Abstract
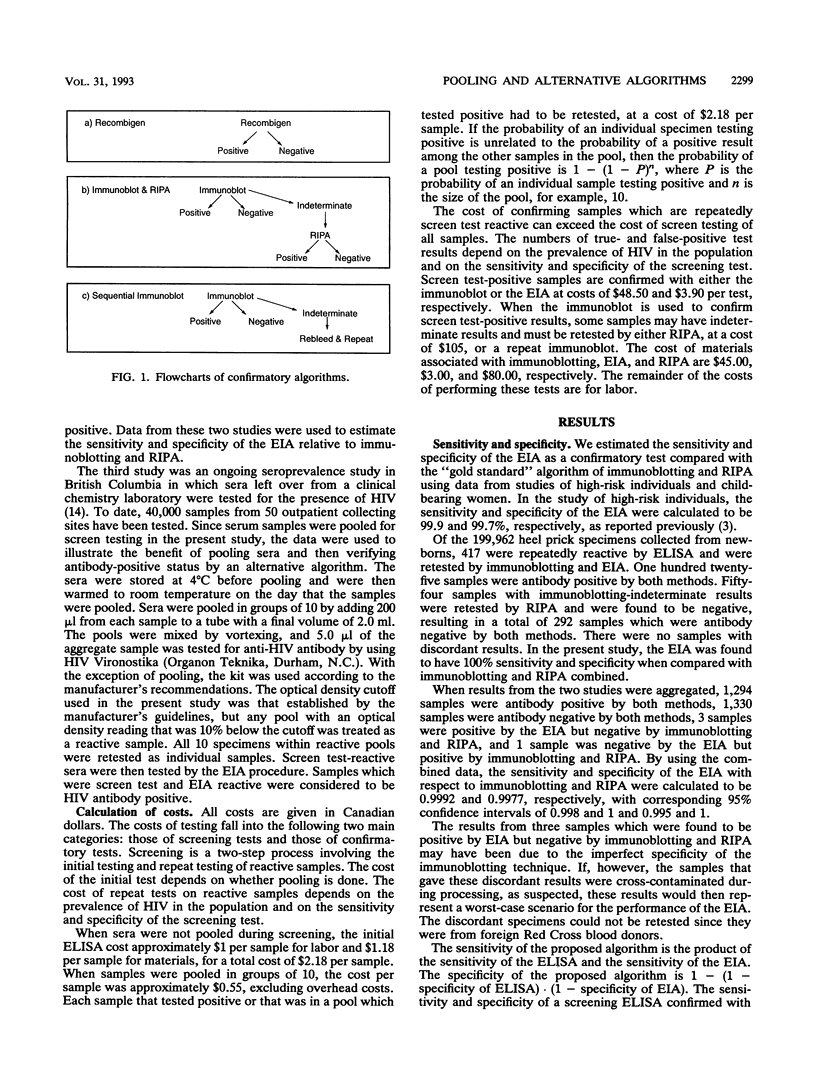
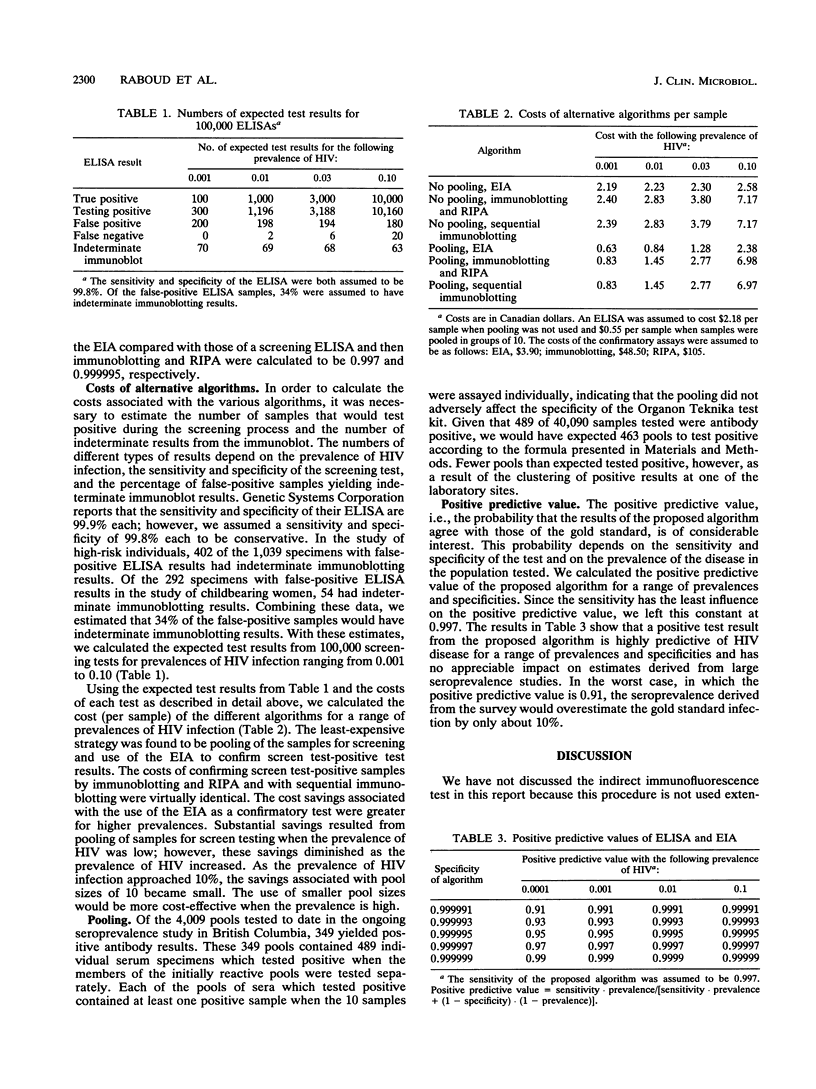
Data from two seroprevalence studies and one comparative study of confirmatory algorithms were used to compare the costs and sensitivities of six algorithms for determining seropositivity to human immunodeficiency virus (HIV). We evaluated confirmatory strategies by using the CBC Recombigen HIV enzyme immunoassay (EIA; Cambridge BioScience, Worcester, Mass.) and immunoblotting followed by radioimmunoprecipitation assay to confirm indeterminate immunoblotting results with and without pooling of samples during screening. The least expensive algorithm was that in which sera were pooled during screening and EIA was used to confirm positive test results. The cost savings associated with this confirmatory test were greater when the prevalence of HIV infection was higher. Savings from pooling of sera for screen testing diminished as HIV prevalence increased. The sensitivity and specificity of EIA with respect to immunoblotting and radioimmunoprecipitation assay were estimated to be 0.9992 and 0.9977, respectively. We found that the implementation of pooling during screening and the use of EIA as the confirmatory test do not affect the statistical reliability of estimates of seropositivity but do result in considerable cost savings.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behets F., Bertozzi S., Kasali M., Kashamuka M., Atikala L., Brown C., Ryder R. W., Quinn T. C. Successful use of pooled sera to determine HIV-1 seroprevalence in Zaire with development of cost-efficiency models. AIDS. 1990 Aug;4(8):737–741. doi: 10.1097/00002030-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Cassol S., Rudnik J., Salas T., Montpetit M., Pon R. T., Sy C. T., Read S., Major C., O'Shaughnessy M. V. Rapid DNA fingerprinting to control for specimen errors in HIV testing by the polymerase chain reaction. Mol Cell Probes. 1992 Aug;6(4):327–331. doi: 10.1016/0890-8508(92)90009-m. [DOI] [PubMed] [Google Scholar]
- Emmanuel J. C., Bassett M. T., Smith H. J., Jacobs J. A. Pooling of sera for human immunodeficiency virus (HIV) testing: an economical method for use in developing countries. J Clin Pathol. 1988 May;41(5):582–585. doi: 10.1136/jcp.41.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. A., Laberge C., Lapointe N., Lai Tung M. T., Racine L., O'Shaughnessy M. HIV infection among Quebec women giving birth to live infants. CMAJ. 1990 Nov 1;143(9):885–893. [PMC free article] [PubMed] [Google Scholar]
- Kline R. L., Brothers T. A., Brookmeyer R., Zeger S., Quinn T. C. Evaluation of human immunodeficiency virus seroprevalence in population surveys using pooled sera. J Clin Microbiol. 1989 Jul;27(7):1449–1452. doi: 10.1128/jcm.27.7.1449-1452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentino J. R., Pachucki C. T., Holzer T. J., Heynen C., Schaaff D., Schaefer M. R., Dorus W. Comparison of radioimmunoprecipitation assay and western blot in the confirmation of sera repeatedly reactive for human T cell lymphotropic virus type I by ELISA. J Infect Dis. 1991 Jun;163(6):1387–1388. doi: 10.1093/infdis/163.6.1387. [DOI] [PubMed] [Google Scholar]
- Lepine D. G., Neumann P. W., Frenette S. L., O'Shaughnessy M. V. Evaluation of a human immunodeficiency virus test algorithm utilizing a recombinant protein enzyme immunoassay. J Clin Microbiol. 1990 Jun;28(6):1169–1171. doi: 10.1128/jcm.28.6.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F. A., Kabeya C. M., Quinn T. C., Ryder R. W., Kifuani N. K., Harris J., Bender T. R., Heyward W. L., Tam M. R., Auditore-Hargreaves K. Performance and cost-effectiveness of a dual rapid assay system for screening and confirmation of human immunodeficiency virus type 1 seropositivity. J Clin Microbiol. 1990 Feb;28(2):303–306. doi: 10.1128/jcm.28.2.303-306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


