Abstract
The percentage of cells with chromosome aberrations or micronuclei induced by low doses of acute (dose rate of 47 cGy/min) or chronic (dose rate of 0.01 cGy/min) gamma-irradiation was studied in vitro in Chinese hamster fibroblasts, human lymphocytes, and Vicia faba seeds and seedlings. The sensitivity of the indicated biological entities to low doses was greater than expected based on linear extrapolation from higher doses. The dose-response curves for cytogenetic damage that were obtained were nonlinear when evaluated over the full range of the doses used. At very low doses, the dose-response curves appeared linear, followed by a plateau region at intermediate doses. At high doses the dose response curves again appeared linear with a slope different from that for the low-dose region. There was no statistically significant difference between the yields of cells with micronuclei induced by low doses of acute versus chronic irradiation. Similar data were obtained both for human lymphocyte culture and for roots and seeds of Vicia faba. Our experiments revealed that the dose range over which the plateau occurs depends on the type of cells irradiated. We have also shown that the modifying effects of the repair inhibitor caffeine and the radioprotector mercaptoethylenamine (MEA) are absent at low doses of gamma irradiation and that caffeine increased the number of cells with cytogenetic damage when evaluated over the plateau region. In the presence of MEA, the upper end of the plateau region was extended from just above 1 Gy to about 2 Gy. We therefore provide direct evidence that a plateau exists in the dose-response curve for the indicated radiation-induced stochastic effects. Furthermore, our results suggest that, for low linear energy transfer radiation, the induction of DNA repair occurs only after a threshold level of cytogenetic damage and that the higher yield of cytogenetic damage per unit dose at low radiation doses is attributable to an insignificant contribution or the absence of DNA repair processes.
Keywords: nonlinear dose response, cytogenetic damage, ionizing radiation, low doses
INTRODUCTION
The linear no-threshold (LNT) hypothesis is the basis for radiation protection standards at low doses. However, questions about the validity of the LNT model remain. Validation of the LNT model is the most important issue related to radiation risk assessment. Studies of cytogenetic effects can provide key information related to the validity of the LNT model. Cyto-genetic effects of ionizing radiation have been studied in vitro in human (Fenech and Morley, 1985; Mitchell and Norman, 1987) and mouse and rat (Erexson et al., 1991) lymphocyte cultures, and in cultures of mammalian cells (Marples and Joiner, 1993; Zaichkina et al., 1995; Shmakova et al., 2002). They have also been studied in vivo in mouse bone marrow cells (Jenssen and Ramel, 1976, 1978; Cole et al., 1981; Jagetia and Ganapathi, 1994) and in plant cells (Oudalova et al., 2002). However, dose-response curves obtained by the different research groups using the various cell types are different. With human lymphocyte cultures, a linear dose–response relationship was found (Fenech and Morley, 1985), whereas in other studies a departure from a linear relationship was reported (Mitchell and Norman, 1987). In mice, a deviation from the linear dose-response curve was revealed for in vivo exposure. Jagetia and Ganapathi (1994) used the micronucleus assay in mice and showed that the dose-response curve had a linear-quadratic form. However, a linear dose-response curve was also obtained by others using the same method (Cole et al., 1981; Uma Devi and Sharma, 1990). Many years ago it was shown by Russel (1965) that the mutation yield per unit dose was higher at low doses of radiation than at high doses. Similar results were obtained by studying radiation-induced cytogenetic damage (Luchnik and Sevankaev, 1976; Pohl-Rulling et al., 1983; Lloyd et al., 1988; Zaichkina et al., 1997), transformation (Oftedal, 1990), and cell survival (Joiner, 1994; Joiner et al., 1996). Joiner et al. (2001) showed that most cell lines have hyper-radiosensitivity to very low radiation doses, which is not predicted by back-extrapolation of the cell survival curve from higher doses. Such nonlinear data have led to the recent view that biological effects of ionizing radiation should not be extrapolated from high to low doses based on the LNT model.
The aim of the present study was to investigate the shape of the dose-response relationship for specific cytogenetic effects after low doses of low-linear-energy-transfer (LET) ionizing radiation using mammalian and plant cells.
MATERIALS AND METHODS
In our previous studies, we initiated investigations of biological responses to ionizing radiation exposure using the occurrence of chromosomal rearrangements as the endpoint scored (Zaichkina and Ganassi, 1984; Zaichkina et al., 1992, 1995). Based on results of those studies, we have subsequently used different radiation dose ranges for the different bioassays associated with our studies of radiation-induced stochastic effects.
In our present study, Chinese hamster fibroblasts (clone 431) were cultivated at 37°C, in a humidified atmosphere under 5% CO2. Cell culture was grown in Dulbecco’s Modified Eagle Medium containing 10% calf serum and antibiotics. The cells were irradiated with acute doses of 0.05–2 Gy of 60Co gamma-rays at a dose rate of 28.2 Gy/h. The chronic gamma-irradiation was carried out for 1, 2, 3, 4, 5, and 6 days at room temperature at a dose rate of 0.0061 Gy/h, with cumulative doses of 0.14, 0.28, 0.42, 0.56, and 0.84 Gy, respectively. The technique for preparation of samples for the micronucleus test has been previously described (Zaichkina and Ganassi, 1984).
When the cells were fixed immediately after chronic irradiation, no damage was observed. To detect cytogenetic damage, the irradiated and control cells kept at room temperature were stored for 24 h at 37°C before fixation. Several experiments were performed for each radiation dose. About 10,000 cells were analyzed for each radiation dose point.
Whole human blood from ten essentially healthy males was irradiated with acute doses of 0.1–0.5 Gy at a dose rate of 28.2 Gy/h. Other blood samples were also chronically irradiated at a dose rate of 0.0061 Gy/h at a 37°C temperature during 0.5, 1, and 2 days with cumulative doses of 0.07, 0.13, and 0.27 Gy, respectively. Whole blood (0.2 ml) was cultivated by the micromethod (Zaichkina et al., 1997). Cytogenetic analysis was carried out based on 150–200 metaphase cells scored from each blood sample. All structural chromosome-type and chromatid-type changes were recorded. Preliminary experiments showed that the conditions of blood-sample irradiation affected neither the background level of chromosomal damage nor radiosensitivity (Zaichkina et al., 1997).
Air-dried seeds of Vicia faba were soaked and germinated at 21°C during 36–48 h until the roots reached 1–1.5 cm in length. The roots of Vicia faba were irradiated with doses of 0.1–2.5 Gy of acute gamma irradiation at a dose rate of 28.2 Gy/h. The end of the 1.5–2-mm roots were fixed 24 h after irradiation and stained by the Fulgen method; 50–100 plants were used for each variant, and 1000 cells from each plant were analyzed.
Air-dried seeds of Vicia faba were also irradiated with doses of 2–40 Gy at a dose rate of 1.4 Gy/min, soaked, germinated, and processed for cytogenetic analysis as described earlier.
All cytological slides were coded blind and analyzed by the investigators independently. Experiments were repeated at least three times. In the figures, results are presented as means +/−standard error.
RESULTS AND DISCUSSION
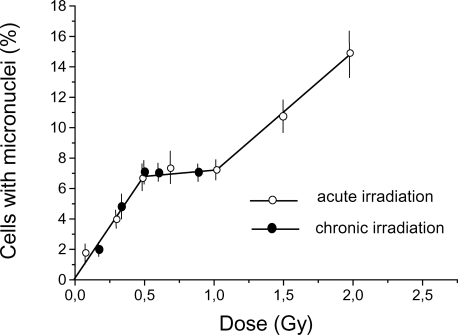
The dose dependence of the yield of cytogenetic damage in Chinese hamster cells after acute and chronic gamma-irradiation is presented in Figure 1. The dose–response curve used is discontinuous. At very low doses, the dependence of cytogenetic damage on dose was fitted by a linear regression with a zero intercept. In the dose range of about 0.5–1 Gy, there is a plateau, and above this range the curve again appears linear but with a different slope. There was no statistically significant difference between the results for the low doses of chronic and acute irradiation for the investigated dose range.
FIGURE 1.
Dose-response curve for micronuclei induction in Chinese hamster fibroblasts by acute (28.2 Gy/h) and chronic (0.0061 Gy/h) gamma-irradiation. Results (means ± standard errors) of six experiments with acute and four experiments with chronic exposure are shown.
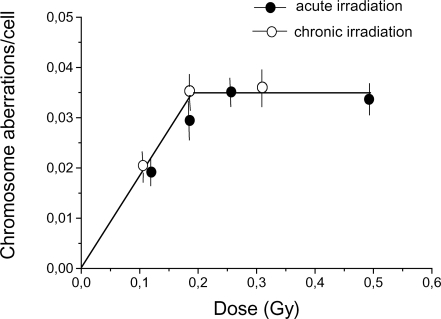
Similar results were obtained for human lymphocyte culture. The dose dependence of the yield of chromosome aberrations in human lymphocytes induced by acute and chronic gamma-irradiation is presented in Figure 2. It can be seen that the obtained high- and low dose rate data for chromosome aberrations were adequately described by single, discontinuous dose-response relationships with an initial linear increase followed by a plateau. Thus, as was the case with Chinese hamster fibroblasts, for the low-dose region, no influence of dose rate was found.
FIGURE 2.
Dose-response curve for chromosomal aberration induction in human lymphocytes. Whole blood received either acute (28.2 Gy/h) or chronic (0.0061 Gy/h) gamma-irradiation. Each point represents the mean ± standard error obtained from blood lymphocytes from 10 healthy donors. The experiment was repeated at least three times.
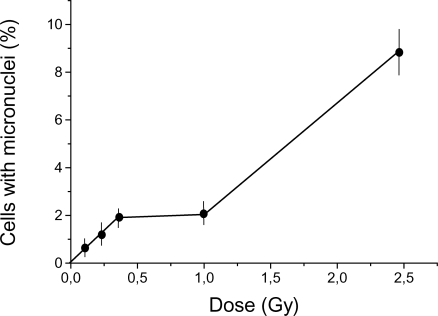
The dose-response curve for micronuclei induction in root cells of Vicia faba by gamma-ray exposure can also be described using a similar discontinuous function, but the plateau spans a different dose range, 0.5–1 Gy (Figure 3), than in Figure 1. Using the radioresistant model of Vicia faba dry seeds, we found that the dose-response curve for micronuclei induction is similar to the dose-response curves in Figure 3, except that the plateau was observed in the dose range of about 8–32 Gy (data not shown). Thus, our experiments revealed that the dose range over which the plateau occurs differs for the different biological systems. For radiosensitive systems, the plateau occurs at lower doses than for radioresistant systems.
FIGURE 3.
Dose-response curve for micronuclei induction in root cells of Vicia faba by acute (28.2 Gy/h) gamma-irradiation. Results (means ± standard errors) are based on at least three experiments.
Our data demonstrate that the dose-response curves for micronuclei induction in Chinese hamster fibroblasts, human lymphocytes, and root cells and dry seeds of Vicia faba exposed to acute and chronic gamma-radiation can all be described by a discontinuous function comprising three parts: (1) a low-dose linear segment (linear segment 1); (2) a plateau at intermediate doses; and (3) a high-dose linear segment (linear segment 2).
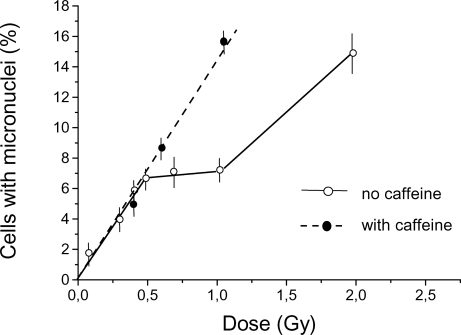
We hypothesized that the indicated complex shape of the dose-response curves relates to the status of DNA repair processes. Previously we have shown that caffeine, an inhibitor of DNA repair, increases the number of cells with micronuclei in linear segment 2 (Zaichkina et al., 1992,Zaichkina et al., 1995). To verify the assumption that low doses of gamma-radiation do not induce repair processes, we studied the influence of the DNA repair inhibitor caffeine on the dose dependence of the induction of chromosome aberrations in Chinese hamster fibroblast cultures. Figure 4 shows the dose-response curves for micronuclei induction in fibroblasts irradiated with low doses of gamma-radiation in the presence of caffeine and without caffeine. Caffeine was added to the culture medium (final concentration 0.08 M, pH 6.5–6.8) prior to irradiation. The cells were kept in the medium with caffeine after irradiation up to fixation. There was no modifying effect of caffeine for linear segment 1; however, for the plateau region and for linear segment 2, caffeine caused significant changes in the dose-response curve. More specifically, caffeine treatment led to the disappearance of the plateau (effectively extending linear segment 1).
FIGURE 4.
Dose-response curve for micronuclei induction in Chinese hamster fibroblasts by acute (28.2 Gy/h) gamma-irradiation with and without caffeine treatment. Caffeine was added to culture medium prior to irradiation at a final concentration of 0.08 M, pH 6.5–6.8. Results (means ± standard errors) of six experiments without caffeine and three experiments with caffeine treatment are shown.
Another approach for validating our hypothesis was to investigate the action of low doses of secondary radiation from 70 GeV protons and neutrons (90%) and of chronic monoenergetic neutrons generated by the Serpukhov charged particle accelerator on cytogenetic damage in mammalian cells. Using inhibitors of DNA repair we have shown that high doses of gamma-radiation induced repair, in contrast to neutrons which inhibit repair of cytogenetic damage (Zaichkina et al., 1995). It was found that, in contrast to the gamma-irradiation, the dose-response curves after exposure to high-LET neutrons for all the cytogenetic endpoints were linear; that is, the plateau was absent (Rozanova et al., 2000). These results suggest that the induction by gamma-rays of DNA repair processes occurs in the dose range corresponding to the plateau. Thus, it can be assumed that the induction of repair by low-LET radiation occurs only after a threshold level of cytogenetic damage and that the increased yield of cytogenetic damage per unit dose at low radiation doses is attributable to an insignificant contribution or the absence of DNA repair. Recently, Rothkamm and Lobrich (2003) have shown a lack of DNA double-strand-break repair in primary human fibroblasts exposed to very low doses (approximately 1 mGy) of ionizing radiation.
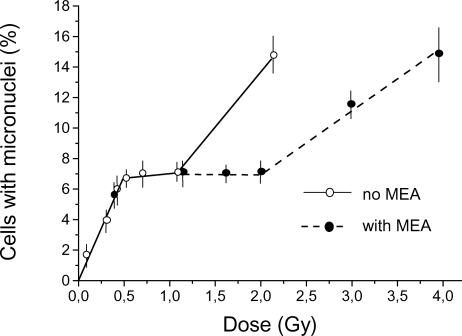
Figure 5 shows the dose-response curves for micronuclei induction in the culture of Chinese hamster fibroblasts irradiated in the presence and without b-mercaptoethylenamine (MEA), a well-known radioprotector. MEA was added (final concentration 0.1 mM) to the culture medium 15 min before irradiation and the cells were kept in the medium with MEA for 2 h after irradiation. As with caffeine, no modifying effect of MEA in linear segment 1 was observed but the plateau region was extended from just above 1 Gy to about 2 Gy. This is direct evidence that a plateau exists and that our interpretation of the experimental results are plausible.
FIGURE 5.
Dose-response curve for micronuclei induction in Chinese hamster fibroblasts by acute (28.2 Gy/h) gamma-irradiation with and without MEA treatment. MEA was added at a final concentration of 0.1 mM to the culture medium 15 min before irradiation and the cells were kept in the medium containing MEA for 2 h after irradiation. Results (means ± standard errors) of six experiments without MEA and three experiments with MEA treatment are shown.
Recently in our study involving irradiated mice, we showed that the in vivo dose-response curve for micronuclei induction in bone marrow cells is also nonlinear and also consists of the three indicated segments (linear segment 1, plateau, linear segment 2) (Zaichkina et al., 1998).
The results we have presented indicate that dose-response curves both in mammalian and plant cells are nonlinear and reflect hypersensitivity of cells to low radiation doses that is not predicted by back-extrapolation of cytogenetic response from higher doses. Although further studies are required to reveal the molecular mechanisms associated with the observed dose-response relationships, our results suggest that the hypersensitivity may be attributed to a lack of induced DNA repair at very low radiation doses. Altogether, our results provide additional evidence that low doses of low-LET radiation could have more significant genetic consequences than would be expected based on extrapolating from high to low doses based on the LNT model
REFERENCES
- Cole RJ, Taylor N, Cole J, Arlett CF. Short-term tests for transplacentally active carcinogens: I. Micronucleus formation in fetal and maternal mouse erythroblasts. Mutat Res. 1981;80:141–147. doi: 10.1016/0027-5107(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Erexson GL, Kligerman AD, Bryant MF, Sontag MR, Halperin EC. Induction of micronuclei by X-radiation in human, mouse, and rat peripheral blood lymphocytes. Mutat Res. 1991;253:193–198. doi: 10.1016/0165-1161(91)90132-r. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley A. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Ganapathi NG. Radiation-induced micronucleus formation in mouse bone marrow after low dose exposure. Mutat Res. 1994;304:235–242. doi: 10.1016/0027-5107(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Jenssen D, Ramel C. Dose response at low doses of X-irradiation and MMS on the induction of micronuclei in mouse erythroblasts. Mutat Res. 1976;41:311–320. doi: 10.1016/0027-5107(76)90104-4. [DOI] [PubMed] [Google Scholar]
- Jenssen D, Ramel C. Factors affecting the induction of micronuclei at low doses of X-rays, MMS, and dimethylnitrosamine in mouse erythroblasts. Mutat Res. 1978;58:51–65. doi: 10.1016/0165-1218(78)90095-2. [DOI] [PubMed] [Google Scholar]
- Joiner MC. Induced radioresistance: An overview and historical perspective. Int J Radiat Biol. 1994;65:79–84. doi: 10.1080/09553009414550111. [DOI] [PubMed] [Google Scholar]
- Joiner MC, Lambin P, Marples P. Abstr. Book of 27th Annual Meeting of the ESRR. Montpellier, France: Les Editions de Physique Les Ulis Press; 1996. Induced radioresistance in mammalian systems; p. 115. [Google Scholar]
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- Lloyd DS, Edwards AA, Leonard D. Frequencies of chromosomal aberrations induced in human blood lymphocytes by low doses of X-rays. Int J Radiat Biol. 1988;53:49–55. doi: 10.1080/09553008814550411. [DOI] [PubMed] [Google Scholar]
- Luchnik NV, Sevankaev AV. Radiation-induced chromosomal aberrations in human lymphocytes. I: Dependence on the dose of gamma-rays and an anomaly at low doses. Mutat Res. 1976;36:363–378. doi: 10.1016/0027-5107(76)90246-3. [DOI] [PubMed] [Google Scholar]
- Marples B, Joiner MC. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat Res. 1993;133:41–51. [PubMed] [Google Scholar]
- Mitchell JC, Norman A. The induction of micronuclei in human lymphocytes by low doses of radiation. Int J Radiat Biol. 1987;52:527–535. doi: 10.1080/09553008714552031. [DOI] [PubMed] [Google Scholar]
- Oftedal P. Low dose radiation effect, holistic model: DNA mutagenesis induced by radiation. In: Amirtaev KG, Kozubek SB, editors. Proc. Workshop on Genetic Effects of Charged Particles. Dubna, Russia: Academic Press; 1990. pp. 11–13. [Google Scholar]
- Oudalova AA, Geraskin SA, Dikarev VG, Nesterov YeB, Dikareva NS. Induction of chromosome aberrations is non-linear within the low dose region and depends on dose rate. Radiat Prot Dosim. 2002;99(1–4):245–248. doi: 10.1093/oxfordjournals.rpd.a006774. [DOI] [PubMed] [Google Scholar]
- Pohl-Ruling J, Ficher P, Haas O, Obe G, Natarajan AT, van Buul PP, Buckton KE, Bianchi NO, Larramendy M, Kucerova M, Polikova Z, Leonard A, Fabry L, Palitti F, Sharma T, Binder W, Mukherjee RN, Mukherjee U. Effect of low-dose acute x-irradiation on frequencies of chromosomal aberrations in human peripheral lymphocytes in vitro. Mutat Res. 1983;110:71–82. doi: 10.1016/0027-5107(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, L¨obrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanova OM, Zaichkina SI, Aptikaeva GF, Akhmadieva Akh, Smirnova EN, Klokov DYu. Cytogenetic effects of neutron radiation in mammalian cells. Advances in Fundamental and Clinical Radiobiology. Abstracts of the 1st International Congress of the South African Radiobiology Society; 10–13 December 2000; University of Stellenbosch South Africa; p. 209. [Google Scholar]
- Russell WL. Effect on the interval between irradiation and conception on mutation frequency in female mice. Proc Natl Acad Sci USA. 1965;54:1552–1557. doi: 10.1073/pnas.54.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakova NL, Fadeeva EA, Nasonova EA, Krasavin EA, Rsynina AV.Cytogenetic effect of low dose radiation in mammalian cells: The analysis of the phenomenon of hypersensitivity and induced radioresistence Radiat Biol Radioecol 200242245–249.in Russian [PubMed] [Google Scholar]
- Uma Devi P, Sharma ASKVS. Mouse bone-marrow response to low doses of whole-body γ-irradiation: Induction of micronuclei. Int J Radiat Biol. 1990;57:97–101. doi: 10.1080/09553009014550371. [DOI] [PubMed] [Google Scholar]
- Zaichkina SI, Ganassi EE. Micronuclear test to estimate chromosomal aberrations induced by different agents. Stud Biophys. 1984;99:203–205. [Google Scholar]
- Zaichkina SI, Aptikaeva GF, Akhmadieva Akh, Livanova IA, Smirnova EN, Antipov AV, Pritutskaia NB, Kuglik P, Shlotova LA, Ganassi EE.Cytogenetic injury realization features in mammalian and plant cells affected by low-level radiation Radiobiology 19923238–41.(in Russian) [PubMed] [Google Scholar]
- Zaichkina SI, Aptikaeva GF, Akhmadieva Akh, Rozanova OM, Smirnova EN, Ganassi EE. Cytogenetic consequences of low-dose radiation. In: Burlakova EB, editor. Radiobiological Disasters: Consequences of Accidents at Nuclear Power Plants. Commack, NY: Nova Science Publishers; 1995. pp. 31–37. [Google Scholar]
- Zaichkina SI, Aptikaeva GF, Rozanova OM, Akhmadieva AK, Smirnova EN, Ganassi EE. Action of chronic irradiation on the cytogenetic damage of human lymphocyte culture. Environ Health Persp. 1997;105:1441–1443. doi: 10.1289/ehp.97105s61441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaichkina SI, Klokov DY, Rozanova OM, Aptikaeva GF, Akhmadieva Akh. Cytogenetic damage to bone marrow polychromatic erythrocytes of mice exposed in vivo to low-dose gamma-radiation. Russ J Genet. 1998;34:1113–1116. [PubMed] [Google Scholar]