Abstract
Background: Implant loosening is associated with inflammatory bone loss induced by ultra-high molecular weight polyethylene wear debris. We hypothesized that a hydroxyapatite-bisphosphonate composite improves periprosthetic bone quality and osseous integration of an intramedullary implant even in the presence of ultra-high molecular weight polyethylene particles in an experimental rat femur model.
Methods: A preliminary in vitro study determined the optimal concentration of zoledronate (50 μM) that would maximally decrease osteoclasts without harming osteoblasts. Hydroxyapatite-coated intramedullary nails were implanted bilaterally in the femora of sixteen rats (the control group), and hydroxyapatite-zoledronate-coated nails were implanted bilaterally in the femora of sixteen rats (the experimental group). Ultra-high molecular weight polyethylene particles were introduced into the femoral canal before implantation. Eight rats from each group were killed at six weeks, and the remaining rats were killed at six months. Periprosthetic bone mass was analyzed by dual x-ray absorptiometry and microcomputed tomography. Osseous integration was examined by biomechanical testing of pullout strength.
Results: The mean bone area (and standard deviation) in the periprosthetic bone region was significantly greater (p < 0.0001) in the hydroxyapatite-zoledronate group (2.388 ± 0.960 mm2) than in the control group (0.933 ± 0.571 mm2). This difference was larger in the six-week group than in the six-month group (p = 0.03). The average peak pullout force for the treated femora (241.0 ± 95.1 N) was significantly greater (p < 0.0001) than that for the controls (55.6 ± 49.0 N). This difference was similar in the six-week and six-month groups. The energy required for nail pullout was significantly greater (p < 0.0001) for the treated femora (521.6 ± 293.8 N-mm) than for the controls (142.2 ± 152.1 N-mm). This difference in energy to pullout was similar in the six-week and six-month groups. Regression analysis demonstrated a high correlation between periprosthetic bone mass and peak pullout force for both the six-week (r = 0.766, p = 0.0005) and six-month (r = 0.838, p < 0.0001) groups.
Conclusions: Surface modification of implants with hydroxyapatite-zoledronate improves periprosthetic bone quality and osseous integration.
Clinical Relevance: Hydroxyapatite-based site-specific delivery of bisphosphonates may be one way of reducing ultra-high molecular weight polyethylene wear particle-induced periprosthetic osteolysis and implant loosening.
The long-term clinical success of total joint replacement depends on stable integration between the host bone and the prosthesis. Common features of implant loosening include periprosthetic inflammatory bone loss, the formation of interface membranes, and the loss of mechanical stability1,2. Several experimental studies have shown improved osseous integration and biomechanical stability of hydroxyapatite-coated implants compared with those without hydroxyapatite coating3. Recently, there has been an effort to use bisphosphonates to im-prove the longevity of hydroxyapatite-coated dental implants4.
Bisphosphonates are antiresorptive agents that have been widely used for the treatment of osteoporosis, metabolic bone diseases, and metastatic bone tumors5. At the tissue level, all bisphosphonates inhibit bone destruction and lead to an increase in bone mineral density by decreasing bone resorption and bone turnover6. At the cellular level, bisphosphonates exert their effect primarily through their action on osteoclasts. It is likely that bisphosphonates are internalized by osteoclasts and interfere with specific biochemical processes that induce apoptosis7. Bisphosphonates avidly bind to bone and other calcium-containing substances both in vitro and in vivo. Several in vitro and animal experiments have demonstrated the potential benefit of topical application of bisphosphonates to improve bone quality in the area of interest. Topical delivery of bisphosphonates has several theoretical advantages such as site-specific delivery to the interface between the prosthesis and host bone, avoidance of systemic exposure to bisphosphonates in younger patients who may not require them, and the simplicity of drug delivery. On the basis of these premises, several animal studies have shown that regional bone density improves after topical application of bisphosphonates8,9.
In this study, we hypothesized that pretreating intramedullary implants with a hydroxyapatite-zoledronate composite enhances periprosthetic bone quality and prosthesis integration with host bone even in the presence of ultra-high molecular weight polyethylene particles. To test our hypothesis, we conducted an in vitro study to determine the optimal therapeutic concentration of zoledronate. Animal experiments were subsequently performed to compare the periprosthetic bone quality and prosthesis integration of implants coated with a hydroxyapatite-zoledronate composite (the study group) and of implants coated with hydroxyapatite without zoledronate (the control group) in the presence of ultra-high molecular weight polyethylene particles.
Materials and Methods
A preliminary study was performed to determine the optimal concentration of zoledronate that reduces the preosteoclast population yet is least harmful to osteoblasts. Details of the methods used and the results of this preliminary study are provided in the Appendix.
Experimental Design
All procedures involving animals were approved by the Institutional Animal Care and Use Committee in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care guidelines. Our study used a rodent femur model of intramedullary nailing with ultra-high molecular weight polyethylene particles instilled into the medullary canal after reaming the femur to stimulate a wear debris reaction. On the basis of a power analysis with pullout strength as the determining measurement parameter, thirty-two six-month-old adult male retired breeder Sprague-Dawley rats (weight, 400 to 500 g) were purchased from Charles River Laboratories, Inc. (Wilmington, Massachusetts). The rats were divided into two groups of sixteen each. Those in the control group received hydroxyapatite-coated nails that had been presoaked in a sterile isotonic saline solution for five minutes and those in the study group received hydroxyapatite-coated nails10 that had been presoaked in a solution of 50 μM of zoledronate for five minutes at room temperature and were air-dried prior to insertion. Eight rats in each group were killed at six weeks after the intramedullary nailing, and the remaining eight rats in each group were killed six months following the surgery.
Surgical Procedure
After the rats were anesthetized with ketamine (60 mg/kg) and xylazine (5 mg/kg), hair over the knees was removed with a clipper. The right and left knee regions were prepared with 70% alcohol and a 1% Betadine (povidone-iodine) solution. A 1-cm longitudinal skin incision was made over the anterior aspect of the distal part of the femur. A medial parapatellar approach was made to expose the intercondylar notch with the knee in flexion. An entry hole was made with a 1.1-mm Kirschner wire. The distal ends of the femora were reamed with an 18-gauge needle, and then ultra-high molecular weight polyethylene particles (Ceridust 3615; Hoechst, Frankfurt, Germany)11,12 that ranged in size from 0.1 to 3 μm and were suspended in sterile saline solution (0.1 mL of 50 volume percent particles) were introduced into the medullary canal prior to implantation. In the control group, a radiolucent intramedullary nail made of ultra-high molecular weight polyethylene and coated with hydroxyapatite was implanted into the distal end of the right femur in a retrograde manner, while a hydroxyapatite-coated stainless steel intramedullary nail was implanted into the distal end of the left femur. The joint capsule was repaired with a 2-0 Vicryl suture (polyglactin; Ethicon, Somerville, New Jersey). The skin was then repaired with metal staples. The rats were allowed weight-bearing as tolerated in their cages while subcutaneous injections of buprenorphine (0.05 mg/kg) were given once a day for two postoperative days for analgesia. Activity level, food intake, the condition of the surgical wound, and clinical signs of infection were monitored daily.
The purpose of the ultra-high molecular weight polyethylene nail was to minimize imaging artifacts inherent with metal implants for a more accurate analysis of dual x-ray absorptiometry and microcomputed tomography. The metallic nail was used for biomechanical testing. The ultra-high molecular weight polyethylene and stainless steel intramedullary nails (Stryker, Mahwah, New Jersey), which were 1.4 mm in diameter and 20 mm in length, were custom-made for this study. The hydroxyapatite coating thickness was approximately 0.02 mm. The coating thickness varied no more than approximately 0.01 mm, both along the nail and circumferentially.
The femora were harvested at six weeks or six months. The animals were killed by carbon dioxide asphyxiation administered by placing the animals in a Plexiglas-covered tank with carbon dioxide administered for five minutes as per our institutional protocol. The retrieved right femora with the ultra-high molecular weight polyethylene nail were wrapped in 0.15-M saline solution-soaked gauze, placed in labeled plastic bags, and stored at −25°C until imaging studies were performed. The retrieved left femora with the stainless steel nail were also fresh-frozen in the same manner until biomechanical testing was performed.
Dual X-ray Absorptiometry
Since metal artifact can have a substantial effect on microcomputed tomographic imaging, the femora were imaged with dual x-ray absorptiometry. The distal region of the bone extending from the distal end of the femur to the proximal end of the implant was selected for density analysis to focus on the region of the femur adjacent to the implant. The bone area (in square millimeters), bone mineral density (in grams per square centimeter), and bone mineral content (in grams) were measured. A correction was made to the bone mineral content and bone mineral density of the left femora to account for the effect of the radiographic artifact introduced by the stainless steel nail. No correction was needed for the right femora because the ultra-high molecular weight polyethylene nails did not produce radiographic artifacts that interfered with dual x-ray absorptiometry. A three-way analysis of variance, with time (six weeks compared with six months) and treatment (control compared with zoledronate) as nonrepeated factors and leg as a repeated factor, was performed for the amount of bone area, bone mineral content, and bone mineral density. Since two dissimilar materials were used in the construction of the nails, the dual x-ray absorptiometry of both the left and right femora allowed an assessment of the effect of nail material on bone density.
Microcomputed Tomography
The right femora with the ultra-high molecular weight polyethylene nails were imaged by quantitative computed tomography (Stratec XCT-3000, running the XCT Series software, version 5.21; Stratec Medizintechnik, Pforzheim, Germany). Slice thicknesses were 1.0 mm. Voxel size was 0.1 × 0.1 × 1.0 mm. Three sequential axial images were obtained at 10 mm, 15 mm, and 20 mm from the distal end of the femur. To focus on the site-specific effect of the zoledronate treatment, the two-dimensional pixel-based images of the microcomputed tomographic slices were analyzed with ImageJ software (National Institutes of Health, Bethesda, Maryland; http://rsb.info.nih.gov/ij/). In the analysis, an anulus was drawn and centered over the nail, with the 1.4-mm diameter of the nail as the inner diameter of the anulus and an outer diameter of 2 mm. Since the average thickness of the hydroxyapatite coating was 0.02 mm, the anulus was chosen to include the small area of the hydroxyapatite coating to account for any periprosthetic osseous ingrowth into the hydroxyapatite coating. The area of bone (millimeters) and bone mineral density (in grams per square centimeter) for each of the images were obtained. The density of bone in the area of the anulus was thresholded at 290 mg/cm3 to differentiate bone from soft tissue. The resulting area of bone within the anulus was obtained for each of the slices. A repeatability study was performed for this technique by repeating the procedure for all three slices on several randomly selected rats from both experimental groups and for both six-week and six-month groups. The estimated thresholded area was found to be repeatable to 0.030 mm2 for the six-week group and 0.025 mm2 for the six-month group, which were 3.5% and 1.7%, respectively, of the mean thresholded areas of the rats used in the repeatability study. A three-way analysis of variance, with time (six weeks compared with six months) and treatment (control compared with zoledronate) as nonrepeated factors and image location (three levels) as the repeated factor, was performed for the area of periprosthetic bone in the anulus.
Biomechanical Testing
The left femora (with the stainless steel intramedullary nail) of the six-week and six-month control and treatment groups underwent biomechanical testing following the imaging studies (dual x-ray absorptiometry and microcomputed tomography). All specimens had a total of three freeze-thaw cycles prior to mechanical testing. The bones were kept moist throughout each imaging study and during mechanical testing with 0.15 M NaCl. During storage and transit between imaging sites, the bones were wrapped with saline solution-soaked gauze and were kept at −78°C. Each femur was potted in a 0.125-in (3.2-mm) galvanized steel threaded pipe nipple. A 1.4-mm hole was drilled through the pipe and bone, and a 1.4-mm wire brad was passed through the pipe and bone proximal to the intramedullary nail. The pipe was filled with Rockite expansion cement (Hartline Products, Cleveland, Ohio) to secure the bone to the pipe. Once potted, the distal end of the femur was carefully ground down until 3.5 mm of intramedullary nail was exposed.
The potted bone was threaded into a testing jig, which allowed translation in two orthogonal directions perpendicular to the long axis of the femur. The testing jig included a ball joint, which allowed internal-external, flexion-extension, and varus-valgus rotations. The five-degree-of-freedom setup ensured that the direction of pull was aligned with the axis of the intramedullary nail. The testing jig was mounted in a servohydraulic materials testing machine (model 858 Bionix; MTS, Eden Prairie, Minnesota), and the exposed nail was gripped by a precision drill chuck attached to a 5-kN load-cell to ensure that the intramedullary nail was centered and that the direction of pull was aligned with the axis of the nail. The intramedullary nail was pulled out at a constant rate of 2 mm/min. Both displacement and load were measured as a function of time throughout the test. Peak force was obtained. Energy to complete pullout of the nail (in newton-millimeters) was obtained by trapezoidal integration of the force-displacement curve. A two-way analysis of variance, with time (six weeks compared with six months) as one factor and treatment (control compared with zoledronate) as the second factor, was performed for the peak pullout force and for the energy to complete pullout. Linear correlations were performed between the area of periprosthetic bone in the anulus and the pullout strength, and between the area and the total energy for pullout, in the six-week and the six-month groups.
Results
Dual X-ray Absorptiometry
The mean area of bone (and standard deviation) for all six-month femora (1.284 ± 0.067 mm2) was found to be significantly greater (p < 0.0029) than that for all six-week femora (1.209 ± 0.079 mm2). No difference in the mean area of bone was detected (p = 0.1222) between the hydroxyapatite control group (1.228 ± 0.080 mm2) and the hydroxyapatite-zoledronate group (1.265 ± 0.081 mm2). There was also no difference (p = 0.5898) in the mean bone area between all left (1.243 ± 0.077 mm2) and all right femora (1.250 ± 0.088 mm2). The mean bone mineral content of all six-month femora (0.462 ± 0.061 g) was significantly greater (p < 0.0001) than that of all six-week femora (0.338 ± 0.057 g). The mean bone mineral content of all femora in the hydroxyapatite-zoledronate group (0.417 ± 0.064 g) was significantly greater (p < 0.0001) than that of the femora in the hydroxyapatite control group (0.333 ± 0.046 g). No difference in the mean bone mineral content was detected between all left femora and all right femora (p = 0.2635). The mean bone mineral density was significantly greater (p < 0.0001) for all six-month femora (0.320 ± 0.040 g/cm2) than for all six-week femora (0.278 ± 0.033 g/cm2). The mean bone mineral density was also significantly greater (p < 0.0001) for all femora in the hydroxyapatite-zoledronate group (0.328 ± 0.036 g/cm2) than for all femora in the hydroxyapatite control group (0.271 ± 0.025 g/cm2). There was no difference (p = 0.1760) in the mean bone mineral density among right and left femora.
Assessment of Bone Area with Use of Microcomputed Tomography
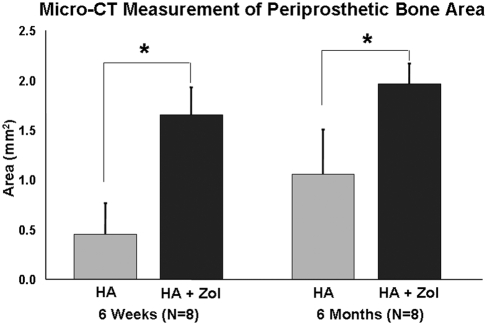
The mean bone area in the periprosthetic region was found to be significantly greater (p < 0.0001) for all femora in the hydroxyapatite-zoledronate group (2.388 ± 0.960 mm2) than for all femora in the control group (0.933 ± 0.571 mm2). The mean bone area was also found to be significantly greater (p < 0.0001) in all six-month femora (1.517 ± 0.571 mm2) than in all six-week femora (1.052 ± 0.673 mm2). The effect of time was found to be more pronounced for the femora in the hydroxyapatite control group than for the femora in the hydroxyapatite-zoledronate group (p = 0.03) (Fig. 1). There was no difference in periprosthetic bone area among the three locations where the images were obtained (p > 0.05).
Fig. 1.
Microcomputed tomography (Micro-CT) results for periprosthetic bone area as a function of treatment and time. The error bars denote one standard deviation. An asterisk denotes a difference (p < 0.05) between the hydroxyapatite-treated (HA) control group and the hydroxyapatite-zoledronate-treated (HA + Zol) group.
Biomechanical Testing
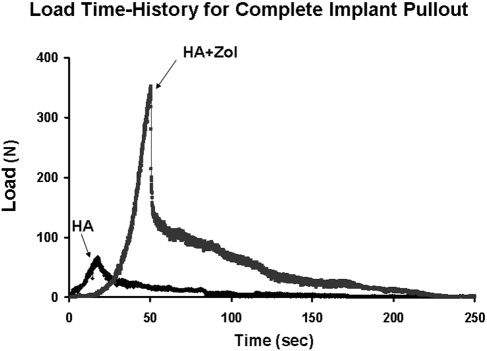
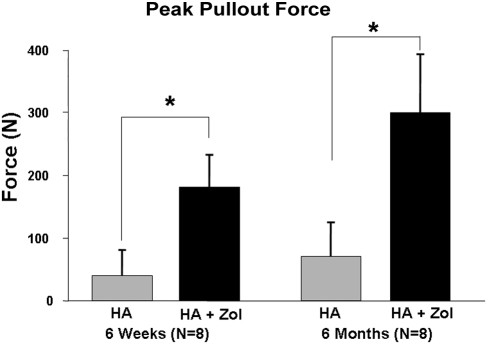
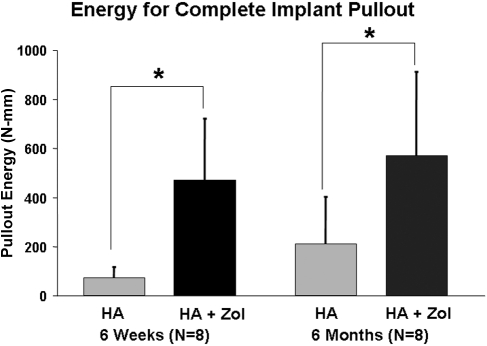
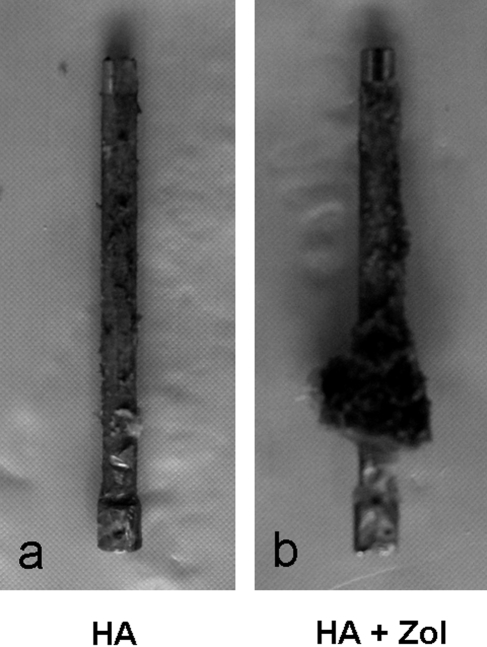
As the loading actuator was displaced at a constant rate of 2 mm/min, an approximately linear increase in the force to the peak pullout force was observed for all specimens (Fig. 2). The average peak pullout force for all femora in the hydroxyapatite-zoledronate group (241.0 ± 95.1 N) was significantly greater (p < 0.0001) than that for all femora in the hydroxyapatite control group (55.6 ± 49.0 N). The average peak pullout force for all six-month femora exhibited a significantly greater (p = 0.0022) average peak force (185.8 ± 139.5 N) than was observed for all six-week femora (110.8 ± 85.5 N). There was no significant difference in the effect of treatment on peak force between the six-week and six-month femora (p = 0.0553) (Fig. 3). The average energy required to completely pull the nail out of the bone was significantly greater (p < 0.0001) for all femora in the hydroxyapatite-zoledronate-treated group (521.6 ± 293.8 N-mm) than for all femora in the hydroxyapatite control group (142.2 ± 152.1 N-mm) (Fig. 4). No significant difference in the average total energy to pullout (p = 0.1616) was found between all six-week femora (272.6 ± 269.1 N-mm) and all six-month femora (391.2 ± 326.1 N-mm). Visually, there was a substantial, but highly variable, amount of bone that remained on the hydroxyapatite-zoledronate-treated nails after pullout, whereas there was virtually no material on any of the hydroxyapatite control nails (Fig. 5).
Fig. 2.
Representative curves of the force versus time required to pull the implant completely out of the femur for a hydroxyapatite-treated control (HA) and a hydroxyapatite-zoledronate-treated (HA + Zol) femur.
Fig. 3.
Peak pullout force as a function of treatment group and time. The error bars denote one standard deviation. An asterisk denotes a significant difference (p < 0.05) between the hydroxyapatite-treated control (HA) and hydroxyapatite-zoledronate-treated groups (HA + Zol).
Fig. 4.
The energy required to pull the stainless steel nail completely out of the femur. The error bars denote one standard deviation. An asterisk denotes a significant difference (p < 0.05) between the hydroxyapatite-treated control (HA) and hydroxyapatite-zoledronate-treated groups (HA + Zol).
Fig. 5.
Close-up photographs of the stainless steel nail after pullout from a femur in the hydroxyapatite-treated control (HA) group (a) and from a femur in the hydroxyapatite-zoledronate-treated (HA + Zol) animals (b), demonstrating the difference in the amount of bone adhering to the nails.
Bone Mass Versus Mechanical Strength
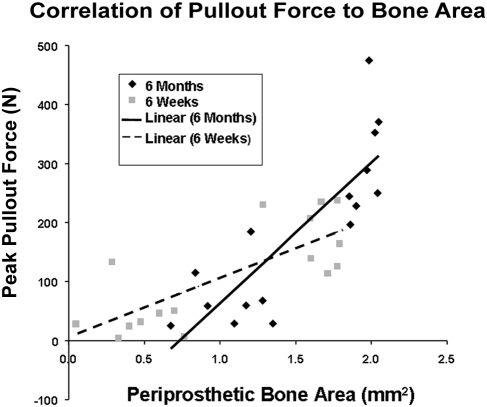
The Pearson correlation coefficient between the bone area in the anulus and the peak pullout force was 0.769 (p = 0.0005) for all six-week femora and 0.838 (p < 0.0001) for all six-month femora. The slope for all six-month femora (238.1 N/mm2) was found to be steeper (p = 0.0336) than the slope for all six-week femora (101.1 N/mm2) (Fig. 6). The Pearson correlation coefficient between the thresholded bone area in the anulus and the total energy for pullout was 0.692 (p = 0.0030) for all six-week femora and 0.595 (p = 0.0152) for all six-month femora. The slope for the six-month femora (394.6 N/mm) and the slope for the six-week femora (286.4 N/mm) were not different (p = 0.9326).
Fig. 6.
Correlation of the peak pullout force from mechanical testing to the amount of periprosthetic bone area determined from computed tomography for both the six-week and six-month femora. The straight lines represent a least-squares linear regression curve-fit.
Discussion
Implant loosening is associated with inflammatory bone loss induced from ultra-high molecular weight polyethylene debris. We hypothesized that the targeted delivery of bisphosphonates, which are commonly used antiresorptive agents, would improve periprosthetic bone quality and osseous integration of an intramedullary implant even in the presence of ultra-high molecular weight polyethylene particles of a size and distribution comparable with wear particles in vivo. In an experimental rat femur model, our findings of higher bone mass and better implant fixation in the study group of hydroxyapatite-zoledronate-coated implants compared with the control group with hydroxyapatite-coated implants without zoledronate support our hypothesis.
Zoledronate has been found to be a potent inhibitor of osteoclastogenesis13. It also has a direct effect on bone mineralization by enhancing osteoblastogenesis14,15. A concern when bisphosphonates are used as a coating material is the possible inhibition of osteoblastic activity and osseointegration since bis-phosphonates may reach a high local concentration. Our in vitro study data showed that this undesirable effect is actually concentration-dependent and avoidable. While zoledronate effectively induced cell death in osteoclast precursor cells at low concentrations, concentrations of >50 μM caused substantial apoptosis of osteoblasts as well. For this study, a 50-μM concentration was found to be optimal for inducing apoptosis in osteoclasts and macrophages, while minimizing osteoblast cell death8,16. Our in vitro study data also showed that zoledronate did not hinder the proliferation and maturation of human mesenchymal stem cells. This study showed that the number of alkaline phosphatase-positive cells, a marker for mature osteoblasts, did not exhibit any zoledronate dose dependency.
There have been recent attempts to improve implant fixation by the application of bisphosphonate both systemically17 and locally by bonding it to hydroxyapatite-coated implants8. Bisphosphonates are known to bind avidly to hydroxyapatite18,19. In a previous study, implants dipped in a low concentration of zoledronate had an increase in pullout force of up to 42% compared with implants without zoledronate8. However, those studies considered only the initial integration between the bone and the implant. In the in vivo situation, implant loosening is frequently associated with osteolysis, even after achieving initial integration, because of wear debris particles that initiate an inflammatory response from the host over time and cause a degradation of osseointegration. Bisphosphonates have been shown to reduce particle-induced osteolysis and implant loosening in animal models20,21 and in humans22 by preventing osteoclastogenesis6,7,23.
For this study, dual x-ray absorptiometry imaging was performed and encompassed the entire femur. Both zoledronate treatment and time were found to significantly increase the bone content and density. However, these effects were not as pronounced as those measured by microcomputed tomographic imaging because the dual x-ray absorptiometry scan measured an average for the entire femur, whereas our postprocessing analysis of the microcomputed tomographic scan measured just bone immediately adjacent to the implant. No difference between the left and right femora was found with regard to bone area, bone mineral content, or bone mineral density, which implies that the material type of the nail, either stainless steel or ultra-high molecular weight polyethylene, had no effect on the response of the bone or on the dual x-ray absorptiometry imaging modality.
The area of peri-implant trabecular bone for the hydroxyapatite-zoledronate treatment group was more than double that of the hydroxyapatite control group as determined from the microcomputed tomography measurements. This could be the result of either increased new bone formation or reduced osteolysis in the zoledronate-treated group. However, previously established experimental models, such as the mouse calvarial osteolysis model24, have suggested that the ultra-high molecular weight polyethylene particles inserted into the femoral canal cause osteolysis. Hence, the increased bone area is most likely due to reduced osteolysis in the study group. This suggests that zoledronate reduced the osteolysis induced by ultra-high molecular weight polyethylene particles in this model. After six months, the area of periprosthetic trabecular bone was still 86% higher for the treated group compared with that of the six-week group, suggesting that zoledronate may have a long-term effect, possibly by a slow release from hydroxyapatite. Although microcomputed tomography is not as accurate as the histomorphometric method in measuring mineralized area, it is a better tool for measuring the mineral density and mineral content of newly formed tissue than is histomorphometry. In addition, microcomputed tomography is simple and a good tool for obtaining an overall value of mineralized area at the implant interfaces.
Pullout testing demonstrated that zoledronate improves the strength of implant fixation, which has been correlated to periprosthetic bone mass. The hydroxyapatite-zoledronate group required significantly higher peak pullout force and pullout energy compared with the control group. We can conclude that the topical addition of zoledronate enhanced osseointegration of the implant in the presence of ultra-high molecular weight polyethylene particles.
It is also interesting to note that, in our study, the peak pullout force was significantly greater in the six-month femora than in the six-week femora. Other investigators have found no significant difference in pullout force for study periods of greater than six weeks because osseous integration of the implant to the host bone was complete by six weeks25. Consistent with the above observation, the control group in our study did not show any significant difference in pullout force between the six-week femora and the six-month femora. Conversely, there was an increase in the peak pullout force from six weeks to six months in the hydroxyapatite-zoledronate-treated femora. This may imply that simply soaking hydroxyapatite-coated implants in a zoledronate solution has a long-term beneficial effect. However, later loosening may occur because of possible zoledronate-related impaired bone-remodeling at the implant surface, and long-term studies will be needed to assess this effect. The large variance in the energy-to-pullout measurements may be explained by the large variation in the amount and pattern of bone remaining on the nails, suggesting variable osseous ingrowth.
The primary limitation of this study was the lack of a continuous supply of ultra-high molecular weight polyethylene particles into the femoral canal, which would have more closely mimicked the clinical setting. In our model, it is likely that the number of ultra-high molecular weight polyethylene particles in the femoral canal decreased over time. We do not know whether the beneficial effects of adding zoledronate to hydroxyapatite-coated implants would have been reduced had there been a continuous supply of ultra-high molecular weight polyethylene particles. Another limitation of the study is the use of the rat femur model itself since its physiologic environment and bone-remodeling potential may be different from those of humans. A previous study has described a turnover rate of trabecular bone in twelve-week-old rats similar to the rate in adult human iliac crest trabecular bone26. However, these results were dependent on the age of the rat, with younger rats having faster bone-remodeling and, furthermore, the pathologic state of inflammatory osteolysis was not assessed. Given these limitations in our current knowledge, clinical trials are necessary to evaluate whether a similar reduction in the rate of osteolysis is achieved in humans. Another potential limitation is the use of hydroxyapatite-coated ultra-high molecular weight polyethylene nails. These nails have different biomechanical properties compared with the hydroxyapatite-coated stainless steel nails. The ultra-high molecular weight polyethylene nails may bend more, load the surrounding bone differently, and affect the amount and type of bone formation around the implant. However, the analysis with dual x-ray absorptiometry, in which the femora with both types of nail were compared, found no significant difference in bone density between them. The ultra-high molecular weight polyethylene nails may also bind hydroxyapatite differently—the hydroxyapatite could loosen from the nails leaving behind a smooth surface not conducive to osseous integration. However, given that the mechanical results (with metal nails) and the microcomputed tomographic results (with ultra-high molecular weight polyethylene nails) showed no significant differences, it is unlikely that this is a major problem.
In summary, site-specific delivery of bisphosphonate by means of hydroxyapatite-coated implants resulted in higher bone mass and better biomechanical performance, even in the presence of ultra-high molecular weight polyethylene particles. Our data suggest that hydroxyapatite alone, as a coating on a stainless steel nail, has limited capacity in maintaining periprosthetic bone mass in the presence of ultra-high molecular weight polyethylene particles. Although the results of the present study support the concept that hydroxyapatite-bisphosphonate improves osseointegration, studies of larger animals and clinical trials will be necessary to verify the beneficial effects of biological modification of the implant-host bone interface.
Supplementary Material
Acknowledgments
Note: The authors thank Dr. Renwen Zhang of Stryker Orthopaedics, Mahwah, New Jersey, for providing the Faxitron images, and Dr. James K. Yeh of Winthrop-University Hospital, Mineola, New York, for providing the dual x-ray absorptiometry scans.
Appendix
A description of the methods and results with supporting figures of a preliminary study performed to determine the optimal concentration of zoledronate that effectively reduces the preosteoclast population, yet is least harmful to osteoblasts, is available with the electronic versions of this article, on our web site at jbjs.org (go to the article citation and click on “Supplementary Material”) and on our quarterly CD-DVD (call our subscription department, at 781-449-9780, to order the CD or DVD). 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from Stryker, the National Institutes of Health (grant 1R01EB006834) and from the Orthopaedic Research and Education Foundation (OREF). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. A commercial entity (Stryker) paid or directed in any one year, or agreed to pay or direct, benefits in excess of $10,000 to a research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which one or more of the authors, or a member of his or her immediate family, is affiliated or associated.
Investigation performed at the Center for Orthopaedic Research, Department of Orthopaedic Surgery, Columbia University, New York, NY
References
- 1.Jacobs JJ, Hallab NJ, Urban RM, Wimmer MA. Wear particles. J Bone Joint Surg Am. 2006;88 Suppl 2:99-102. [DOI] [PubMed] [Google Scholar]
- 2.Hirakawa K, Jacobs JJ, Urban R, Saito T. Mechanisms of failure of total hip replacements: lessons learned from retrieval studies. Clin Orthop Relat Res. 2004;420:10-7. [DOI] [PubMed] [Google Scholar]
- 3.Lappalainen R, Santavirta SS. Potential of coatings in total hip replacement. Clin Orthop Relat Res. 2005;430:72-9. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinari M, Oda Y, Ueki H, Yokose S. Immobilization of bisphosphonates on surface modified titanium. Biomaterials. 2001;22:709-15. [DOI] [PubMed] [Google Scholar]
- 5.Lipton A. New therapeutic agents for the treatment of bone diseases. Expert Opin Biol Ther. 2005;5:817-32. [DOI] [PubMed] [Google Scholar]
- 6.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s-6230s. [DOI] [PubMed] [Google Scholar]
- 7.Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Peter B, Pioletti DP, Laib S, Bujoli B, Pilet P, Janvier P, Guicheux J, Zambelli PY, Bouler JM, Gauthier O. Calcium phosphate drug delivery system: influence of local zoledronate release on bone implant osteointegration. Bone. 2005;36:52-60. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res. 2006;444:229-34. [DOI] [PubMed] [Google Scholar]
- 10.Dumbleton J, Manley MT. Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86:2526-40. [DOI] [PubMed] [Google Scholar]
- 11.Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376-83. [DOI] [PubMed] [Google Scholar]
- 12.von Knoch M, Jewison DE, Sibonga JD, Sprecher C, Morrey BF, Loer F, Berry DJ, Scully SP. The effectiveness of polyethylene versus titanium particles in inducing osteolysis in vivo. J Orthop Res. 2004;22:237-43. [DOI] [PubMed] [Google Scholar]
- 13.Cheer SM, Noble S. Zoledronic acid. Drugs. 2001;61:799-806. [DOI] [PubMed] [Google Scholar]
- 14.Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K, Zannettino AC. The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE). J Bone Miner Res. 2004;19:147-54. [DOI] [PubMed] [Google Scholar]
- 15.Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S, Evdokiou A, Lynch K, Atkins GJ, Zannettino AC. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone. 2004;34:112-23. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schünemann H, Einhorn TA. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2005;87:293-301. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli A, Henderson C, Grant MH, Meikle ST, Lloyd AW, Goldie I. The interactions of bisphosphonates in solution and as coatings on hydroxyapatite with osteoblasts. J Mater Sci Mater Med. 2002;13:923-31. [DOI] [PubMed] [Google Scholar]
- 19.Josse S, Faucheux C, Soueidan A, Grimandi G, Massiot D, Alonso B, Janvier P, Laïb S, Pilet P, Gauthier O, Daculsi G, Guicheux JJ, Bujoli B, Bouler JM. Novel biomaterials for bisphosphonate delivery. Biomaterials. 2005;26:2073-80. [DOI] [PubMed] [Google Scholar]
- 20.Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84:236-49. [DOI] [PubMed] [Google Scholar]
- 21.Shanbhag AS, Hasselman CT, Rubash HE. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33-43. [PubMed] [Google Scholar]
- 22.Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhav EM. Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty: a prospective randomized study. J Bone Miner Res. 2001;16:2126-31. [DOI] [PubMed] [Google Scholar]
- 23.Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Mönkkönen J, Auriola S, Chilton KM, Russell RG. Molecular mechanisms of action of bisphosphonates. Bone. 1999;24(5 Suppl):73S-79S. [DOI] [PubMed] [Google Scholar]
- 24.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopaedic implant osteolysis. Am J Pathol. 1999;154:203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: an experimental in vivo study in the rat. Biomaterials. 1997;18:969-78. [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Tross R, Vignery A. Evidence of sequential remodeling in rat trabecular bone: morphology, dynamic histomorphometry, and changes during skeletal maturation. Anat Rec. 1984;208:137-45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.