonoids are naturally occurring polyphenolic compounds found ubiquitously in vegetables and fruits. These compounds demonstrate potent antioxidant effects in a variety of in vitro and in vivo models using endogenous or exogenous oxidant stress (Gao et al., 1999; Haramaki et al., 1994; Sakano et al., 2005; Wang et al., 2006b). Specifically, flavonoids have been investigated for their ability to prevent oxidant injury caused by reperfusion of ischemic tissue, a scenario similar to clinical conditions such as stroke and myocardial infarction. Flavonoids have been typically administered as a pretreatment for several days and a delayed antioxidant protective effect in an ischemia/reperfusion model has been evaluated (Pataki et al., 2002; Sato et al., 2001). Although chronic treatment with flavonoids has shown induction of enhanced antioxidant protection, it may not always be feasible to administer flavonoids as a pretreatment. Therefore, protective role of flavonoids in an acute treatment model of ischemia/reperfusion, i.e. administration of flavonoids concurrently with ischemia/reperfusion or just at the point of reperfusion, is of greater interest.
The type of reactive oxygen species generated during ischemia and reperfusion have been demonstrated to be phase-dependent in our cardiomyocyte model of ischemia/reperfusion (Becker et al., 1999; Lebuffe et al., 2003; Vanden Hoek et al., 1997). Superoxide anion is mainly produced during ischemia or hypoxemia as a result of increased electron leak from the impaired mitochondrial electron transport chain. Upon reperfusion, mitochondrial respiratory chain is overwhelmed with the increased oxygen tension from hypoxic to normoxic conditions and results in production of massive quantity of superoxide from complex III of the mitochondrial electron transport chain (Zweier et al., 1994). The superoxide produced during reperfusion, however, appears to be rapidly converted to hydrogen peroxide (H2O2). The H2O2 is in turn converted to hydroxyl radical through Fenton reaction that is stimulated by the presence of free iron accumulated during ischemia (Vanden Hoek et al., 1997). Production of both H2O2 and hydroxyl radical constitute the accelerated reactive oxygen species production in the first few minutes of reperfusion, also known as the reperfusion oxidant burst. Lipid peroxides and NADH/NADPH reducing equivalents may accumulate during ischemia and could also help drive reactive oxygen species generation at reperfusion (Wang et al., 2006a). Other oxidants such as peroxynitrite are also produced, however, superoxide and H2O2/hydroxyl radical are generally regarded as the primary reactive oxygen species produced during ischemia/reperfusion (Li and Jackson, 2002). The highly reactive hydroxyl radical formed at reperfusion oxidizes cellular lipids, proteins and DNA, and is therefore very detrimental (Wang et al., 1999). Superoxide produced during ischemia also contributes to the overall ischemia/reperfusion injury. Thus, the reactive oxygen species generated during ischemia and reperfusion damage the cell. Antioxidant therapy aiming at mitigating ischemia/reperfusion injury, therefore, should effectively scavenge these specific reactive oxygen species produced during both ischemic and reperfusion phases so as to eliminate the oxidant injury.
In this study, we compared the oxidant scavenging capacity of 5 specific flavonoids (wogonin, baicalin, baicalein, catechin and procyanidin B2, Fig. 1), the major natural antioxidant components frequently found in vegetables, fruits, tea, and herbs. The scavenging of superoxide and hydroxyl radical (the main reactive oxygen species associated with ischemia/reperfusion injury) with these flavonoids was measured. We then evaluated the protective efficacy in different treatment protocols: flavonoids were administered (1) as a 72-h pretreatment, (2) during ischemia and reperfusion, and (3) only at reperfusion, in an established chick cardiomyocyte model of ischemia/reperfusion. The correlation between the protective efficacy and the oxidant scavenging potency of the flavonoids was also discussed.
Fig. 1.
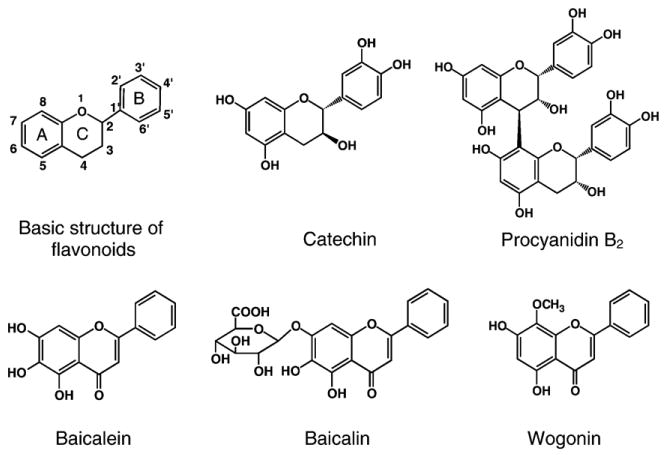
Illustration of the chemical structures of flavonoids.
2. Materials and methods
2.1. Chemicals and solutions
The following chemicals were purchased: baicalin, baicalein, catechin, procyanidin B2, propidium iodide, hydrogen peroxide, ammonium iron (II) sulfate hexahydrate, xanthine, diethylene-triaminepentaacetic acid (DTPA), 1,1-diphenyl-2picrylhydrazyl (DPPH), and spin-trap 5, 5-dimethyl N-oxide pyrroline (DMPO), CH3CN from Sigma Co. (St. Louis, MO); wogonin from Wako Pure Chemical Industries Ltd. (Japan); xanthine oxidase from Roche Applied Science (Indianapolis, IN). Balanced salt solution contained 117 mM NaCl, 4.0 mM KCl, 18 mM NaHCO3, 0.8 mM MgSO4, 1.0 mM NaHPO4, 1.21 mM CaCl2 and 5.6 mM glucose. To mimic the energy deprivation and hyperkalemic conditions in clinical ischemia, the ischemic balanced salt solution contained no glucose but 2-deoxyglucose 20 mM to inhibit glycolysis, and [K+] was raised to 8.0 mEq/L.
2.2. Electron spin resonance (ESR) spectrometric assay
The free radical scavenging by the five flavonoids (wogonin, baicalin, baicalein, catechin and procyanidin B2) was analyzed using electron spin resonance (ESR) spectrometry (Varian E-109 X-Band, ESR Spectrometer. Varian Inc., Palo Alto, CA) in 3 radical producing systems: DPPH, superoxide (DMPO–xanthine/xanthine oxidase) and hydroxyl radical (DMPO–H2O2/FeSO4) using a cell-free, in vitro system. The flavonoid concentration used for the different radicals was varied to demonstrate the maximal radical scavenging efficacy, but was kept constant per radical for comparison. Each experiment was repeated 3 times.
2.2.1. DPPH radical scavenging activity by ESR
The scavenging activity for DPPH, a stable radical, by flavonoids was estimated using ESR according to the method by Yamaguchi et al. (2000). Stock solution was made with flavonoids in CH3CN and DPPH in ethanol. Reaction mixture contained 50 μl test sample in phosphate buffered saline with 5% CH3CN (volume/volume) and 50 μl DPPH (0.5 mM) in ethanol. The final concentration of all flavonoids used was 50 μM. ESR signal was recorded at room temperature, 2 min after sample mixing. Scavenging effect was determined by comparison with a solvent-treated control group.
2.2.2. Superoxide radical scavenging activity by ESR
The method of superoxide radical detection using spin trap DMPO were essentially the same as that described by Zhao et al. (2001). Superoxide radicals were produced by reaction of the xanthine/xanthine oxidase system and reacted with spin trap DMPO. Stock solutions were made with flavonoids in CH3CN and the rest chemicals in phosphate buffered saline. The DMPO–OOH adducts were detected using ESR spectrometry. ESR signal was recorded 1 min after 20 μl xanthine oxidase (0.5 U/ml, fresh made) was mixed with 20 μl xanthine (5 mM), 20 μl DTPA (0.5 mM), 20 μl DMPO (250 mM), 15 μl phosphate buffered saline and 5 μl flavonoids in CH3CN, with the final flavonoid concentration at 25 μM.
2.2.3. Hydroxyl radical scavenging activity by ESR
The ESR spin-trapping technique was used to evaluate the hydroxyl radical scavenging activity (Noda et al., 1999). Hydroxyl radicals were generated by Fenton reaction and reacted rapidly with spin-trap DMPO. Stock solutions were made with flavonoids in CH3CN and the rest in phosphate buffered saline. The resultant DMPO–OH adducts were detected using ESR spectrometry. ESR signal was recorded at room temperature, 10 min after 20 μl FeSO4 (1 mM, fresh made) was mixed with 20 μl DMPO (150 mM), 20 μl H2O2 (1 mM), 20 μl EDTA (1 mM) and 20 μl reference (CH3CN) or samples (1 mM) in CH3CN, with the final flavonoid concentration at 200 μM.
2.3. Cardiomyocyte culture
Chick cardiomyocytes were isolated from 10-day chick embryos as previously described (Vanden Hoek et al., 1996). In brief, the hearts were removed and the ventricles were minced and enzymatically dispersed with 0.025% trypsin (Invitrogen, Grand Island, NY). The cell suspension containing trypsin inhibitor was centrifuged and then resuspended in the culture medium (54% balanced salt solution, 40% medium 199, 6% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin). The cells were pipetted onto 25 mm glass coverslips with approximately 0.7×106 cells per coverslip, and incubated at 37 °C. The purity of the cardiomyocytes was confirmed by anti-myosin heavy chain monoclonal antibodies. All experiments were performed using the 4–5 day cultured cells, by which time a synchronously contracting layer of cells could be visualized with viability exceeding 95%.
2.4. Simulated ischemia/reperfusion
Cardiomyocyte culture medium was replaced with balanced salt solution and cardiomyocytes were incubated at 37 °C for 30 min. The cells were then placed into a hypoxic chamber (37 °C, 1% O2, 20% CO2 and 79% N2) with balanced salt solution immediately changed to ischemia balanced salt solution preincubated overnight in the hypoxic chamber. The ischemic conditions were simulated and verified as before in our cardiomyocyte model (PO2 3–5 Torr, PCO2 144 Torr and pH 6.8) (Vanden Hoek et al., 1996). The coverslips were kept in the hypoxic chamber for 1 h, subsequently removed with an immediate change of the ischemic balanced salt solution to standard balanced salt solution, and incubated at 37 °C, 21% O2 and 5% CO2 for 3 h.
2.5. Therapeutic intervention
Five flavonoid antioxidants, wogonin, baicalin, baicalein, catechin and procyanidin B2 (all at 25 μM), were each tested in 3 different treatment protocols. For chronic treatment group, cells were treated with the tested flavonoids for 72 h and continued through the 1-h ischemia and 3-h reperfusion. For acute treatment groups, the tested flavonoids were added during both ischemia and reperfusion phases, or at reperfusion phase only. For ischemia/reperfusion control group, cells were subjected to 1-h simulated ischemia and 3-h reperfusion. In negative control group, cells were kept in balanced salt solution for 4.5 h. The protocols of all therapeutic interventions were shown in Fig. 2.
Fig. 2.
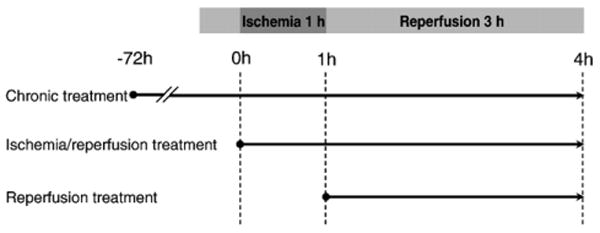
Experimental protocols for cell viability assay in ischemia/reperfusion injury. Using an established chick cardiomyocyte model of simulated ischemia/reperfusion, tested flavonoids were: incubated since 72 h before ischemia and continued through ischemia/reperfusion (chronic treatment), treated during ischemia and reperfusion (ischemia/reperfusion treatment), or administered at reperfusion only (reperfusion treatment).
2.6. Viability assay
Cell viability was assessed with the exclusion fluorescent dye propidium iodide (5 μM) measured at excitation 540 nm/emission 590 nm using a Nikon TE 2000-U inverted phase/epifluorescent microscope (Bond et al., 1991). The dye was used throughout the entire experiment, exhibiting minimal toxicity in control cells even after a ten-hour exposure (Vanden Hoek et al., 1997). At the end of experiment, all cells on the coverslip were permeabilized with digitonin (300 μM). Measurement of propidium iodide was done in 3 random fields of each coverslip at the end of 3-h reperfusion and after 1-h digitonin exposure. The fluorescence values were averaged. Percentage cell death (propidium iodide uptake) was expressed as the propidium iodide fluorescence relative to the maximal value seen after digitonin exposure (100%). Three to five coverslips were used per group with each coverslip representing n of one. Each experiment was repeated for at least 3 times in different batches of cells.
2.7. Statistical analysis
All results were presented as means ± S.E.M. Comparison among different treatment groups was analyzed by one-way ANOVA with post-hoc examination by Tukey test. Difference was considered statistically significant if P<0.05.
3. Results
3.1. DPPH scavenging capacity of the flavonoids
Determining the quenching effect of the stable DPPH free radical is a rapid method to evaluate the radical scavenging activity of the tested compound. The scavenging capacity of the flavonoids was initially evaluated using the DPPH radical. As shown in Fig. 3. The DPPH scavenging capacity of the flavonoids at 50 μM concentration was in the order of catechin (99±1%), procyanidin B2 (92±5%), baicalein (55±4%), baicalin (17±4%), and wogonin (5±3%). Catechin and procyanidin B2 exhibited excellent DPPH quenching effects with ESR signals almost completely eliminated.
Fig. 3.
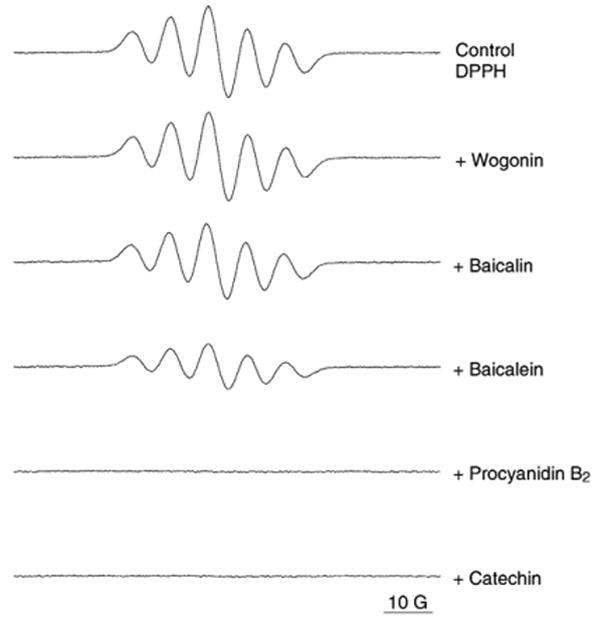
Electron spin resonance (ESR) spectra of DPPH and DPPH radical-scavenging activity of different flavonoids: wogonin, baicalin, baicalein, procyanidin B2, and catechin at the same concentration of 50 μM.
3.2. Superoxide scavenging capacity of the flavonoids
Employing DMPO as the trapping agent, we evaluated the generation of superoxide using the xanthine/xanthine oxidase system and the superoxide scavenging effect of the flavonoids. As shown in Fig. 4, addition of the tested flavonoids to xanthine/xanthine oxidase plus DMPO system (final concentration 25 μM) reduced the DMPO–OOH signals to different levels compared to that in control. The superoxide scavenging capacity of the five flavonoids was as follows: catechin (90±3%), baicalein (69±5%), procyanidin B2 (68±5%), baicalin (42±4%), and wogonin (8±3%).
Fig. 4.
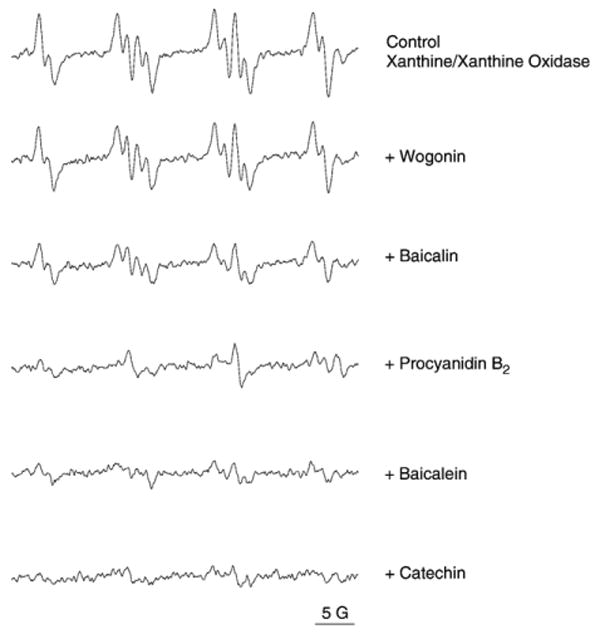
Electron spin resonance (ESR) spectra of DMPO–OOH and superoxide radical-scavenging activity of different flavonoids: wogonin, baicalin, procyanidin B2, baicalein, and catechin at the same concentration of 25 μM.
3.3. Hydroxyl radical scavenging capacity of the flavonoids
The hydroxyl radical scavenging effect of the flavonoids was tested using a Fenton reaction system with DMPO as the trapping agent. Fig. 5 showed the typical 1:2:2:1 four-line ESR signal (with hyperfine splitting parameter aN=aH=14.9 G) of the DMPO–OH adducts. The addition of flavonoids (final concentration 200 μM) resulted in differential quenching effects of the DMPO–OH adduct as demonstrated by reduction in the ESR signal intensity. In general, the scavenging effect of the flavonoids on hydroxyl radicals was weaker than those on DPPH or superoxide even with higher concentration (200 μM): baicalein (56±4%), catechin (31±2%), procyanidin B2 (28±3%), wogonin (25±5%), and baicalin (15±3%). Only baicalein showed an ESR reduction rate of >50%.
Fig. 5.
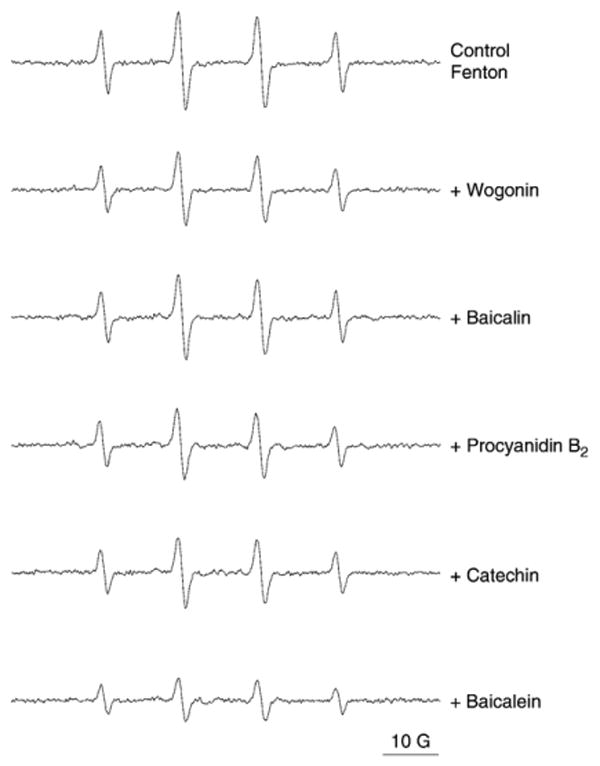
Electron spin resonance (ESR) spectra of DMPO–OH and hydroxyl radical-scavenging activity of different flavonoids: wogonin, baicalin, procyanidin B2, catechin, and baicalein at the same concentration of 200 μM.
The scavenging capacities of the 5 flavonoids in 3 different free radicals were summarized in Table 1.
Table 1.
Oxidant scavenging potency of flavonoids
| Oxidant scavenging (% reduction) | Wogonin | Baicalin | Baicalein | Catechin | Procyanidin B2 |
|---|---|---|---|---|---|
| DPPH | 5±3 | 17±4 | 55±4 | 99±1 | 92±5 |
| DMPO–OOH (superoxide) | 8±3 | 42±4 | 69±5 | 90±3 | 68±5 |
| DMPO–OH (hydroxyl radical) | 25±5 | 15±3 | 56±4 | 31±2 | 28±3 |
3.4. Protective efficacy of the flavonoids against ischemia/reperfusion injury
We used an established chick cardiomyocyte model of simulated ischemia/reperfusion and tested the differential protective effects using 3 treatment protocols.
3.4.1. Chronic treatment
Cardiomyocytes subjected to 1-h ischemia followed by 3-h reperfusion resulted in 55.2±2.9% cell death measured using the exclusion of propidium iodide (Fig. 6). In contrast, in cells incubated with balanced salt solution for 4.5 h, the percentage of cell death was 6.3±1.7% (n=6, P<0.001), suggesting a significant detrimental effect of ischemia/reperfusion injury as we have previously described (Qin et al., 2004; Vanden Hoek et al., 1996). In chronic treatment group in which 25 μM flavonoids were administered for 72 h and through ischemia/reperfusion, most of the tested compounds showed protective effect against ischemia/reperfusion injury, except wogonin in which the cell death was not reduced significantly from the ischemia/reperfusion control (47.5±4.1% vs. 55.2±2.9%, n=6, P=NS; Fig. 6). The level of protection in the other four flavonoids varied, with baicalein exhibiting the best protective effect (cell death 20.1±1.9%, n=6, P<0.001 vs. ischemia/reperfusion control), followed by procyanidin B2 (29.5±4.0%, n=6, P<0.001), baicalin (41.0±2.9%, n=6, P<0.01) and catechin (43.7±2.1%, n=6, P<0.01).
Fig. 6.
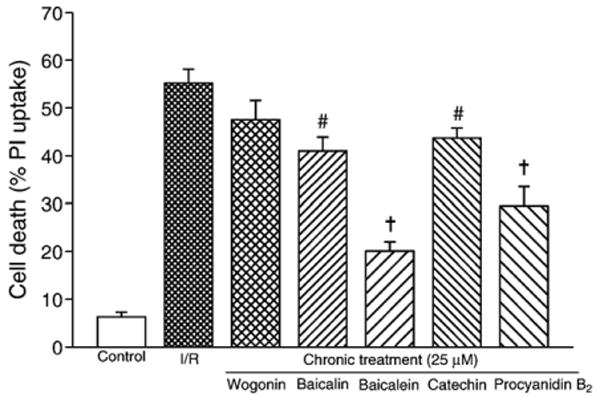
Protective efficacy of flavonoids against cardiomyocyte ischemia/reperfusion injury in chronic treatment protocol. The tested flavonoids (25 μM) were treated for 72 h prior to ischemia and continued through ischemia and reperfusion. #P<0.01, +P<0.001 compared to ischemia/reperfusion (I/R).
3.4.2. Treatment during ischemia and reperfusion
If the treatment with flavonoids was initiated from the beginning of ischemia and continued through reperfusion (ischemia/reperfusion treatment), the cardiomyocyte death was not attenuated as dramatically as with the chronic treatment. For example, the cell death for baicalein (25 μM) ischemia/reperfusion treatment was 36.9±2.1%, though lower than that of ischemia/reperfusion control (56.7±1.5%, n=6, P<0.001; Fig. 7), was significantly higher compared to the chronic treatment (20.1±1.9%, n=6, P<0.001). Similar condition was also observed with procyanidin B2 treatment (ischemia/reperfusion treatment 43.3±2.6% vs. chronic treatment 29.5±4.0%, n=6, P<0.005). For catechin, the cell death in ischemia/reperfusion treatment (46.9±2.3%) was lower than that of the ischemia/reperfusion control (P<0.05; Fig. 7), but was not statistically significant from the chronic treatment group (43.7±2.1%, n=6, P=NS). For baicalin and wogonin, the cell death was not significantly reduced compared to that of the ischemia/reperfusion control (51.9±1.3% for baicalin and 53.1±2.3% for wogonin vs. 56.7±1.5% in ischemia/reperfusion, n=6, P=NS; Fig. 7).
Fig. 7.
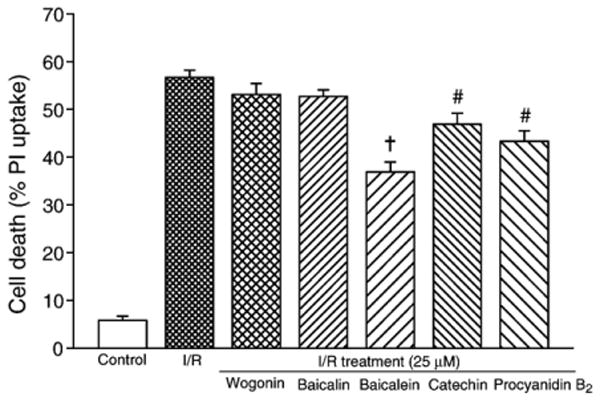
Protective efficacy of flavonoids against cardiomyocyte ischemia/reperfusion injury in ischemia/reperfusion treatment protocol. The tested flavonoids (25 μM) were administered during both ischemic and reperfusion phases. #P<0.01, +P<0.001 compared to ischemia/reperfusion (I/R).
3.4.3. Treatment during reperfusion only
For flavonoids given just at reperfusion (reperfusion treatment), the protective effect against ischemia/reperfusion injury was even less. While cell death after 3 h of reperfusion was still reduced in baicalein and procyanidin B2 treatment groups (cell death 46.3±2.6% and 47.8±3.7%, respectively, vs. 57.5±2.2% in ischemia/reperfusion control, n=6, both P<0.05; Fig. 8), the level of protection was lower than those of chronic and ischemia/reperfusion treatments (Figs. 6 and 7). For catechin, baicalin and wogonin, no significant protective effect was seen with reperfusion treatment (cell death 54.6±1.7%, 54.6±2.3% and 52.9±2.2%, respectively, n=6, P=NS vs. ischemia/reperfusion control; Fig. 8). This suggests that flavonoid treatment at reperfusion only may not be effective in rescuing the ischemic tissues or cells from reperfusion injury.
Fig. 8.
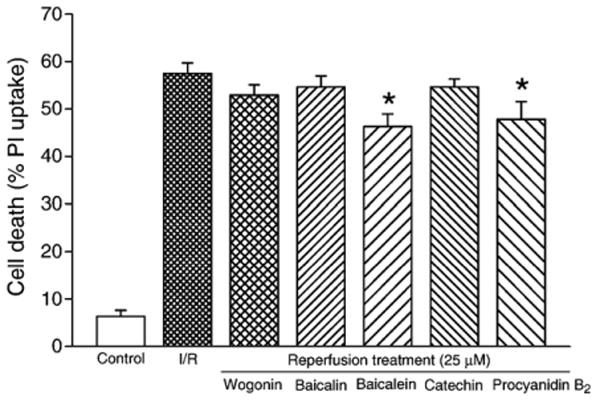
Protective efficacy of flavonoids against cardiomyocyte ischemia/reperfusion injury in reperfusion treatment protocol. The tested flavonoids (25 μM) were administered at reperfusion phase only. *P<0.05 compared to ischemia/reperfusion (I/R).
4. Discussion
Flavonoids have attracted great attention in the prevention and treatment of cardiovascular diseases. With epidemiological evidence supporting that flavonoids reduce the risk of coronary artery disease (Hertog et al., 1993; Renaud and de Lorgeril, 1992), recent interest has been directed toward their therapeutic potential in the acute stages of the diseases (Rossi et al., 2003; Schultke et al., 2003). Our current study shows that five distinct flavonoids confer varied cardioprotection against ischemia/reperfusion injury, depending on the treatment timing. The chronic treatment offers the best effect, while the reperfusion treatment has much lesser protective effect. We also found that the variant oxidant-scavenging capacity of flavonoids may partly contribute to their differential protective effects against ischemia/reperfusion injury.
4.1. Chemical characteristics determining the oxidant scavenging capacity
In general the oxidant scavenging capacity of flavonoids is related to the hydrogen donating ability and the stability of the phenoxyl radicals formed (Rice-Evans, 2004). Several factors determine the antioxidant capacity of flavonoids, such as the number and position of the phenolic hydrogens, the saturation status of the heterocyclic C ring, and any substitution of the phenolic hydrogens by glycoside or alkyl groups (Rice-Evans et al., 1996). For example, the ortho-dihydroxyphenolic structure of the B ring has been considered important for the oxidant scavenging activity (Fig. 1). The 3-OH group in the C ring, in conjunction with the adjacent 2,3-double bond and 4-oxo group, further potentiates the radical scavenging effect. The 3- and 5-OH groups in the A ring also contribute in maximizing the radical scavenging potential. These structural characteristics affect the ability of electron delocalization from the aryloxyl radical on the B ring to the A ring and are thus responsible for the resonance effect of the aromatic nucleus that stabilizes the resulting phenoxyl radicals (Bors et al., 1990; Rice-Evans et al., 1996). Also, these characteristics contribute to the metal chelating activity, an important component of the flavonoids' antioxidant capacity (Sugihara et al., 1999).
In this study, catechin and procyanidin B2 exhibited the best scavenging potency for DPPH and superoxide radicals as determined by ESR (Figs. 3, 4 and Table 1). This can largely be attributed to the abundant hydroxyl groups distributed in A, B and C rings that mediate hydrogen donation and electron delocalization. As procyanidin B2 structurally is 2 epicatechin molecules linked from 4 position of the C ring in one molecule to 8 position of the A ring in the other (Fig. 1), its oxidant scavenging capacity is similar to that of catechin. Wogonin, on the other hand, was found to be poor in scavenging free radicals. This can probably be explained by the absence of hydroxyl groups in the B ring, even though there are 2,3 double bond and 4-oxo group in the C ring and two hydroxyl groups in the A ring. For baicalein, the absence of hydroxyl groups in the B ring is not different from that in wogonin (Fig. 1). However, the three hydroxyl groups in the A ring and the 2,3-double bond plus 4-oxo group in the C ring may account for its better oxidant scavenging capacity. For baicalin, as the 7-OH group in the A ring is substituted by glycoside (Fig. 1), the antioxidant potential is attenuated as compared to that of baicalein (Bors et al., 1990; Rice-Evans et al., 1996).
For hydroxyl radical, the scavenging capacity of the tested flavonoids was generally of lesser magnitude than those of DPPH and superoxide radicals, even if the concentration used for hydroxyl radical ESR experiment (200 μM) was significantly higher than those used for DPPH (50 μM) and superoxide (25 μM). This might in part explain why the protective effect is unsatisfactory if the flavonoids are administered only at reperfusion, since hydroxyl radical constitutes the major reactive oxygen species produced in this phase. One exception is baicalein, whose hydroxyl radical scavenging capacity is comparable to that for DPPH or superoxide (Table 1). Theoretically, the absence of OH groups in baicalein's B ring would attenuate the oxidant scavenging potency, making elimination of the hydroxyl radical poorer than other flavonoids with such a structure (e.g. catechin or procyanidin B2). Nevertheless, the ESR result shows that, in contrast to DPPH or superoxide, baicalein's potency in hydroxyl radical scavenging is paradoxically better than the other flavonoids. This is probably due to the specific structural differences between baicalein and catechin that enables greater iron chelation and hydroxyl radical scavenging (Afanas'ev et al., 1989; Halliwell, 1995; Melidou et al., 2005). Iron chelation results in inhibition of hydroxyl radical production via Fenton reaction, thus contributing significantly to the overall antioxidant activity of the flavonoids.
4.2. Phase-dependent oxidant scavenging and protection against ischemia/reperfusion injury
We have previously reported in this chick cardiomyocyte ischemia/reperfusion model that superoxide generation increases during ischemia and declines rapidly in the first minutes of reperfusion (Becker, 2004; Becker et al., 1999). The oxidant burst at early reperfusion is composed mainly of H2O2 and hydroxyl radical (Li and Jackson, 2002), and is associated with accelerated cell death after reperfusion (Levraut et al., 2003; Vanden Hoek et al., 2003; Vanden Hoek et al., 1996). Thus there appears to be a phase-specific oxidant production during ischemia and reperfusion. In cardiomyocytes receiving chronic flavonoid treatment, the protective efficacy against ischemia/reperfusion injury is in the order of baicalein>procyanidin B2>baicalin>catechin (Fig. 6). For ischemia/reperfusion treatment, the protective efficacy is baicalein>procyanidin B2>catechin (Fig. 7). Since these two protocols involve treatment with flavonoids during both ischemic and reperfusion phases, the results are consistent with their superoxide- and hydroxyl radical-scavenging capacity shown by ESR (Figs. 4, 5 and Table 1), thus protecting the cells from oxidant injury. The only exception is catechin, which exhibits high superoxide- and moderate hydroxyl radical-scavenging capacity but less satisfactory cytoprotection against ischemia/reperfusion injury at the tested concentration. The low lipid solubility of catechin, attributed to lack of 4-carbonyl group and 2,3-double bond in the C ring, may explain this discrepancy (Melidou et al., 2005). The ESR experiments are performed in the aqueous phase, while the ischemia/reperfusion experiments in cardiomyocytes are interactions with cell membrane and other lipophilic intracellular components. A differential antioxidant activity of flavonoids between aqueous and lipophilic phases has been demonstrated earlier. It is suggested that the lack of 4-carbonyl group and 2,3-double bond in the heterocyclic C ring of catechin may explain the weaker oxidant scavenging effect in the lipophilic phase, though such structure exhibits less important role in the antioxidant potency in aqueous system (Rice-Evans et al., 1996).
In cardiomyocytes treated with flavonoids during reperfusion only, the protective efficacy against ischemia/reperfusion injury is significant only with baicalein and procyanidin B2. Since large quantity of hydroxyl radical is generated intracellularly in the early phase of reperfusion, the hydroxyl radical-scavenging capacity of the lipid-soluble flavonoids would become the determinant of the protective effect. Though the level of cytoprotection is less compared to that of ischemia/reperfusion treatment and chronic treatment, the order of protective effect is consistent with the ESR results (Fig. 5 and Table 1). Again discrepancy is noted between catechin's protective effect and its hydroxyl radical-scavenging capacity, which is probably due to similar factors discussed above. Baicalein's high lipid-solubility, affording a rapid intracellular access (Saija et al., 1995), and its free iron-chelating ability (Afanas'ev et al., 1989; Halliwell, 1995), which in turn reduces hydroxyl radical formation, may explain its superior protective effect even when administered at reperfusion only (Shao et al., 2002). For procyanidin B2, an increased capacity to prevent membrane injury due to availability of several hydroxyl group per molecule and an increased lipophilicity due to a compact conformation of the dimer, apart from its metal chelating activity, may explain the protective effect (da Silva Porto et al., 2003; Maffei Facino et al., 1996; Wang et al., 2000). Future studies measuring reactive oxygen species in situ using a more sensitive ESR technique need to be conducted to further support our observations.
4.3. Other possible protective mechanisms in chronic treatment strategy
Compared to acute treatment protocols, the protective effect against ischemia/reperfusion injury is most significant with chronic flavonoid treatment. This is consistent with previous studies and clinical observations suggesting that prevention is better than emergency treatment in face of acute oxidant injury (Hertog et al., 1997; Renaud and de Lorgeril, 1992). Administration of flavonoids prior to the ischemic insult would allow time for the flavonoids to accumulate intracellularly, which enables efficient oxidant scavenging from the beginning of ischemia. Other factors such as activation of the endogenous antioxidant system may also contribute to the better protection seen in chronic treatment (Pataki et al., 2002). While 72-h pretreatment allows upregulation of the antioxidant proteins, which prepares the cell for an eventual ischemic insult, such activation is not possible in ischemia/reperfusion treatment or reperfusion treatment. Moreover, flavonoids have demonstrated potentials in modulating a number of second messengers and other intracellular processes that are involved in oxidant stress and cell death (Rice-Evans, 2001, 2004; Williams et al., 2004). Preadministration of flavonoids accomplishes these before the ischemia/reperfusion insult, thus preparing the cell in advance. Further work is needed for better understanding the alterations in endogenous antioxidant enzymes and chemicals that may be responsible for protection from oxidant injury in the chronic treatment model.
In conclusion, flavonoids' antioxidant protection against ischemia/reperfusion injury is dependent on the oxidant scavenging capacity for the reactive oxygen species generated during ischemia/reperfusion, the timing of flavonoid treatment, and the membrane permeability to flavonoids. The flavonoid-specific oxidant-scavenging capacity may simply be explained on the basis of their chemical structures. However, the cytoprotective effects of the flavonoids are probably determined, apart from their scavenging capacity, by intracellular bioavailability of the flavonoids and their potential pharmacodynamic effects within the cardiomyocytes. The flavonoids with high scavenging capacity for oxidant species produced during ischemia (superoxide) and reperfusion (hydroxyl radical), in addition to possessing higher lipid solubility, exhibit best protective efficacy. Thus, when administered as an acute treatment, only those flavonoids with favorable chemical characteristics ensuring rapid intracellular access and scavenging of relevant toxic reactive oxygen species will be efficacious in protecting against ischemia/reperfusion injury.
Acknowledgments
This work was supported in part by NIH grants HL68951, HL72734, AT002176, AT003255 and AT01575-01.
References
- Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Bond JM, Herman B, Lemasters JJ. Recovery of cultured rat neonatal myocytes from hypercontracture after chemical hypoxia. Res Commun Chem Pathol Pharmacol. 1991;71:195–208. [PubMed] [Google Scholar]
- Bors W, Heller W, Michel C, Saran M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- da Silva Porto PA, Laranjinha JA, de Freitas VA. Antioxidant protection of low density lipoprotein by procyanidins: structure/activity relationships. Biochem Pharmacol. 2003;66:947–954. doi: 10.1016/s0006-2952(03)00458-1. [DOI] [PubMed] [Google Scholar]
- Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidant characterization. Methodology and mechanism. Biochem Pharmacol. 1995;49:1341–1348. doi: 10.1016/0006-2952(95)00088-h. [DOI] [PubMed] [Google Scholar]
- Haramaki N, Aggarwal S, Kawabata T, Droy-Lefaix MT, Packer L. Effects of natural antioxidant ginkgo biloba extract (EGB 761) on myocardial ischemia-reperfusion injury. Free Radic Biol Med. 1994;16:789–794. doi: 10.1016/0891-5849(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol: Heart Circ Physiol. 2003;284:H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- Levraut J, Iwase H, Shao ZH, Vanden Hoek TL, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am J Physiol: Heart Circ Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol: Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Maffei Facino R, Carini M, Aldini G, Berti F, Rossoni G, Bombardelli E, Morazzoni P. Procyanidines from Vitis vinifera seeds protect rabbit heart from ischemia/reperfusion injury: antioxidant intervention and/or iron and copper sequestering ability. Planta Med. 1996;62:495–502. doi: 10.1055/s-2006-957956. [DOI] [PubMed] [Google Scholar]
- Melidou M, Riganakos K, Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radic Biol Med. 2005;39:1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Noda Y, Kohno M, Mori A, Packer L. Automated electron spin resonance free radical detector assays for antioxidant activity in natural extracts. Methods Enzymol. 1999;299:28–34. doi: 10.1016/s0076-6879(99)99006-7. [DOI] [PubMed] [Google Scholar]
- Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–899. doi: 10.1093/ajcn/75.5.894. [DOI] [PubMed] [Google Scholar]
- Qin Y, Vanden Hoek TL, Wojcik K, Anderson T, Li CQ, Shao ZH, Becker LB, Hamann KJ. Caspase-dependent cytochrome c release and cell death in chick cardiomyocytes after simulated ischemia-reperfusion. Am J Physiol: Heart Circ Physiol. 2004;286:H2280–H2286. doi: 10.1152/ajpheart.01063.2003. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoids and isoflavones: absorption, metabolism, and bioactivity. Free Radic Biol Med. 2004;36:827–828. doi: 10.1016/j.freeradbiomed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Rossi A, Serraino I, Dugo P, Di Paola R, Mondello L, Genovese T, Morabito D, Dugo G, Sautebin L, Caputi AP, Cuzzocrea S. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic Res. 2003;37:891–900. doi: 10.1080/1071576031000112690. [DOI] [PubMed] [Google Scholar]
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19:481–486. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- Sakano K, Mizutani M, Murata M, Oikawa S, Hiraku Y, Kawanishi S. Procyanidin B2 has anti- and pro-oxidant effects on metal-mediated DNA damage. Free Radic Biol Med. 2005;39:1041–1049. doi: 10.1016/j.freeradbiomed.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–737. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Schultke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BH. Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2003;20:583–591. doi: 10.1089/089771503767168500. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Yuan CS. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol: Heart Circ Physiol. 2002;282:H999–H1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- Sugihara N, Arakawa T, Ohnishi M, Furuno K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with alpha-linolenic acid. Free Radic Biol Med. 1999;27:1313–1323. doi: 10.1016/s0891-5849(99)00167-7. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol. 1996;270:H1334–H1341. doi: 10.1152/ajpheart.1996.270.4.H1334. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol: Heart Circ Physiol. 2003;284:H141–H150. doi: 10.1152/ajpheart.00132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Schuschke DA, Kang YJ. Metallothionein-over-expressing neonatal mouse cardiomyocytes are resistant to H2O2 toxicity. Am J Physiol. 1999;276:H167–H175. doi: 10.1152/ajpheart.1999.276.1.H167. [DOI] [PubMed] [Google Scholar]
- Wang JN, Chen YJ, Hano Y, Nomura T, Tan RX. Antioxidant activity of polyphenols from seeds of Vitis amurensis in vitro. Acta Pharmacol Sin. 2000;21:633–636. [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006a;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun. 2006b;346:367–376. doi: 10.1016/j.bbrc.2006.05.156. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Zhang H, Karoui H, Kalyanaraman B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic Biol Med. 2001;31:599–606. doi: 10.1016/s0891-5849(01)00619-0. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Thompson-Gorman S, Klunk D, Lutty GA. Measurement and characterization of free radical generation in reoxygenated human endothelial cells. Am J Physiol. 1994;266:C700–C708. doi: 10.1152/ajpcell.1994.266.3.C700. [DOI] [PubMed] [Google Scholar]


