Abstract
Background and objectives
Recombinant human growth hormone (rhGH) has been on the list of forbidden substances since availability of its recombinant form improved in the early 1990s. Although its effectiveness in enhancing physical performance is still unproved, the compound is likely used for its potential anabolic effect on the muscle growth, and also in combination with other products (androgens, erythropoietin, etc.). The degree of similarity between the endogenous and the recombinant forms, the pulsatile secretion and marked interindividual variability makes detection of doping difficult. Two approaches proposed to overcome this problem are: the indirect method, which measures a combination of several factors in the biological cascade affected by administration of GH; and the direct method, which measures the difference between the circulating and the recombinant (represented by the unique 22 kD molecule) forms of GH. This article gives an overview of what is presently known about hGH in relation to sport. The available methods of detection are also evaluated.
Methods
Review of the literature on GH in relation to exercise, and its adverse effects and methods of detection when used for doping.
Results and conclusion
The main effects of exercise on hGH production and the use and effects of rhGH in athletes are discussed. Difficulties encountered by laboratories to prove misuse of this substance by both indirect and direct analyses are emphasised. The direct method currently seems to have the best reliability, even though the time window of detection is too short. hGH doping is a major challenge in the fight against doping. The effect of exercise on hGH and its short half‐life are still presenting difficulties during doping analysis. To date the most promising method appears to be the direct approach utilising immunoassays.
Keywords: hGH, doping, sport, athlete, abuse
The human growth hormone (hGH) is a naturally occurring peptide hormone secreted by the pituitary gland.1 Although the hormone in the body is rather heterogeneous, the major component is made up of 191 amino acids, stabilised by two disulphide bonds and reaching a molecular weight of 22 kDa.2,3 Previously, the only source of hGH was human cadavers, but the contamination that led to Creutzfeldt–Jakob disease made this form of treatment obsolete. In the late 1980s, recombinant hGH (rhGH) was developed through genetic engineering and has been used with good results in the treatment of patients with hGH deficiency—allowing bone growth and impacting on the patient's final stature. This form of hGH has a sequence identical to the naturally occurring 22 kDa hormone. Its misuse has been suspected in sport because of its anabolic properties. Athletes and bodybuilders claim that hGH increases lean body mass and decreases the fat mass.
The use of hGH in sport today is not only based on its anabolic properties, but also on its effect on carbohydrate and fat metabolism. rhGH has been found in swimmers and also in players taking part in major sports events. International federations and the International Olympic Committee have had hGH on the list of forbidden compounds since 1989, when it became obvious that the development of biotechnology products based on the recombination of DNA made hGH much more easily available on the regular and black markets.
In the 2006 Prohibited List, hGH in listed under class S2 of hormones and related substances. Erythropoietin (EPO) and corticotrophin as well as insulin‐like growth factor (IGF)‐1 and insulin also belong to the same category of peptide hormones. During the 2004 Olympics in Athens, for the first time, the so‐called direct method of Wu et al4 was used. None of the positive serum samples were finally declared positive; this was because of the too‐short time window of detection of the test and the short half‐life of circulating GH (about 20 minutes).5 Studies have shown that GH concentrations returned to baseline 8–16 hours after intramuscular injection and 11–20 hours after subcutaneous injection.6
Growth hormone and exercise
The effect of acute exercise on production of GH in the body has been widely described in the literature.7 The concentration of hGH in blood increases with time for a given work intensity and can increase 10‐fold during prolonged moderate exercise. During more intensive exercise (with accumulation of lactate at 70% Vo2 max for a short term period such as 10–20 minutes) hGH will increase by 5–10‐fold.8 With short exercise durations, levels of GH will generally peak at 15–30 minutes after the exercise. Furthermore, it appears that hGH response is more closely related to the peak intensity of exercise than the total work output.9 Endurance training generally amplifies the pulsatile release of growth hormone, elevating the GH amplitude. This appears evident when the training is very hard and above the aerobic threshold.10
Apart from exercise related increase, hGH secretion can be affected by other factors—for example, GH secretion is increased in hypoglycaemia, increased temperature, and stress, whereas it decreases in obesity, or with a carbohydrate‐rich diet and intake of β2 adrenergic agonists. Thus, it is hard to differentiate between the physiological increase in hGH levels seen in exercise and what can be from external hGH administration (as in doping). This problem makes the purely quantitative approach of measuring directly the total circulating GH not feasible in case of doping, except if the conditions of collection of biological samples are well controlled.
Effect of hGH in the body
Somatotrope cells in the anterior pituitary secrete hGH in a pulsatile fashion. The secretion is regulated by two hypothalamic peptides, growth hormone releasing hormone, which stimulates hGH secretion, and somatostatin, which inhibits hGH secretion by back regulation. hGH exerts its biological effects on target cells by binding to specific receptors present throughout the whole body.
Secretion of hGH is slightly higher in women than in men,11 with the highest levels observed at puberty. Secretion decreases with age by around 14% per decade.12 Moreover, secretion varies with normal physiological and pathological conditions. hGH levels are higher during slow wave sleep and are increased by exercise, stress, fever, fasting and, with some amino acids (leucine and arginine). Some drugs, such as clonidine, l‐dopa and γ‐hydroxybutyrate, increase its secretion, as do androgens and estrogens.
hGH exerts its effects through target cells by binding to specific membrane receptors found in abundance throughout the body.13 It has both direct and indirect effects on the tissues; the indirect effects are mediated by IGF‐1, which is generated in the liver in response to GH.14
Therapeutic use of hGH
Human GH is prescribed for both childhood and adulthood hGH deficiency and for girls with Turner's syndrome. High doses of hGH are used for relief from excessive burns or other thermal injuries.15 Nevertheless, Takala et al16 showed that supraphysiological doses of GH administered to critically ill patients increased mortality when compared with placebo. Since the late 1950s, children with GH deficiency have been treated with hGH extracted from cadaver pituitary glands. Recently, due to the better availability of rhGH, hGH deficiency in adults has been recognised as a clinical syndrome and studied in clinical trials. In 1989, two major contributions were published describing the beneficial effects of GH treatment in GH‐deficient adults, related on their body composition and metabolism.17,18 These seminal studies showed that rhGH treatment for a period of four to six months had favourable effects on body composition, exercise aptitude, renal and cardiac function, and in general, led to improvement in the quality of life. Long term GH administration studies have shown an increase in bone mass and persistence of the positive effects of hGH therapy.
The positive effects on the body composition are essentially due the anabolic, lipolytic, and antinatriuretic properties of GH. Among the effects that have been observed are: increase in the body cell mass (muscles) and total body water (extracellular); and decrease in the body fat with its redistribution from central to peripheral depots. The hGH dose in adults is generally individualised, but the typical dose is 1–2 IU/day administered subcutaneously every evening. With therapeutic doses, no adverse side effects have been observed.19,20,21
hGH as a doping agent
GH has been considered as an ergogenic drug since the late 1980s. Since that time, official and non‐official sources have reported that misuse in sport has steadily increased. The attractiveness of the product is based on popular knowledge that it is efficient, hard to detect, and without major side effects if well dosed. GH misusers primarily try to benefit from the known anabolic action of the drug, to increase their muscle mass and power.
The frequency of use and the dosage are hard to evaluate, but underground information suggests that the athletes misusing hGH take 10–25 IU/days three to four times a week to increase their lean body mass. We think that the mean dose is about 4 IU/day in combination with other doping agents, such as anabolic steroids in power sports or EPO in endurance sport. GH is often taken in cycles of four to six weeks, as is the case for anabolic steroids in bodybuilding. In endurance sport, little is known about the optimum utilisation of hGH doping in combination with other products. It is highly individual and empirical.
The effectiveness of rhGH in the improvement of sport performance is still under debate among users. The positive effects described in hGH deficient adults are not that clear among athletes. Although many of these underground reports indicate some positive effect on muscle mass, it is difficult to differentiate benefits obtained when hGH is taken in combination with anabolic steroids or even if the hGH used was a less effective product. The use of hGH as an anabolic agent still seems to be widespread, but it is difficult to investigate the extent of the phenomenon. It has been reported that 5% of male American high‐school students used or have used hGH as an anabolic agent.22 It is unknown how popular hGH is among female athletes, but some use has been reported because of the low risk of androgenic side effects that are seen with anabolic steroids. Not only is the anabolic effect of hGH favored by high power output athletes, but its use is also gaining acceptance in endurance sport in combination with methods for enhancing oxygen transport. Although there are anecdotal reports on the so‐called dramatic increases in muscle mass and strength after large doses of hGH (especially among bodybuilders) their effectiveness under controlled conditions is generally less impressive.
As the results of controlled studies are generally not in agreement with subjective underground reports by misusers, it is difficult to draw any definite conclusions regarding the effects of excessive hGH administration on skeletal muscle function. It must be stressed that the regimen of hGH use in sport is designed to fulfil purposes other than just an increase in athletes' muscle mass. The doses involved are certainly specific to a discipline, its training model, and tailored to the regimen of other ergogenic substances being used concurrently.
GH misuse is still expensive and the high costs and difficulty in finding the “right” clean drug have certainly pushed some athletes to use products claimed to enhance GH production. Among these are the amino supplements such as arginine, ornithine, lysine, and tryptophan, but there are no clearly established results. The effectiveness of rhGH is also widely discussed among its users in the underground literature or in internet chat rooms without a clear positive position. Several aspects can be debated, but because of its price, some proportionality in the effects is to be expected by the users. Certainly disappointments are due to bad dosing, not combining with anabolic steroids, or a too‐short duration of use.
There are few controlled studies on the effectiveness of GH on the performance of top level athletes. In general these studies have been performed with supraphysiological dosages but not with the large amounts claimed to be effective, for instance, by bodybuilders. The results of most of these controlled studies are generally less impressive than the claims of those who misuse the substance. A study of volunteers under heavy resistance training found decrease of free fatty mass but no difference in the muscle strength.23 With weight lifters, it has been shown that short term GH treatment does not increase muscle protein synthesis more than placebo24 or other factors such as maximal voluntary strength (biceps or quadriceps).25
These results conflict with reality, which is that rhGH misuse seems to exist in top‐level sport, because the compound is often found in police raids related to doping affairs. We believe that most of the time misusers will take rhGH as a part of their cocktail of specific preparations, rather than considering rhGH as a unique pharmaceutical preparation. The effects of GH on the metabolism are so widespread that one can be certain that this is taken in combination with other products. And the final effect generally occurs elsewhere, rather than in what is tested in the laboratories.
Adverse effects of hGH
The long term risks of hGH use are not well known since epidemiological data regarding this type of treatment in healthy sportsmen are unavailable. Acromegaly, which results from a pathological increase in endogenous production of GH, is often cited as one of the major risks associated with excessive use of hGH. The major symptoms are swelling of the hands and feet, coarsened facial appearance, dentition problems, arthralgias, fluid retention, and excessive sweating. Acromegalic patients have an increased risk for diabetes mellitus and hypertension that can lead to premature mortality from cardiovascular diseases.26 It can be argued that long term hGH doping with high dosages will probably result in misusers experiencing symptoms of fluid retention and increased risk of development of diabetes mellitus and hypertension. There is also a risk of cardiomyopathy, osteoporosis, menstrual irregularities, and impotence. Some of these side effects are reversible after withdrawal of the drug. Furthermore, hGH misuse can disturb the lipid profile with decreased high density lipoprotein (HDL)‐cholesterol.
As hGH is administered by injection, if syringes are non‐sterile or contaminated, there is a risk of cross‐infection, such as HIV/AIDS and hepatitis. Even though cadaveric GH is now rare in the black market, its use is associated with a high risk of developing Creutzfeldt–Jakob disease, which is characterised by slowly progressive dementia.
Detection of hGH doping
Until the 2004 Olympic Games in Athens, hGH doping was considered undetectable. Growth hormone is a peptide with a very short half‐life in blood and low concentration in urine. The peptidic nature of the substance forced analysts to investigate other methods than those used in the classic analyses for anabolic steroids or stimulants with relatively low molecular weights. The amino acid sequence of the recombinant molecule is identical to the major 22 kDa isoform secreted by the pituitary gland. There is no way of using a post‐transcription modification of the molecule to find out the difference between the recombinant and the natural forms.
Secretion of hGH by the pituitary gland is pulsatile, leading to highly fluctuating levels in the circulation. Moreover, hGH is considered to be a stress hormone regulated by factors such as sleep, nutritional status, exercise, and emotion. Thus, there is high intraindividual and interindividual variability in the secretion of hGH. Quantifying the hormone itself is not sufficient to detect exogenous rhGH. More stable serum variables implied in the biological cascade produced by hGH secretion, or a doping application, may be the route of successful detection of hGH. The growth factor IGF‐1 and some of its transport proteins (IGFBP‐3), have been proposed as possible candidates for indirect detection of hGH doping. But the interindividual variability is quite high and makes it hard to precisely define a quantitative cut‐off level.
What is already known on this topic
hGH doping is a major challenge in sport. This hormone is used by some athletes in combination with either anabolic steroids to increase their muscle mass or EPO to increase their aerobic power. Detection of rhGH is still controversial, but it appears that the direct method based on the ratio of several circulating forms is the most promising one.
The urine strategy
Most anti‐doping samples consist of urine collected out of competition or after effort. Because of its convenient availability and relatively unlimited volume, attempts have been made to use urine for peptide detection. For example, urine has been used for successful detection of EPO because of the glycosylated form of this hormone. However, the only way to detect hGH in urine is to use an extremely sensitive immuno‐test to quantify the total amount of the hormone in urine. The average urine concentration of hGH is between 100 and 1000 times less than in blood. One notion has been to develop a screening test for out‐of‐competition testing in order to benefit from a relatively longer time window of detection.27 The limitations of this test have been clearly shown, because of the large influence of the process of renal excretion on the concentration measured in urine. The lack of discrimination and specificity of the result made the urinary test less promising than a blood test. Nevertheless, today, improvements in the organisation of target testing are quite obvious. It is considered feasible to do a urine test for GH in the morning, with an unannounced urine test outside any exercise session for other hormonal analyses. This may eventually be a solution for effective screening.
The blood strategies
Two main strategies are currently being followed to detect hGH doping using blood: the indirect and direct approaches.
The indirect approach
Increasing knowledge about the naturally occurring variability of several hGH dependent factors (that is, IGF‐1, the different IGF binding proteins (IGFBPs), or several markers of the bone turnover), individually or in combination, could provide a database of normal ranges for the concentration of these factors. This may lead to establishment of cut‐off levels and description of so‐called abnormal values outside the normal constellation of measures.
This approach, proposed in the mid‐1990s, was investigated by an international panel of endocrinologists, but did not lead to a final solution for detection of hGH doping.28,29,30 The advantage of an indirect approach to target GH use is certainly that these biological factors are less variable or less sensitive than GH itself and should have a longer half‐life in the body. A main objective of the study was to investigate the variation of these secondary variables during or after exercise. IGF‐1 and IGFBP‐3 in the hGH biological cascade as well as selected peptides involved in bone metabolism (for example the N‐terminal peptide from the pro‐collagen named PIIIP) or osteocalcin were considered as good biological markers of GH doping. These variables showed slight but significant changes after acute exercise. Moreover, the interindividual variability in the reaction to GH administration makes the use of indirect measurements almost impossible in a forensic description of GH misuse. It is obvious that rather than depending on the observation of a single value, a solution may be found in an algorithm combining all the biological variables from the cascade. Nevertheless, all these investigations clearly show that the indirect approach can certainly be used for screening and targeting purposes when a biological follow up of athletes will be acceptable in the sport community. But it cannot stand in front of a court as an absolute proof of doping. The regular evaluation of individual normal ranges in sportspeople could in fact lead to, as is currently done with haematological substances, better screening and targeting of the athletes and direct detection of hGH misuse as proposed in the following section.
What this study adds
The direct method of detection, based on double immunological tests needs to be well evaluated and validated. This review has described the difficulty sports authorities will face to prove hGH doping. At present, the short time window of detection of any method and the effect of exercise on natural hGH secretion still make any approach quite risky.
The direct approach
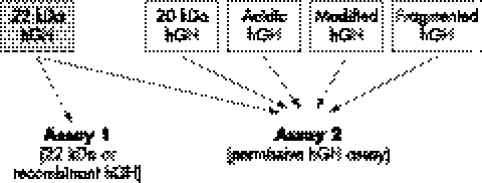
The Strasburger–Bidlingmaier group in Munich developed a so‐called direct method for the detection of hGH doping.4,6 Two specific immunoassays have been developed to quantify several types of hGH isoform. Recombinant hGH is exclusively represented by the native 22 kDa form whereas the circulating hGH in the human blood is present in several forms (table 1). When the recombinant form is injected in the body, this increases, for a period of time, the proportion of the 22 kDa form in comparison with all other circulating forms. Moreover, with long term use, classic back regulation of the endogenous secretion of natural hGH occurs, which favours the proportion of the major 22 kDa sequence.
Table 1 Relative abundance of hGH molecular forms in circulation (adapted from Bidlingmaier et al6). The percentages are approximate.
| Isoforms | Per cent |
|---|---|
| 22 kDa monomer | 48 |
| 20 kDa monomer | 9 |
| Modified hGH (dimers and oligomers) | 30 |
| Acidic hGH (desaminated and acylated forms) | 7 |
| Fragmented hGH (17, 12, 5, 30 kDa) | Variable |
The proposed test was used during the Olympics in Athens (2004) and in Torino (2006). To fulfil the requirements of the World Anti‐Doping Agency (WADA) code and the standards for laboratories, two double tests were applied to serum samples: the first test quantified specifically the 22 kDa form and the second test was a comprehensive assay measuring all forms present in the serum (see fig 1). The ratio was established and a cut‐off defined to differentiate normal subjects (negative samples) from those having a significant higher proportion of 22 kDa hGH (positive samples). A second double sample test was used for confirmation purposes. The time window of detection for these tests is claimed to be between 24 hours and 36 hours after the last injection, depending on the dosage used. It is thought that hGH doping, to be efficient, needs multiple injections. Environmental influences, such as exercise, have been evaluated by Wallace et al.31 These authors measured total, pituitary, 22 kDa, recombinant, non‐22 kDa, 20 kDa, and immunofunctional GH. They concluded that all isoforms increased during exercise, peaked at the end, and declined after exercise. At peak exercise, 22 kDa GH was the predominant isoform. After exercise, the ratio of non‐22 kDa/total GH increased and that of recombinant/pituitary GH decreased. But it is considered that these changes will not invalidate the test after competition. Moreover, even if GH was used out of competition, this test should act a deterrent for its use. Since the test was introduced in 2004, no adverse analytical findings have been declared from any of the WADA laboratories that have validated the tests.
Figure 1 Molecular basis of direct detection of hGH abuse by differential immunoassay. A ratio is then calculated between the signal given by assay 1 to the signal given by assay 2. Two of these double tests must be applied in case of positive serum sample to confirm the result (adapted from Bidlingmaier et al6).
Conclusion
With new biotechnology products on the market, such as rGH and its precursors, the fight against doping must evolve with new analytical techniques and strategies based on different biological matrices. These new methods will need to be fully validated forensically before being submitted to any court challenge.
Abbreviations
GH - growth hormone
IGF - insulin‐like growth factor
rhGH - recombinant human GH
Footnotes
Competing interests: none declared
References
- 1.Heally M L, Russel‐Jones D. Growth hormone and sport: abuse, potential benefits and difficulties in detection. Br J Sports Med 199731267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C H, Dixon J S. Human pituitary growth hormone. The primary structure ot the hormone: revision, Arch Biochem Biophys 1971233–236. [DOI] [PubMed]
- 3.Niall H D. Revised primary structure for human growth hormone. Nat New Biol 197123090–91. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Bidlingmaier M, Dall R.et al Detection of doping with human growth hormone. Lancet 1999353895. [DOI] [PubMed] [Google Scholar]
- 5.Holl R W, Schwarz U, Schauwecker P.et al Diurnal variation in the elimination rate of human growth hormone (GH): the half‐life of serum GH is prolonged in the evening, and affected by the source of the hormone, as well as by body size and serum estradiol. J Clin Endocrinol Metab 199377216–220. [DOI] [PubMed] [Google Scholar]
- 6.Bidlingmaier M, Strasburger J W C. Test method: GH. Baillère Clin Endocr Metab 20001499–109. [DOI] [PubMed] [Google Scholar]
- 7.Sutton J, Lazarus L. Growth hormone in exercise. Comparison of physiological and pharmacological stimuli. J Appl Physiol 197641523–527. [DOI] [PubMed] [Google Scholar]
- 8.Felsing N E, Brasel J A, Cooper D M. Effect of low and high intensity on circulating growth hormone in men. J Clin Endocrinol Metab 199275157–162. [DOI] [PubMed] [Google Scholar]
- 9.Vanhelder W P, Goode R C, Radomski M W. Effect of anaerobic and aerobic exercise on circulating growth hormone in men. Eur J Appl Phys 198452257–257. [DOI] [PubMed] [Google Scholar]
- 10.Weltman A, Weltman J Y, Schurrer R.et al Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. J Appl Phys 1992722188–2196. [DOI] [PubMed] [Google Scholar]
- 11.Stolar M W, Baumann G. Secretory patterns of growth hormone during basal period in man. Metabolism 198635883–888. [DOI] [PubMed] [Google Scholar]
- 12.Iranmanesh A, Lizzaralde G, Veldhuis J D. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half‐life of endogenous GH in healthy men. J Clin Endocrinol Metab 1991731081–1088. [DOI] [PubMed] [Google Scholar]
- 13.Kelly P A, Djiane J, Postel‐Vinay M C.et al The prolactin/growth hormone receptor family. Endocr Rev 199112235–251. [DOI] [PubMed] [Google Scholar]
- 14.Daughaday W H, Rotwein P. Insulin‐like growth factor I and II. Peptide messenger ribonucleic acid and gene structure, serum and tissue concentration. Endocr Rev 19891068–91. [DOI] [PubMed] [Google Scholar]
- 15.Wilmore D W, Moylan J A, Jr, Bristow B F.et al Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet 1974138875–878. [PubMed] [Google Scholar]
- 16.Takala J, Ruokonen E, Webster N R.et al Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 1999341785–792. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen J O, Pedersen S A, Laurberg P.et al Effects of growth hormone therapy on thyroid function of growth hormone‐deficient adults with and without concomitant thyroxine‐substituted central hypothyroidism. J Clin Endocrinol Metab 1989691127–1132. [DOI] [PubMed] [Google Scholar]
- 18.Salomon F, Cuneo R C, Hesp R.et al The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 19893211797–1803. [DOI] [PubMed] [Google Scholar]
- 19.Johannsson G, Rosen T, Bosaeus I.et al Two years of growth hormone (GH) treatment increases bone mineral content and density in hypopituitary patients with adult‐onset GH deficiency. J Clin Endocrinol Metab 1996812865–2873. [DOI] [PubMed] [Google Scholar]
- 20.Rosen T, Johannsson G, Bengtsson B A. Consequences of growth hormone deficiency in adults, and effects of growth hormone replacement therapy. Acta Paed Supplement 199439921–4 discussion 25. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson B A, Eden S, Lonn L.et al Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 199376309–317. [DOI] [PubMed] [Google Scholar]
- 22.Rickert V I, Pawlak‐Morello C, Sheppard V.et al Human growth hormone: a new substance of abuse among adolescents? Clin Ped 199231723–726. [DOI] [PubMed] [Google Scholar]
- 23.Yarasheski K E, Campbell J A, Smith K.et al Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Phys 1992262(3 Pt 1)E261–E267. [DOI] [PubMed] [Google Scholar]
- 24.Yarasheski K E, Zachweija J J, Angelopoulos T J.et al Short‐term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Phys 1993743073–3076. [DOI] [PubMed] [Google Scholar]
- 25.Deyssig R, Frisch H, Blum W F.et al Effect of growth hormone treatment on hormonal parameters, body composition and strength in athletes. Acta Endocrinol 1993128313–318. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson B A, Eden S, Ernest I.et al Epidemiology and long‐term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand 1988223327–335. [DOI] [PubMed] [Google Scholar]
- 27.Saugy M, Cardis C, Schweizer C.et al Detection of growth hormone doping in urine. J Chrom B Biomed Appl 1996687201–211. [DOI] [PubMed] [Google Scholar]
- 28.Longobardi S, Keay N, Ehrnborg C.et al Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo‐controlled study. The GH‐2000 Study Group. J Clin Endocrinol Metab 2000851505–1512. [DOI] [PubMed] [Google Scholar]
- 29.Wallace J D, Cuneo R C, Lundberg P A.et al Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adult males. J Clin Endocrinol Metab 200085124–133. [DOI] [PubMed] [Google Scholar]
- 30.Wallace J D, Cuneo R C, Baxter R.et al Responses of the growth hormone (GH) and insulin‐like growth factor axis to exercise, GH administration, and GH withdrawal in trained adult males: a potential test for GH abuse in sport. J Clin Endocrinol Metab 1999843591–3601. [DOI] [PubMed] [Google Scholar]
- 31.Wallace J D, Cuneo R C, Bidlingmaier M.et al The response of molecular isoforms of growth hormone to acute exercise in trained adult males. J Clin Endocrinol Metab 200186200–206. [DOI] [PubMed] [Google Scholar]