Abstract
Nandrolone and other 19‐norsteroid potent anabolic steroids have been prohibited in sports for 30 years. The detection of the main urinary metabolite—19‐norandrosterone—in amounts greater than 2 ng/ml constitutes an adverse analytical finding. The presence in nutritional sport supplements of steroids not listed on the label has undoubtedly resulted in positive tests, but inadvertent consumption of meat containing residues of hormonal treatment should not realistically cause apprehension. Although highly improbable, athletes should prudently avoid meals composed of pig offal in the hours preceding the test since the consumption of edible parts of a non‐castrated pig, containing 19‐nortestosterone, has been shown to results in the excretion of 19‐norandrosterone in the following hours. Norsteroid metabolites are formed during pregnancy and excreted as minor metabolites of norethisterone, and minute amounts have been identified in some male and female samples when using more sensitive techniques of detection. Whereas exercise does not seem to be a significant factor in 19‐norandrosterone excretion, some rare urine samples were found to be a suitable medium for in situ 19‐demethylation of urinary metabolites.
Keywords: 19‐norandrosterone, 19‐norsteroids, excretion
Nandrolone (19‐nortestosterone, 17β‐hydroxyestr‐4‐en‐3‐one) was first synthesised in the 1950s.1 Its anabolic properties have prompted investigations to assess its clinical potential in male fertility control,2,3 patients on haemodialysis,4,5,6,7 aplastic anaemia (its main legitimate use),8 asthenia in AIDS patients,9 recovery following trauma or surgery,10 protecting the immune system following cancer treatments,11,12 osteoporosis,13,14 and cachexia.15 Anabolic steroids and 19‐nortestosterone are used in medical veterinary practice for retarding degenerative processes and promoting tissue repair; they are also used—legitimately in some countries but not in others—as growth promoters in the farming industry. Nandrolone is perhaps best known as a performance enhancing substance, not devoid of adverse health effects, for increasing muscle strength and mass, and to speed up recovery, which it found its way in the sporting world (human and animal) as soon as it became available.16,17,18 Surveys revealed use of anabolic steroids among adolescents not only for performance purposes but also to improve body image.19,20 The use of anabolic androgenic steroids has been prohibited in “amateur” sports since the 1970s. A vast number of 19‐nortestosterone human and veterinary preparations are available worldwide, most frequently for parenteral administration (intramuscular), as long‐chain ester derivatives in the 17‐O position (phenpropionate, decanoate) in vegetable oil; the pharmacokinetics are well described.21 Further to the adoption of the Dietary Supplement and Health Education Act in the USA, several steroids and prohormones became available for oral self‐administration worldwide and on the internet, including 19‐norandrostenedione (estr‐4 (or ‐5)‐en‐3,17‐dione) and 19‐ norandrostenediol (3,17‐dihydroxyestr‐4 (or ‐5)‐en); since January 2005 the distribution of those commercial products has finally been regulated/prohibited in many countries. Nandrolone and other 19‐norsteroids have been banned to athletes for years and are listed in section S1, anabolic agents in the World Anti‐Doping Agency list of prohibited substances and methods.22
Norsteroids: metabolism, excretion, and detection in human urine samples
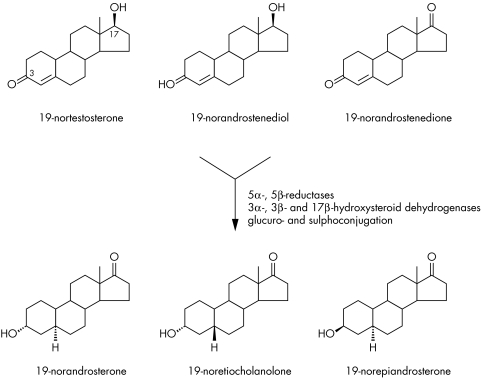
The principal urinary metabolites formed following the administration of 19‐nortestosterone (19‐NT), were rapidly identified as 19‐norandrosterone (19‐NA; 3α‐hydroxy‐5α‐androstan‐17‐one), 19‐noretiocholanolone (19‐NE; 3α‐hydroxy‐5β‐androstan‐17‐one) and 19‐norepiandrosterone (19‐NEA; 3β‐hydroxy‐5α‐androstan‐17‐one). The latter, possessing a 3β‐hydroxyl group, is almost exclusively sulphoconjugated, whereas the other two are predominantly excreted as their glucuronide derivatives (fig 1).23,24,25 The same metabolites are produced from “prohormones”, 19‐norandrostenedione and 19‐norandrostenediol, often referred to as precursors of 19‐NT.26,27,28 When orally administered, due to extensive first pass metabolism, the metabolites are rapidly and massively excreted in the initial hours following administration. The enzymatic reactions involved are well known: phase I involving 5α‐ and 5β‐reductases, 3α‐, 3β‐, and 17β‐hydroxysteroid dehydrogenases, followed by phase II conjugative reactions with glucuronic acid or sulphate. Although after parenteral or oral administration of 19‐NT and 19‐norandrost‐4‐endione the formation of 19‐NA (3α‐OH, 5α‐H) is clearly favoured over 19‐NE (3α‐OH, 5β‐H), inverted ratios of the metabolites have been reported for 19‐norandrost‐4‐en‐3β,17β‐diol, Δ5‐norsteroids and at the end of the excretion period following oral ingestion of nandrolone sulphate.25,26,27,28,29,30,31
Figure 1 Metabolism of norsteroids in humans: 19‐norandrosterone and 19‐noretiocholanolone are predominantly glucuroconjugated, 19‐epiandrosterone is sulphoconjugated.
The metabolites are easily detected in human urine samples following isolation through solid‐phase extraction, enzymatic hydrolysis, liquid‐liquid extraction, chemical formation of derivatives, usually per‐trimethylsilylation, and gas chromatographic‐mass spectrometric (GC‐MS) analysis.32,33,34,35 Parenteral administration of the long‐chain esters of 19‐nortestosterone may be detected for months, with anecdotal evidence pointing to more than 18 months past the last injection. Metabolites formed after oral administration of norsteroids remain detectable for only a few days. Since strong interindividual variability exists in the excretion of metabolites linked to different rates of absorption and rapid elimination of metabolites following oral ingestion, it seems almost impossible to determine from the results of a single test what exact preparation was used, let alone the time, dosage,or mode of administration. It appears that high‐intensity exercises do not influence the excretion of 19‐norsteroids administered to trained athletes.36
The analysis of urinary metabolites by gas chromatography/combustion/isotope ratio mass spectrometry (GC/C/IRMS) has been shown to be useful in determining their origin, endogenous or exogenous.37,38,39,40 It is routinely used for confirmation in a few laboratories; major improvements were needed with regard to sample purification and instrumental conditions to reach the level of sensitivity required for the analysis of low levels of 19‐norsteroid urinary metabolites.
19‐NA in athletes' urine samples
The Medical Commission of the International Olympic Committee (IOC) and, since 2003, the World Anti‐Doping Agency (WADA) are collecting results of testing done on athletes' samples in accredited laboratories. Since 1988 it has appeared that testosterone, 19‐nortestosterone (or precursors), stanozolol, and methandienone are the anabolic androgenic steroids most frequently found in athletes' samples. Each year, the presence of 19‐NA is recurrently detected in an average of 0.23% of all samples tested (table 1).41,42
Table 1 Results of international testing in IOC/WADA accredited laboratories41,42.
| Year | Total number of tests | Nandrolone adverse findings reported | Per cent |
|---|---|---|---|
| 1988 | 47069 | 304 | 0.65 |
| 1989 | 52371 | 224 | 0.43 |
| 1990 | 71341 | 192 | 0.27 |
| 1991 | 84088 | 165 | 0.20 |
| 1992 | 87808 | 152 | 0.17 |
| 1993 | 89166 | 227 | 0.25 |
| 1994 | 93680 | 207 | 0.22 |
| 1995 | 93938 | 212 | 0.23 |
| 1996 | 96454 | 232 | 0.24 |
| 1997 | 106561 | 262 | 0.25 |
| 1998 | 105250 | 259 | 0.25 |
| 1999 | 118259 | 293 | 0.25 |
| 2000 | 117314 | 325 | 0.28 |
| 2001 | 125701 | 304 | 0.24 |
| 2003 | 151210 | 256 | 0.17 |
| 2004 | 169187 | 339 | 0.20 |
While testing athletes' samples, the laboratories must follow WADA's requirements as detailed in the International Standard for Laboratories including technical documents describing criteria for identification and reporting.43 Adverse analytical findings occur when the concentration of 19‐NA is greater than 2 ng/ml, with consideration being given to the estimated uncertainty of the measure; the threshold, introduced in 1998,44 is adjusted to the specific gravity of the specimen. Verifications are made to exclude pregnancy and the legitimate use of norethisterone contraceptive medicines, which have been found to result in minor metabolites to low levels of 19‐NA and 19‐NE.
19‐NA normally found in human urine samples
The threshold being fixed at 2 ng/ml, the methods used in laboratories should permit testing with a required performance level of 1 ng/ml.45 It appears that the low excretion of endogenous 19‐norandrosterone is usually not detected in human urine samples during routine doping control testing because the limit of detection (LOD) of the methods employed is usually in the order of 0.3–0.5 ng/ml. Exception should be made for specimens collected during pregnancy, in which levels can reach approximately 15 ng/ml.46 The presence of 19‐nortestosterone and 19‐norandrostenedione in human follicular fluids and of 19‐nortestosterone in the plasma of pregnant women was reported in 1984 and 1987, respectively, but no 19‐NT was detectable in the plasma of males and non‐pregnant women. The hypothesis of a placental origin of 19‐NT—for example, by an alternative aromatising pathway converting testosterone to estradiol by 19‐demethylation, was presented.47,48 The synthesis of 19‐NT in the human ovary was described in 2002.49
More sensitive instrumentation such as GC or high performance liquid chromatography (HPLC) coupled with high resolution mass spectrometry or tandem mass spectrometry, larger volumes of urine, and extensive purification by HPLC or ion exchange and partition chromatography were needed to detect, identify, and quantify endogenous 19‐norandrosterone. Between 1997 and 1999, three studies described under such experimental conditions, the presence of 19‐NA in male specimens (sportsmen and volunteers). The levels were described at around 0.01–0.32 ng/ml (mean 0.08 ng/ml) in non‐fractionated 24 hour urine,50 0.02 (LOD) to 0.6 ng/ml,51 and lower than 0.5 ng/ml.52 Although insulin stress had no effect on 19‐NA excretion in males, gonadal stimulation with human chorionic gonadotropin was found to increase the level by 250%. The maximal concentration reached post‐stimulation was 0.43 ng/ml.53 In samples collected from non‐pregnant females, the level of endogenous 19‐NA seems also relatively low (below 1 ng/ml), varying during the menstrual cycle and correlating with higher plasma levels of 17β‐estradiol,54,55 or with increased excretion of luteinising hormone. In the latter study, a threefold to fourfold increase was measured with urinary concentrations peaking at a maximum of 0.8 ng/ml in mid‐cycle.56,57
Ingestion from contaminated commercial products
In the past five years, numerous studies have confirmed that products sold as nutritional or sport supplements are not properly labelled and contain, whether that is deliberate, steroids and prohormones including 19‐norsteroids or stimulants such as caffeine and ephedrine, all of which do not appear on the list of ingredients. The ingestion of such products was shown to result in positive tests in the next hours.58,59,60,61,62,63,64,65 Some athletes who tested positive were able to link the test result to a mislabelled commercial product.66
Ingestion of non‐castrated pig offal
Notwithstanding some reports suggesting a link existing between the consumption of meat contaminated by residual hormonal treatment and potential positive tests,67,68 it is generally regarded as highly improbable since residues in meat, when measured, are very low; there is no indication that such a case happened.50,51
19‐nortestosterone, the 17β‐isomer, is present in the intact boar. A first report described the ingestion of a substantial amount of non‐castrated pig meat (375 g) resulting in the excretion of 19‐norandrosterone in amounts reaching 3–7.5 ppb in the following hours.69 Another study confirmed that in spite of interindividual differences, 19‐NA and 19‐NE were always measured in specimens provided in the hours following the improbable ingestion (non‐castrated pig meat being difficult to find) of such meat‐rich meal composed of non‐castrated pig tissues, with levels even reaching 160 ng/ml in one case. No trace of norsteroid metabolites was found in urine samples collected before or after the ingestion of castrated pig edible parts. As expected, the GC/C/IRMS analysis confirmed endogenous‐like 13C/12C value of metabolites.70
What is already known about this topic
Nandrolone and other 19‐norsteroids have been prohibited in sports for 30 years. Although the detection of their main urinary metabolite, 19‐norandrosterone, in amounts greater than 2 ng/ml constitutes an adverse analytical finding, there have been ongoing discussions on the influence of exercise as well as the consumption of nutritional supplements and pig offal on its excretion in the urine.
The effect of exercise
In the past years, different groups have tested selected athletes' samples to determine whether exercise played a role in the excretion of norsteroid metabolites and apparently, it is not the case. One group analysed samples taken from two cohorts of football players, amateurs and professionals, and noted that up to 6% of the samples collected post‐match—in uncontrolled conditions—contained traces of 19‐NA; the authors prudently attributed the findings to either endogenous production, release from the fatty tissues of a previous intake of 19‐NT, or intake just prior to the match of a product containing nandrolone.71 Another group reported the results of 385 samples from 40 football players and described concentrations of 19‐NA as significantly higher than those before match,72 while again in uncontrolled conditions, traces of 19‐NA were found in some professional football players' samples but were absent in samples from the control group.73
Under controlled conditions, baseline levels of 19‐NA in male athletes have been found to be either undetectable or reaching a maximum level of 0.25 ng/ml (mean 0.048 ng/ml). Exercise was not found to influence 19‐NT secretion or excretion of 19‐NA.74 There was no impact on the excretion of norsteroid metabolites of controlled exercise sessions in young male athletes; only one sample contained measurable amount of 19‐NA (0.13 ng/ml).75,76
In situ 19‐demethylation of urinary steroids
Recently, formation of trace amounts of norsteroid metabolites by 19‐demethylation of etiocholanolone (3α‐hydroxy‐5β‐androstan‐17‐one) and androsterone (3α‐hydroxy‐5α‐androstan‐17‐one) was observed in athletes' samples following incubation. The reaction being favoured in 5β‐isomers, 19‐NA and 19‐NE appear in ratios lower than that of their respective urinary precursors, androsterone and etiocholanolone.77 Urine samples in which 19‐demethylation activity was observed were often very concentrated, and turbidity was noted. This crucial observation, which was confirmed by two different groups, prompted the revision of the criteria for reporting adverse findings, to include verification steps in the relatively rare samples showing characteristic criteria of unstable urines.78
Efforts have been made to permit the reliable analysis of urinary 19‐norandrosterone in trace amount by GC/C/IRMS which requires better instrumental sensitivity and improved sample purification. The origin of 19‐NA present in amounts as low as 2–3 ng/ml can now be determined in a relatively low volume of urine, making it suitable for confirmation of athletes' samples (Hebestreit M et al, personal communication, 2006; Fakirian A et al unpublished data).
What this article adds
Nowadays, the use of IRMS identifies the endo‐ or exogenous origin of the urinary metabolites of 19‐nortestosterone, even low quantities. Whereas exercise does not seem to significantly increase their excretion, athletes should refrain from taking nutritional supplements to avoid positive testing. Recently, the finding that in some unstable urine samples metabolites may be formed by in situ 19‐demethylation, has prompted the inclusion of defined verification steps before reporting an adverse result.
Conclusion
Administration of 19‐nortestosterone does not represent a major analytical challenge and it has been tested relatively easily for almost 30 years in human athletes' urine through its characteristic metabolites, 19‐norandrosterone and 19‐noretiocholanolone. Norsteroids can be formed as side products during the conversion by aromatisation of steroids such as testosterone to estradiol and have been identified in some animal species, including humans, albeit in minute amounts. Following technical improvements in instrumental analytical technology rendering possible the detection of traces of metabolites, a threshold was fixed for 19‐norandrosterone reporting. However, in recent years, the presence of 19‐norsteroid metabolites in levels approaching and even exceeding the threshold were noted in some urine samples; the metabolites were formed by 19‐demethylation of urinary steroids. IRMS provides conclusive evidence of the origin, endogenous or exogenous, of the urinary metabolites, even when found in low quantities.
Abbreviations
19‐NA - 19‐norandrosterone
19‐NE - 19‐noretiocholanolone
19‐NT - 19‐nortestosterone
GC - gas chromatography
IRMS - isotope ratio mass spectrometry
LOD - limit of detection
MS - mass spectrometry
WADA - World Anti‐Doping Agency
Footnotes
Competing interests: none declared
References
- 1.Birch J A. Hydroaromatic steroid hormones 1. 19‐nortestosterone. J Chem Soc 1950367–368.
- 2.Wilds A L, Nelson N A. The facile synthesis of 19‐nortestosterone and 19‐norandrostendione from estrone. J Am Chem Soc 1953755366 [Google Scholar]
- 3.Junkmann K. Long‐acting steroids in reproduction. Recent Prog Horm Res 195713389–419. [PubMed] [Google Scholar]
- 4.Schürmeyer T, Knuth U A, Belkien L.et al Reversible azoospermia induced by the anabolic steroids 19‐nortestoserone. Lancet 198425417–420. [DOI] [PubMed] [Google Scholar]
- 5.Williams J S, Stein J H, Ferris T F. Nandrolone decanoate therapy for patients receiving hemodialysis. Arch Intern Med 1974134289–292. [PubMed] [Google Scholar]
- 6.Deicher R, Horl W H. Hormonal adjuvants for the treatment of renal anaemia. Eur J Clin Invest 20053575–84. [DOI] [PubMed] [Google Scholar]
- 7.Johansen K L, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA 19992811275–1281. [DOI] [PubMed] [Google Scholar]
- 8.Barton Pai A, Chrétien C, Lau A H. The effects of nandrolone decanoate on nutritional parameters in hemodialysis patients. Clin Nephrol 20025838–46. [DOI] [PubMed] [Google Scholar]
- 9.Daiber A, Herve L, Con I.et al Treatment of aplastic anemia with nandrolone decanoate. Blood 197036748–753. [PubMed] [Google Scholar]
- 10.Sattler F R, Jaque S V, Schroeder E T.et al Effects of pharmacological doses of nandrolone decanoate and progressive resistance training in immunodeficient patients infected with human immunodeficiency virus. J Clin Endocrinol Metab 1999841268–1276. [DOI] [PubMed] [Google Scholar]
- 11.Tweedle D, Walton C, Johnston I D. Effect of an anabolic steroid on postoperative nitrogen balance. Br J Clin Pract 197327130–132. [PubMed] [Google Scholar]
- 12.Pentycross C R, Toussis D, McKinna J A.et al Effect of hormone therapy on mitogenic responses of lymphocytes from patients with cancer of the breast. Lancet 19732177–179. [DOI] [PubMed] [Google Scholar]
- 13.Evans J T, Elias E G. Erythropoietic response to anabolic therapy in patients receiving radiotherapy. J Clin Pharmacol New Drugs 197212101–114. [DOI] [PubMed] [Google Scholar]
- 14.Need A G, Horowitz M, Bridges A.et al Effects of nandrolone decanoate and antiresorptive therapy on vertebral density in osteoporotic postmenopausal women. Arch Intern Med 198914957–60. [PubMed] [Google Scholar]
- 15.Geusens P. Nandrolone decanoate: pharmacological properties and therapeutic use in osteoporosis. Clin Rheumatol 19951432–39. [DOI] [PubMed] [Google Scholar]
- 16.Herrington A M, Herrington J D, Church C A. Pharmacologic options for the treatment of cachexia. Nutr Clin Pract 199712101–113. [Google Scholar]
- 17.Haupt H A, Rovere G E. Anabolic steroids: a review of the literature. Am J Sports Med 198412469–484. [DOI] [PubMed] [Google Scholar]
- 18.Haupt H A. Anabolic steroids and growth hormone. Am J Sports Med 199321468–474. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers H. Anabolic steroids: side effects. In: Fahey TD, ed. Encyclopedia of Sports Medicine and Science. Internet Society for Sport Science, 1998. Available at: http://sportsci.org (accessed 7 March 2006)
- 20.Committee on Sports Medicine and Fitness Adolescents and anabolic steroids: a subject review. Pediatrics 199799904–8 (and references cited herein). [PubMed] [Google Scholar]
- 21.Canadian Centre for Ethics in Sport National School Survey on Drugs and Sport. Available at: www.cces.ca/pdfs/CCES‐PAPER‐NationalSchoolSurvey‐E.pdf (accessed 31 March 2006)
- 22.Minto C F, Howe C, Wishart S.et al Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume. J Pharmacol Exp Ther 199728193–102. [PubMed] [Google Scholar]
- 23.World Anti‐Doping Code List of Prohibited Substances 2006. Available at: www.wada‐ama.org/rtecontent/document/2006_LIST.pdf (as accessed 31 March 2006)
- 24.Engel L L, Alexander J, Wheeler M. Urinary metabolites of administered 19‐nortestosterone, J Biol Chem1958231159. [PubMed] [Google Scholar]
- 25.Dimick D F, Heron M, Baulieu E E.et al Comparative study of the metabolic fate of testosterone, 17‐methyltestosterone, 19‐nortestosterone, 17‐methyl‐19‐nortestosterone and 17‐methyl‐estr‐5‐ene‐17‐ol‐3‐one in normal males. Clin Chim Acta 1961663–71. [DOI] [PubMed] [Google Scholar]
- 26.Schanzer W. Metabolism of anabolic androgenic steroids. Clin Chem 1996421001–1020. [PubMed] [Google Scholar]
- 27.Schanzer W, Breidbach A, Geyer H van Kuk C.et al Metabolism of nortestosterone, norandrostenedione and norandrostenediol: Identification of 3α‐hydroxyestr‐4‐en‐17‐one glucuronide and 3α,16α‐dihydroxy‐5α‐estran‐17‐one glucuronide and sulfate. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis, Proceedings of the Manfred Donike Workshop, 18th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 2000155
- 28.Uralets V P, Gillette P A. Over‐the‐counter anabolic steroids 4‐androsten‐3,17‐dione; 4‐androsten‐3,17‐diol and 19‐nor‐4‐androsten‐3,17‐dione: excretion studies in men. J Anal Toxicol 199923357–366. [DOI] [PubMed] [Google Scholar]
- 29.Uralets V P, Gillette P A. Over‐the‐counter delta5 anabolic steroids 5‐androsten‐3,17‐dione; 5‐androsten‐3beta, 17beta‐diol; dehydroepiandrosterone; and 19‐nor‐5‐androsten‐3,17‐dione: excretion studies in men. J Anal Toxicol 200024188–193. [DOI] [PubMed] [Google Scholar]
- 30.Masse R, Laliberte C, Tremblay L.et al Gas chromatographic/mass spectrometric analysis of 19‐nortestosterone urinary metabolites in man. Biomed Mass Spectrom 198512115–121. [DOI] [PubMed] [Google Scholar]
- 31.Kintz P, Cirimele V, Ludes B. Norandrostérone et norétiocholanolone… les métabolites révélateurs. Acta Clin Belg Suppl 1999168–73. [PubMed] [Google Scholar]
- 32.Massé R, Bi H, Ayotte C.et al Studies on anabolic steroids. V. Sequential reduction of methandienone and structurally related steroid A‐ring substituents in humans: gas chromatographic‐mass spectrometric study of the corresponding urinary metabolites, J Chromatogr 1991562323–340. [PubMed] [Google Scholar]
- 33.Ward R J, Shackleton C H L, Lawson A M. Gas chromatographic‐mass spectrometric methods for the detection and identification of anabolic steroid drugs. Br J Sports Med 1975993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björkhem I, Hakan E. Detection and quantitation of 19‐norandrosterone in urine by isotope dilution‐mass spectrometry. J Steroid Biochem 198217447–451. [DOI] [PubMed] [Google Scholar]
- 35.Massé R, Ayotte C, Dugal R. Studies on anabolic steroids I. Integrated methodological approach to the gas chromatographic‐mass spectrometric analysis of anabolic steroid metabolites in urine. J Chromatogr 198948923–50. [PubMed] [Google Scholar]
- 36.Ayotte C, Goudreault D, Charlebois A. Testing for natural and synthetic anabolic agents in human urine. J Chromatogr 19966873–25. [DOI] [PubMed] [Google Scholar]
- 37.Baume N, Avois L, Sottas P E.et al Effects of high‐intensity exercises on 13C‐nandrolone excretion in trained athletes. Clin J Sport Med 200515158–166. [DOI] [PubMed] [Google Scholar]
- 38.Aguilera R, Becchi M, Casabianca H.et al Improved method of detection of testosterone abuse by gas chromatography/combustion/isotope ratio mass spectrometry analysis of urinary steroids. J Mass Spectrom 199631169–176. [DOI] [PubMed] [Google Scholar]
- 39.Becchi M, Aguilera R, Farizon Y.et al Gas chromatography/combustion/isotope‐ratio mass spectrometry analysis of urinary steroids to detect misuse of testosterone in sport. Rapid Commun Mass Spectrom 19948304–308. [DOI] [PubMed] [Google Scholar]
- 40.Horning S, Geyer H, Machnik M.et al Detection of exogenous testosterone by 13C/12C analysis. In: Schänzer W, Geyer H, Gotzmann A, et al, eds. Recent Advances in Doping Analysis. Koln: Verlag Sport und Buch Strauβ, 1997275
- 41.Mathurin J C, Herrou V, Bourgogne E.et al Gas chromatography‐combustion—isotope ratio mass spectrometry analysis of 19‐norsteroids: application to the detection of a nandrolone metabolite in urine. J Chromatogr B Biomed Sci Appl 2001759267–275. [DOI] [PubMed] [Google Scholar]
- 42.IOC Medical Commission Statistics of IOC Accredited Laboratories. Communication to the IOC accredited laboratories for doping control analyses. International Olympic Committee, Lausanne, Switzerland (1988 to 2002)
- 43.World Anti‐Doping Agency 2004 Adverse analytical findings reported by accredited laboratories. www.wada‐ama.org/rtecontent/document/LABSTATS_2004.pdf (accessed 9 May 2006)
- 44.World Anti‐Doping Agency International Standard for Laboratories (version 4, August 2003) and Technical Documents TD2003IDCR (Identification criteria for qualitative assays incorporating chromatography and mass spectrometry) and TD2004NA (Reporting norandrosterone findings). Montreal: World Anti‐Doping Agency, 2003, Available at: www.wada‐ama.org/en/(accessed 31 March 2006)
- 45.Pr Alexandre de Mérode, Président of the Medical Commission of the IOC Analytical criteria for reporting low concentrations of anabolic steroids. Letter to IOC Accredited Laboratories, 7 August 1998
- 46.World Anti‐Doping Agency International Standard for Laboratories. Technical Document TD2003MRPL. Montreal: World Anti‐Doping Agency, 2004
- 47.Mareck‐Engelke U, Schultze G, Geyer H.et al 19‐norandrosterone in pregnant women. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis (8), Proceedings of the 18th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 2000145–154.
- 48.Dehennin L, Silberzahn P, Reiffsteck A.et al Présence de 19‐norandrostenedione et de 19‐nortestosterone dans les fluides folliculaires humain et équin. Pathol Biol 198432828 [Google Scholar]
- 49.Reznik Y, Herrou M, Dehennin L.et al Rising plasma levels of 19‐nortestosterone throughout pregnancy: determination by radioimmunoassay and validation by GC/MS. J Clin Endocrinol Met 1987641086. [DOI] [PubMed] [Google Scholar]
- 50.Lund H S, Jathun S, Fedorcsak P.et al Synthesis of nandrolone in the human ovary. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis, Proceedings of the Manfred Donike Workshop, 18th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 200023
- 51.Dehennin L, Bonnaire Y, Plou P. Urinary excretion of 19‐norandrosterone of endogenous origin in man: quantitative analysis by gc/ms. J Chromatogr B Biomed Appl 1999721301–307. [DOI] [PubMed] [Google Scholar]
- 52.Le Bizec B, Monteau F, Gaudin I.et al Evidence for the presence of endogenous 19‐norandrosterone in human urine. J Chromatogr B Biomed Sci Appl 1999723157–172. [DOI] [PubMed] [Google Scholar]
- 53.Jeanneau T, Kintz P, Cirimele V.et al Determination of the physiological concentrations of nandrolone metabolites in human urine by GC/MS. Toxicorama 1999XI25 [Google Scholar]
- 54.Reznik Y, Dehennin L, Coffin C.et al Urinary nandrolone metabolites of endogenous origin in man: a confirmation by output regulation under human chorionic gonadotropin stimulation. J Clin Endocrinol Metab 200186146–150. [DOI] [PubMed] [Google Scholar]
- 55.Ciardi M, Ciccoli R, Barbarulo M V.et al Presence of norandrosterone in “normal” urine samples, In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis (6), Proceedings of the 16th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 199897–104.
- 56.Van Eenoo P, Delbeke F T, de Jong F H.et al Endogenous origin of norandrosterone in female urine: indirect evidence for the production of 19‐norsteroids as by‐products in the conversions from androgen to estrogens, J Steroid Biochem Mol Bio 200178351–357. [DOI] [PubMed] [Google Scholar]
- 57.Hemmersbach P, Hagensen A H, Misund J. Determination of urinary norandrosterone excretion in females during one menstrual cycle by GC/MS. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis, Proceedings of the Manfred Donike Workshop, 18th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 2000141
- 58.Hemmersbach P, Hagensen J A H, Lund H S. Determination of urinary norandrosterone excretion in females during one menstrual cycle by gas chromatography/mass spectrometry. Biomed Chromatogr 2006 (in press) [published online, DOI: 10.1002/bmc.586] [DOI] [PubMed]
- 59.Ayotte C. Nutritional supplements and doping controls. IAAF New Studies in Athletics. 1999;14: 37, Available at: www2. iaaf. org/TheSport/Science/Nsa.html (accessed 9 May 2006)
- 60.Geyer H, Mareck‐Engelke U, Reinhart U.et al Positive doping cases with norandrosterone after application of contaminated nutritional supplements. Dtsch Z Sportmed 200051378–382. [Google Scholar]
- 61.Geyer H, Mareck‐Engelke U, Reinhart U.et al The analysis of “non‐hormonal” nutritional supplements for prohormones. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis, 18th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 2001, 63. Report available at: http://multimedia.olympic.org/pdf/en_report_324.pdf (accessed on 31 March 2006)
- 62.Ayotte C, Levesque J F, Cleroux M.et al Sport nutritional supplements: Quality and doping controls. Can J Appl Physiol 200126(suppl)S120–S129. [DOI] [PubMed] [Google Scholar]
- 63.Kamber M, Baume N, Saugy M.et al Nutritional supplements as a source for positive doping cases? J Int Sport Nutr Exerc Metab 200011258. [DOI] [PubMed] [Google Scholar]
- 64.Catlin D H, Leder B Z, Ahrens B.et al Trace contamination of over‐the‐counter androstenedione and positive urine test results for a nandrolone metabolite. JAMA 20002842618–2621. [DOI] [PubMed] [Google Scholar]
- 65.Maughan R J. Contamination of dietary supplements and positive drug tests in sport. J Sports Sci 200523883–889. [DOI] [PubMed] [Google Scholar]
- 66.van der Merwe P J, Grobbelaar E. Unintentional doping through the use of contaminated nutritional supplements. J Afr Med J 200595510–511. [PubMed] [Google Scholar]
- 67.See for example Aanes vs. FILA (CAS 2001/A/317). Arbitral Award, Court of Arbitration for Sport, Lausanne, Switzerland; USADA vs. Moninger (AAA No. 30 190 001 100 03). Arbitral Award. American Association of Arbitrators, NY, USA
- 68.Debruyckere G, de Sagher R, Van Peteghem C. Clostebol‐positive urine after consumption of contaminated meat. Clin Chem 1992381869–1873. [PubMed] [Google Scholar]
- 69.Debruyckere G, de Sagher R, Van Peteghem C. Influence of the consumption of meat contaminated with anabolic steroids on doping tests. Anal Chim Acta 199327549–56. [Google Scholar]
- 70.Le Bizec B, Gaudin I, Monteau F.et al Consequence of boar edible tissue consumption on urinary profiles of nandrolone metabolites. I. Mass spectrometric detection and quantification of 19‐norandrosterone and 19‐noretiocholanolone in human urine. Rapid Commun Mass Spectrom 2000141058–1065. [DOI] [PubMed] [Google Scholar]
- 71.Ayotte C, Guay C, Cléroux M.et al Origin of elevated levels of norandrosterone in human urine: Half‐truths vs. facts. In: Schanzer W, Geyer H, Gotzman A, et al, eds. Recent Advances in Doping Analysis, 20th Cologne Workshop on Dope Analysis. Cologne: Sport & Buch Strauβ, 200213
- 72.Robinson N, Taroni F, Saugy M.et al Detection of nandrolone metabolites in urine after a football game in professional and amateur players: a Bayesian comparison, Forensic Sci Int 2001122130–135. [DOI] [PubMed] [Google Scholar]
- 73.Le Bizec B, Bryand F, Gaudin I.et al Endogenous nandrolone metabolites in human urine. Two‐year monitoring of male professional soccer players, J Anal Toxicol 20022643–47. [DOI] [PubMed] [Google Scholar]
- 74.Gambelunghe C, Sommavilla M, Rossi R. Testing for nandrolone metabolites in urine samples of professional athletes and sedentary subjects by GC/MS/MS analysis. Biomed Chromatogr 200216508–512. [DOI] [PubMed] [Google Scholar]
- 75.Schmitt N, Flament M M, Goubault C.et al Nandrolone excretion is not increased by exhaustive exercise in trained athletes. Med Sci Sports Exerc 2002341436–1439. [DOI] [PubMed] [Google Scholar]
- 76.de Geus B, Delbeke F, Meeusen R.et al Norandrosterone and noretiocholanolone concentration before and after submaximal standardized exercise. Int J Sports Med 200425528–532. [DOI] [PubMed] [Google Scholar]
- 77.Grosse J, Anielski P, Hemmersbach P.et al Formation of 19‐norsteroids by in situ demethylation of endogenous steroids in stored urine samples. Steroids 200570499–506. [DOI] [PubMed] [Google Scholar]
- 78.World Anti‐Doping Agency Stability of 19‐norandrosterone Findings in Urine. Technical note. Montreal: World Anti‐Doping Agency, 2005