Abstract
The tumor control effects by total-body irradiation (TBI) or half-body irradiation (HBI) on tumor-bearing mice and human cancer were investigated. In fundamental studies using a murine experimental system, mice that received 10 or 15 cGy of TBI showed a high value of TD50 (number of tumor cells required for successful transplantation to a half group of injected sites) compared with nonirradiated control mice. The combination of low doses of TBI and local irradiation on tumor-bearing mice demonstrated enhanced tumor cell killing compared with only local irradiation, but this tumor-cell killing effect was not observed following 10 or 15 cGy of TBI alone. However, the suppression of distant metastasis of tumor cells was observed following low doses of TBI alone. Immunological studies on these effects suggested that TBI or HBI caused immunopotentiating effects. In clinical studies, malignant lymphoma (non-Hodgkin’s lymphoma) was selected as the first disease for clinical trial. The results were promising for tumor control applications, except for advanced cases and very aged patients.
Keywords: total-body irradiation, tumor immunology, murine squamous carcinoma, TD50, non-Hodgkin’s lymphoma
INTRODUCTION
Total-body irradiation (TBI) with X-rays is generally considered to be a method of suppressing immunological responses in organisms and has been used to eliminate immunological ability from experimental animals. The immunological study group asked us, from time to time, to irradiate mice to suppress their immunological ability. We irradiated sublethal; however, the smallest dose required to suppress immunological responses was not clear (Hellstrom and Hellstrom, 1978; Tilkin et al., 1981; Anderson et al., 1982). The experiments described here were started about 25 years ago to determine the smallest dose. And then we investigated whether tumor cell transplantation could be accomplished with a small number of cells after TBI. On the contrary, it became clear that TBI of 10 or 15 cGy caused a rejection effect for the transplantation of tumor cells, and the rejection effect was remarkable when tumor cells were transplanted between 10 and 15 h after TBI. Thereafter, many kinds of experiments were designed to determine the mechanism of these effects, and the results are presented in this review.
There are clinical publications indicating that low doses of TBI are effective against some malignant lymphoma or chronic myelogeneous leukemia (Chaffey et al., 1976; Choi et al., 1979). For more than 25 years we have been studying the effects of low dose of TBI on normal or tumor-bearing mice, especially the influences on their immunological abilities. After carrying out these fundamental studies for almost 10 years, clinical trials were started. Human patients with advanced tumors were treated by employing TBI or by the combined therapy of TBI and local irradiations. In the present paper, the results of a clinical trial for non-Hodgkin’s lymphoma are presented. This trial was not performed as a randomized trial.
EXPERIMENTAL STUDIES
Materials and Methods
Mice and Tumor
Male or female mice of strain WHT/Ht albino mice were used as tumor hosts in the TD50* experiments. The tumor was transplantable keratinizing squamous carcinoma, which arose spontaneously in a WHT/Ht mouse and has since been maintained by serial passage as a subcutaneous tumor. A full description of the tumor and its radiobiological characteristics has already been reported (Hewitt et al., 1967; Hewitt and Sakamoto, 1971).
Irradiation
X-rays were generated by a therapy machine operated at 250 kV and 20 mA with filtration of 0.5 mm Cu and 1.0 mm A1. The exposure dose rate was 0.95 Gy/min.
Tumor cells were transplanted into the hind legs for local irradiation and then exposed to X-rays when the tumor size reached 0.8–1.0 cm in diameter. During local irradiation, the other parts of the body were covered with a 4-mm thickness of lead. The scattered dose to the other organs, including the spleen, was less than 0.2% of the tumor dose. The dosimetry was performed using the standard X-ray exposure meter made by National Physical Laboratory (England) and BeO thermoluminescent dosimeters manufactured by National Electric Industrial Co. (Japan).
Survival of Tumor Cells
The tumors were excised after irradiation, and then single-cell suspensions of tumor cells were prepared by a method described previously (Hewitt et al., 1967). Transplantation assays of counted suspensions were performed by the technique described by Hewitt (1966) and 20 mice were used in each assay. TD50 and 95% confident limits were calculated from the results by the method of Litchfield and Wilcoxon (1949).
Preparation of Spleen Cells
The mice were sacrificed by cervical dislocation and their spleens were resected at 12 h after low-dose TBI or sham irradiation. Then single-cell suspensions of splenocytes were prepared from each spleen. The technical procedure for preparation of single-cell suspensions of spleen cells has been described elsewhere (Miyamoto and Sakamoto, 1987).
In Vivo Assay System for Mitogenic Activity of Spleen Cells
For these experiments, aliquots (0.3 ml each) of spleen cell suspensions were prepared, and rIL-2 response, Con A response, PHA response, and mixed lymphocyte reaction were investigated.
rIL-2 Response. Human recombinant IL-2 was added to the culture media of spleen cells, to a final concentration of 400 U/ml, and cultured in wells of a Nune micrometer plate (0.5 μg/well) at 37°C in a CO2 incubator. DNA synthesis in response to IL-2 was estimated by the 3H-thymidine uptake in spleen cells in each well, measured after collecting cells with a cell harvester.
Con A Response. Concanavarin A was added to the culture of spleen cells, adjusting the final concentration to be 5 μg/ml. The remaining procedure was the same as for the aforementioned rIL-2 response.
PHA Response. The phytohemagultinin response was studied by adding phytohemagultinin to the culture of spleen cells at a final concentration of 0.05%. The remaining procedure was the same as for the rIL-2 response.
Mixed Lymphocyte Reaction (MLR). MLR was studied by adding 0.1 ml of spleen cell suspension (5.0 × 106/ml) from C57BL/6 mice to a spleen cell suspension from WHT/Ht mice and incubating for 120 h. The remaining procedure was the same as for the rIL-2 response.
Procedure for Investigating Effect of TBI on Metastasis. Lung-colony-forming abilities were studied to determine the effect of TBI on the metastasis of tumor cells in WHT/Ht mice that were injected through their tail vein or in another experimental system of WHT/Ht mice in which spontaneous metastasis occurred after tumor-cell transplantation into the axilla.
To form artificial metastasis in the lung, 103 tumor cells were injected into the tail vein along with 1.0 × 106 heavily irradiated (100 Gy by Softex) tumor cells. Many lung colonies appeared 14 days after the injection. In experiments with spontaneous metastasis, mice were injected with 1 × 106 tumor cells into both axilla. Lung metastases were observed 15 days after the tumor-cell inoculations.
Results
Dependence of TD50 on TBI Dose
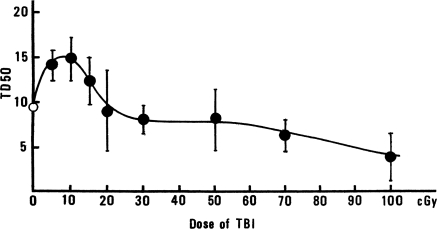
The effect of TBI dose, delivered 30 min before tumor-cell injection, on the TD50 values of murine epithelioma cells in WHT/Ht mice is shown in Figure 1. The TD50 values increased as the TBI dose increased from 0 to 10 cGy, and then gradually decreased and returned to the control value (0 cGy) when the TBI dose reached ∼20 cGy. The TD50 value of non-irradiated controls was significantly different from the TD50 value of the 10-cGy-irradiated group. It appears that a dose of 10 cGy TBI caused tumor-cell rejection.
FIGURE 1.
Changes of TD50 value with dose in WHT/Ht mice inoculated with epithelioma cells, 30 min after TBI (K. Sakamoto, unpublished data).
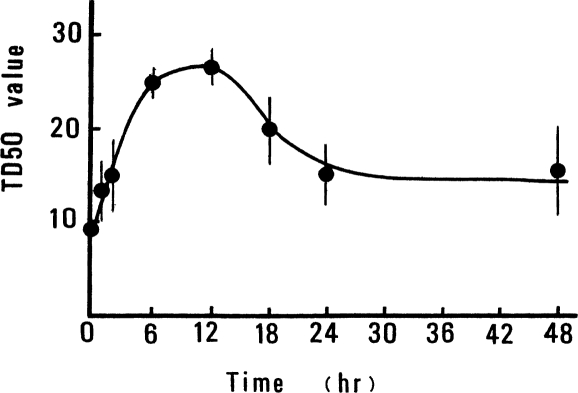
The dependence of the TD50 value on the time of tumor-cell injection, after a TBI dose of 10 cGy, was also investigated. The results, shown in Figure 2, indicate that tumor-cell rejection was maximum, a factor of ∼2.5 times that for controls, when the mice were injected with tumor cells at about 12 h after 10 cGy of TBI (Sakamoto and Miyamoto, 1987; Sakamoto et al., 1987a, 1987b, 1997).
FIGURE 2.
Change of TD50 value in WHT/Ht mice inoculated with epithelioma cells at different times after 10cGy of TBI (Sakamoto et al., 1987a).
Survival of Tumor Cells Irradiated Locally or in Combination with TBI
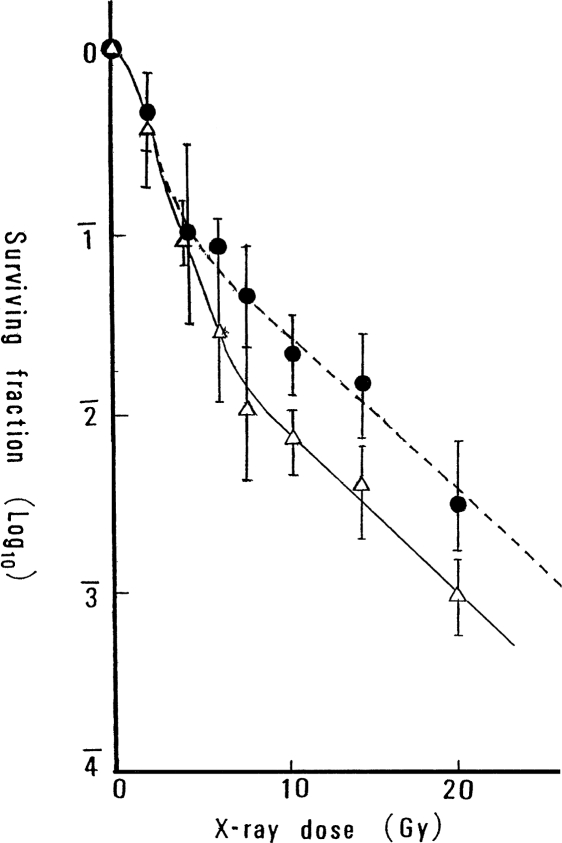
Figure 3 shows the in situ survival curves of tumor cells irradiated in vivo, to a local dose ranging from 1 to 20 Gy, compared to the survival of tumor cells in mice that received 10 cGy of TBI followed 12 h later by the local dose to the tumor cells. These survival curves demonstrate that the combined treatment of TBI and local irradiation is more effective for tumor-cell killing than local irradiation alone, for doses greater than ∼5 Gy. Local irradiation with a dose of only 10 cGy did not produce a detectable tumor-cell killing effect; therefore, it appears that TBI of 10 cGy enhanced tumor-cell killing after local irradiation in this large dose range.
FIGURE 3.
Survival curves of epithelioma cells exposed to graded dose of X-rays locally to the tumor, with or without TBI of 10cGy (Sakamoto et al., 1987b): •, high-dose local irradiation; ▵, combined method of TBI and high-dose local irradiation.
Effect of TBI on Tumor Regrowth
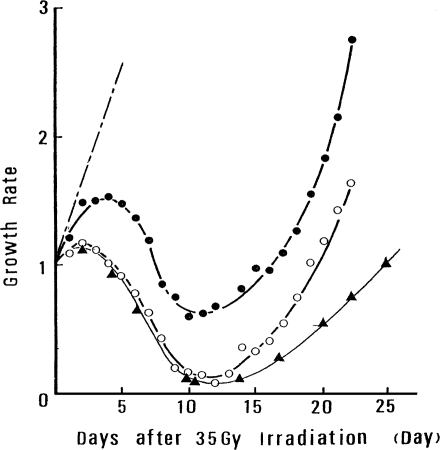
Figure 4 shows regrowth curves for tumors (5 to 6 mm in diameter) in mice that received local tumor irradiation (35 Gy) alone or various combined treatments of low-dose TBI followed by local tumor irradiation. The tumors in the mice that received the combined treatment decreased more rapidly in volume and regrew more slowly, compared with the tumors that received local irradiation only. For the combined treatment, tumor growth rate declined to about 15% of the original rate compared with the decline to about 65% for the tumors that received only local irradiation. The suppressive effect of the combined treatment on regrowth is greater when five treatments of TBI were given at 8-h intervals, as seen by the slower regrowth rate observed 15 days after the treatment. The suppressive effect was not observed following five treatments of TBI at 8-h intervals given without local irradiation.
FIGURE 4.
Regrowth curve of tumors exposed to 35 Gy locally or to combined treatment (Miyamoto and Sakamoto, 1987): • tumors irradiated locally; ○, tumors irradiated locally, 12 h after TBI; ▴ tumors irradiated locally after five TBI treatments spaced 8 h apart; –·– nonirradiated control mice.
Tumor Control by Combined Treatments Compared to Local Irradiation Only
Figure 4 shows that 10 cGy TBI enhanced the tumor suppression effects of local irradiation and delayed the regrowth of tumors exposed locally. To investigate whether these effects could be employed for improved tumor control, tumors were treated by local irradiation or by combined exposure when they reached 4 to 5 mm in diameter and observed for 30 days. The results shown in Table 1 are a coarse indication because the observation time was not long and number of mice was not large. They suggest that the combined treatment of five TBIs repeated every 6 h and followed at 12 h by 35 Gy of local irradiation was more effective than the other two treatments for curing tumors.
TABLE 1.
Effect of Irradiation Method on Tumor Cure Rates (Miyamoto and Sakamoto, 1987)
| Number of tumors
|
|||
|---|---|---|---|
| Irradiation | Irradiated | Cured | Tumor Cure Rate, % |
| 10 cGy TBI and 35 Gy local | 18 | 7 | 39 |
| Five TBIs and 35 Gy local | 14 | 7 | 50 |
| 35 Gy local only | 13 | 3 | 23 |
Identification of Organs Involved in Low-Dose TBI-Induced Cancer Suppression
As just described, low-dose TBI exposures enable increased cell killing in tumors subsequently irradiated with a high dose, but cell killing by TBI of 10 or 15 cGy was not apparent. So experiments were performed to identify specific organs that might play a role in this TBI effect, by local irradiation to various organs.
The results, shown in Table 2, indicate that 10-cGy irradiation of the spleen alone produced the same effect as TBI, but 10-cGy irradiation of the other organs and regions, except the spleen, did not show the same effects. The spleen seems to be one of the critical organs involved in producing the effects of TBI of low doses, suggesting that the effects of TBI may relate to the modification of the immunological background in mice. Therefore, immunological studies concerning the effects of TBI were carried out.
TABLE 2.
Survival Fractions of Tumor Cells after Combined Exposures (Sites Not Shielded from 10 cGy Followed by 10 Gy of Local Irradiation; Miyamoto and Sakamoto, 1987)
| Regions exposed to 10 cGy | Survival fraction (log10) |
|---|---|
| Total body except spleen | 2.17 ± 0.32 |
| Tumor | 2.12 ± 0.27 |
| Spleen | 3.75 ± 0.36 |
| Total body | 3.66 ± 0.30 |
| Upper thoracic region | 2.07 ± 0.27 |
| 10 Gy of local irradiation only | 2.09 ± 0.30 |
Immunological Studies of the Effect of Low-Dose TBI
In immunological studies, it is important to investigate the effects of irradiation to the thymus only. However, there are technical difficulties in irradiating the thymus without exposing other organs. Therefore, in the present studies, only the function of spleen cells was investigated.
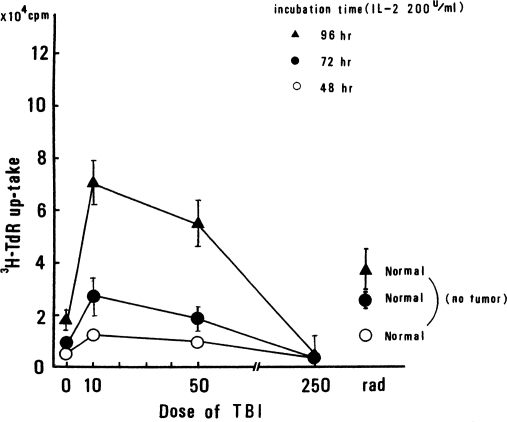
IL-2 Response of Spleen Cells. The IL-2 response of spleen cells from tumor-bearing WHT/Ht mice was investigated by incorporation of 3H-TdR, as shown in Figure 5. Each symbol represents a different spleen cell incubation time. The symbols on the right-hand side are the control values for non-tumor-bearing mice. It is clear that 10 cGy of TBI increased the IL-2 response of spleen cells in tumor-bearing mice. This was especially apparent in the case of 96 h of incubation; the increase in IL-2 response was almost twice as much as the increase for the controls.
FIGURE 5.
IL-2 response of spleen cells in tumor-bearing WHT/Ht mice. IL-2 response estimated by 3H-TdR uptake. Symbols in right corner are control values (non-tumor-bearing mice; Sakamoto et al., 1987b).
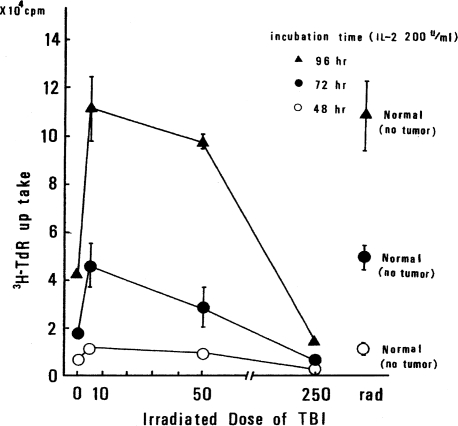
To be sure that the observed effect was not a special phenomenon that occurs only in WHT/Ht mice, the same kind of experiment was carried out using C57 black mice and B16 melanoma. The results are shown in Figure 6. The enhancement of IL-2 response in C57 black mice with B16 melanoma is also demonstrated, although the control values differ from the corresponding values in the WHT/Ht mice.
FIGURE 6.
IL-2 response of spleen cells in C57 black mice bearing B16 melanoma; symbols at right-hand side are control values (Sakamoto et al., 1987b).
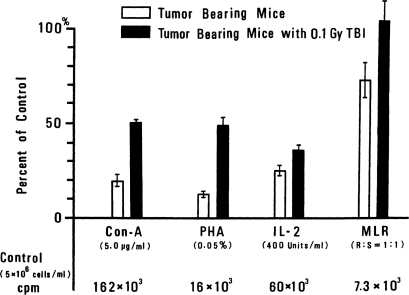
Effects of TBI on Con-A, PHA, and MLR Response. The responses of spleen cells to Con-A, PHA, MLR, and IL-2 are shown in Figure 7. All of these responses were augmented by 10 cGy of TBI. In other experiments, enhancement in the activities of allospecific CTL cells and NK cells was observed.
FIGURE 7.
Change in immunoresponse of splenocytes in tumor-bearing mice (from response in non-tumor-bearing mice) without TBI and after TBI (Miyamoto and Sakamoto, 1987).
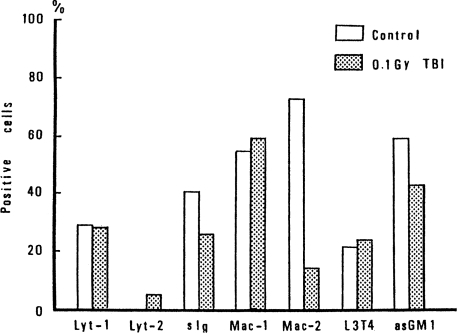
Effect of TBI on the Ratios of Phenotypes of Spleen Cells
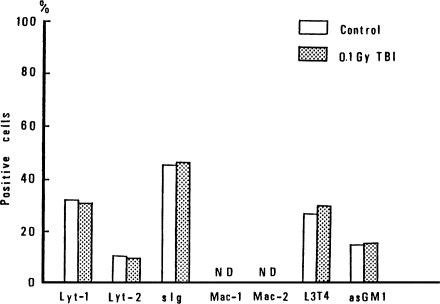
It is important to identify the types of spleen cells affected by 10 cGy of TBI. Therefore, phenotypes of spleen cells in spleen exposed to 10cGy of TBI were analyzed by Fluorescence-activated cell sorter (FACS) analysis at 12 h after TBI. The results for tumor-bearing mice are shown in Figure 8, and the results for non-tumor bearing mice are shown in Figure 9. TBI is seen to suppress Mac-2 positive cells, slg positive cells and asGM1 positive cells in tumor-bearing mice. However, in non-tumor-bearing control mice, the phenotypes of Mac-1 and Mac-2 positive cells were not detectable, as shown in Figure 9, and the other cells were not affected by TBI (Miyamoto and Sakamoto, 1987).
FIGURE 8.
Change of phenotype of spleen cells in tumor-bearing mice caused by 10 cGy of TBI (Sakamoto et al., 1987b).
FIGURE 9.
Change of phenotype of spleen cells in non-tumor-bearing mice due to 10 cGy of TBI (Sakamoto et al., 1987b).
Effect of TBI on Metastasis
The effect of TBI on metastasis is described in detail elsewhere (Hosoi and Sakamoto, 1993) and is summarized only briefly here.
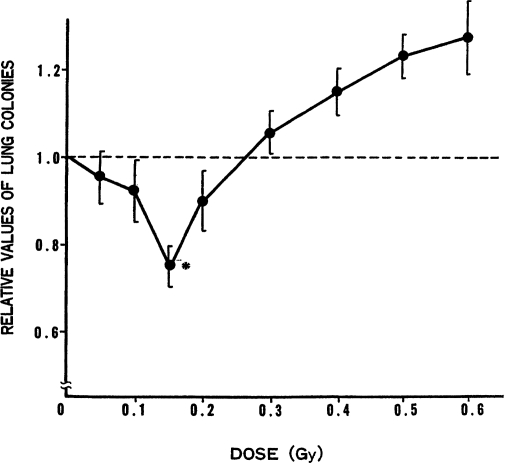
The Suppressive Effect of Low Doses of TBI on Formation of Artificial Metastases. To investigate the influence of low dose of TBI on the formation of metastases, lung colonies produced by tumor-cell injection through the tail vein were measured. As shown in Figure 10, the lung-colony-forming abilities of tumor cells, injected before exposing the mice to TBI, were observed to decrease gradually as dose increased. At about 15 cGy, the suppressive effect of the radiation peaked and, as the dose increased further, the ability to form lung colonies returned to the control level (nonirradiated mice) at about 25 cGy.
FIGURE 10.
Relative values of lung colonies in mice versus exposed dose of X-ray given 9 h before tumor-cell injection into tail vein (Hosoi and Sakamoto, 1993);* p ≤ 0.05.
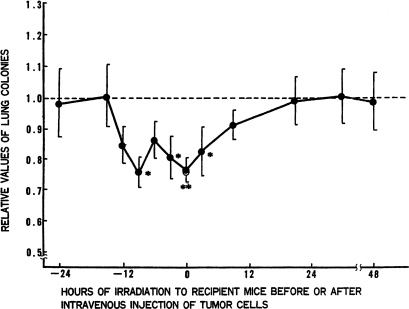
Experiments were performed to determine the duration of the suppressive effect caused by TBI on lung-colony formation abilities. Figure 11 gives the ratio of the number of lung colonies in the mice that received 15 cGy of TBI to the number in the nonirradiated control mice. The suppressive effects of TBI on colony formation were apparent from 12 h before cell injection to 12 h after cell injection.
FIGURE 11.
Effect of 15 cGy of TBI on artificial lung-colony formation: TBI given at various times before or after tumor-cell injection into tail vein (Hosoi and Sakamoto, 1993);* p ≤ 0.05, ** p ≤ 0.01.
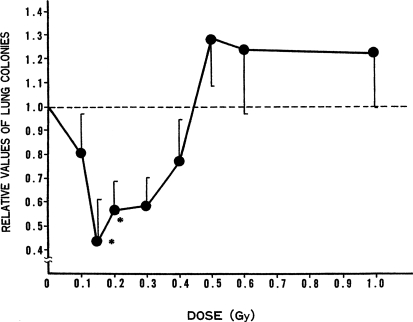
The effect of TBI on formation of spontaneous metastases. The results of the investigation of the effects of TBI on artificial metastasis by the introduction of tumor cells though a blood vessel may not reflect the effects of TBI on spontaneous metastasis because metastases from human tumors originate through lymphatic flow. Therefore, the suppressive effect of low dose of TBI on spontaneous lung metastasis in WHT/Ht mice was investigated. In these experiments, mice were subjected to TBI of various doses 12 days after tumor-cell injection into their groins. Lung colonies were counted 20 days after TBI occurred. The results are shown in Figure 12. The data suggest that effectiveness for suppression of lung-colony formation peaked around 15 cGy and then declined with increasing dose, but doses in the range from 10 to 40 cGy were all effective.
FIGURE 12.
Effect of TBI dose on spontaneous lung metastasis: TBI given 12 days after tumor-cell transplantation into groin (Hosoi and Sakamoto, 1993); * p ≤ 0.05.
CLINICAL STUDIES
Two Preliminary Cases
Before starting programmed clinical studies, we tried to treat by 10 cGy TBI and half-body irradiation (HBI) two advanced-case cancer patients with local and distant metastases. They were unable to find any other proper treatment.
The first patient was a 49-year-old woman with an ovarian tumor. Her gynecologist wanted to treat her by intraoperative radiotherapy, in cooperation with radiotherapists. However, other gynecologists recommended against intraoperative radiotherapy because the exploratory operation revealed that tumor cells had infiltrated into the sigmoidal, rectal, and peritoneal regions with ascites. The gynecologists removed only the main tumor and then asked the radiotherapist to give the patient palliative radiotherapy for the residual tumors, including the regional lymph nodes. The gynecologists judged that it was impossible for the patient to live more than 6 months after the operation, even if radiotherapy was able to allay the patient’s distress.
A combined treatment of TBI (10 cGy) and local irradiation was planned for this patient. It consisted of a single dose of 1.5 Gy to the whole abdominal region, given 5 or 6 h after 10 cGy of TBI. This combined treatment was given three times a week and repeated for 5 weeks. The total dose of TBI did not exceed 150 cGy, and the total dose of local irradiation was 37.5 Gy. The results were successful. The value of CA125 (Kabawat et al., 1983a, 1983b), which is used as the tumor marker for ovarian tumors, was 140 before treatment. During the course of the treatment, its value decreased to 35 (normal value ≤50) and was maintained there for 3 months after completion of the therapy. Two or three months later, the CA125 value began to increase gradually. A second course of treatment, 10 cGy of HBI, repeated six times at an interval of 7 days, was delivered. The value of CA125 decreased and remained at the normal level. The patient lived more than 2 years after the radiotherapy, even though there had been many distant metastases. The patient died by colon stenosis, which might have been caused by the high-dose irradiation to the whole abdominal region; however, no tumors were detected in any region of the body during the pathological autopsy.
The second case was a 52-year-old woman who underwent surgery for sigmoidal colon cancer, but 4 years later metastasis to the liver and vagina were detected. Her gynecologist administered antitumor drugs. One year later, the patient suddenly experienced genital bleeding and pain in the sacral region. Severe jaundice was identified. The gynecologist then requested the radio-therapist to carry out palliative radiotherapy on the patient. When admitted, examination of the patient also revealed lung metastasis and liver metastasis, palpably very large and very hard.
Initially, the patient was given conventional radiotherapy, specifically 200 cGy/day, 10 Gy/week, for 5 weeks—a total dose of 50 Gy. However, the tumors did not show a regressive response. At that point, therapy by HBI was commenced. As the first step, an HBI dose of 10 cGy was given three times a week, and this was repeated on the second week—a total of six HBI treatments. After four of these HBI doses were delivered, the hard liver metastases became palpably very soft and very small. The severe jaundice also appeared to be getting better. After six of these HBI treatments, the liver metastases became smaller and softer, and finally could no longer be felt. The jaundice disappeared completely.
Metastasis of the ishium and sacrum were subsequently treated for 2 months by conventional radiotherapy. Thereafter, the therapy of 10 cGy HBI was repeated nine times over a period of 3 weeks to achieve a lasting response, even though enlargement of the liver or the appearance of jaundice was not observed. The patient was discharged from hospital, but was readmitted 4 months later due to the recurrence of liver metastasis. She died several months later in spite of a repeated attempt at a cure by HBI treatment.
The two patients just mentioned were very advanced cases of cancer, and the TBI or HBI treatment employed achieved excellent results. Subsequently, the combination treatment of TBI or HBI and local irradiation was tried on several patients with advanced tumors in various regions and the results indicated a good prognosis. Therefore, a clinical study was designed and malignant lymphoma was selected as the first choice among various tumor types to be subjected to trials.
Clinical Trial on Malignant Lymphoma
Selection of Tumor Types
Malignant lymphoma was selected to be the tumor type for the first clinical study because it is generally considered to have a high likelihood of distant metastasis when the primary tumor is found. From the fundamental studies, it was clear that 10 or 15 cGy doses of TBI or HBI prevented distant metastasis and also enabled enhanced cell killing and enhanced suppression of tumor regrowth when combined with local irradiation, compared with local irradiation alone. In addition, the effects of radiation therapy on many of the malignant lymphomas could be readily assessed by inspection.
Treatment Schedule
Radiotherapy was performed using X-rays produced by a 6-MV linear accelerato. The treatment schemes were as follows.
Treatment Scheme I. TBI or HBI therapy was given three times a week when 10 cGy was used as the dose fraction or twice a week when 15 cGy was used as the dose fraction. The weekly treatment was repeated for 5 weeks, giving a total TBI or HBI dose of 1.5 Gy. Local irradiation therapy was carried out after completion of the TBI or HBI treatment schedule. Local irradiation of 60 Gy (2 Gy, five times a week, for 6 weeks) was delivered by conventional radiotherapy.
Treatment Scheme II. In this scheme, local irradiation was carried out 6 h after TBI or HBI, and local irradiation alone was given on days in which day TBI or HBI was not planned. The total dose of the local irradiation was 60 Gy (2 Gy given five times each week for 6 weeks). TBI or HBI, 10 or 15 cGy, was used three or two times in a week, for 5 weeks, as in scheme I.
In these schemes, HBI was used in all of the cases of combined therapy except for special cases, because no significant differences in effect were observed between TBI and HBI treatments in the fundamental experimental studies, described earlier in this paper. Some patients were given chemotherapy a few months later as a booster therapy.
Immunological examination by two-color methods (by Special Reference Laboratories, Inc., Japan) was carried out three times just before TBI or HBI treatments at midday and also after TBI or HBI treatments.
Results
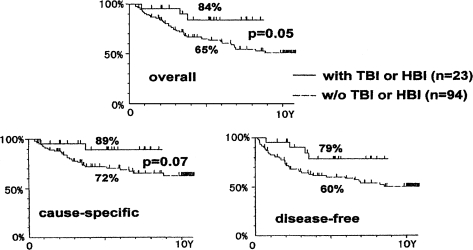
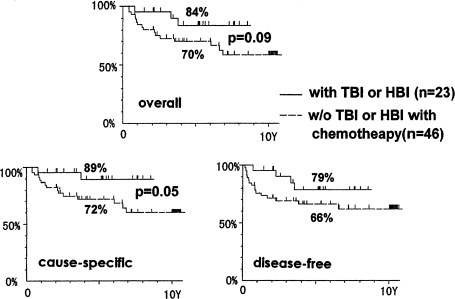
The survival data of patients with non-Hodgkin’s lymphoma, stage I and II, who were treated by local irradiation alone or by the combined treatment of TBI and local irradiation, are shown in Figure 13. The upper panel in Figure 13 indicates the overall survivals. Comparing 5-year survivals, the combined therapy achieved an 84% survival rate vs. 65% for the local irradiation therapy alone. The uncertainty factor in these results is 5%.
FIGURE 13.
Survival of stage I and II non-Hodgkin’s lymphoma patients treated by the combined treatment of low-dose TBI and high-dose local irradiation only (Takai et al., 1991; Sakamoto et al., 1997).
The lower-left panel of the figure is the cause-specific survival† curve and the lower-right panel is the disease-free survival‡ curve. These data demonstrate that combined treatments achieve an increased 5-year survival rate. The difference between the two groups is quite significant in the disease-free survivals.
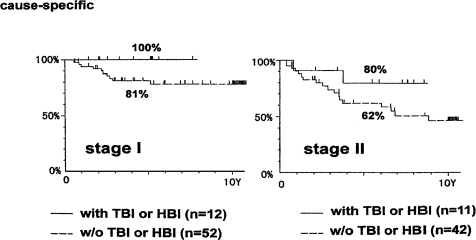
Figure 14 shows the cause-specific survival curves for patients with non-Hodgkin’s lymphoma, stage I and II. The treatment results for stage I are better than for stage II. For each stage, the results suggest that combined therapy will yield a better survival rate than local irradiation therapy alone, even though the total number of patients who had combined treatment may not be sufficient for an adequate statistical determination.
FIGURE 14.
Cause-specific survival for non-Hodgkin’s lymphoma: stage I, patients treated by the combined treatment or by local irradiation only, and for stage II patients (Takai et al., 1991; Sakamoto et al., 1997).
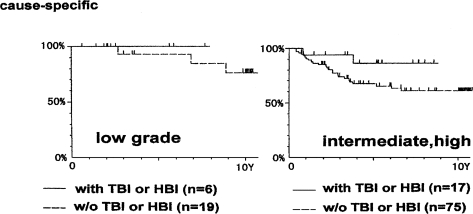
Figure 15 indicates the cause-specific survival curves for patients with non-Hodgkin’s lymphoma of low grade and of intermediate or high grade, histologically in stages I and II. It is clear that the survival rate of patients with low-grade lymphoma is higher than those with intermediate or high-grade lymphoma, and the combined therapy seems to be more effective than the local irradiation alone.
FIGURE 15.
Cause-specific survivals for histologically low-grade patients with non-Hodgkin’s lymphoma, stage I and II, treated by the combined treatment or by local irradiation only, and for intermediate, high-grade patients (Takai et al., 1991; Sakamoto et al., 1997).
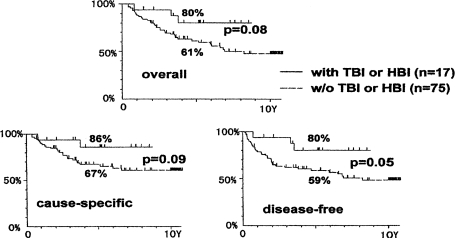
Figure 16 provides the results of the statistical analysis of the treatments given for intermediate and high-grade lymphoma, in stages I and II. The upper, lower-left, and lower-right panels show overall, cause-specific, and disease-free survivals, respectively. In each analysis, the results indicate that the combined treatment of TBI or HBI and local irradiation provides better long-term survivals than local irradiation alone.
FIGURE 16.
Survivals of patients with non-Hodgkin’s lymphoma of stage I and II and with histologically intermediate and high-grade tumors, treated by the combined treatment or by local irradiation (Takai et al., 1991; Sakamoto et al., 1997).
Figure 17 shows a comparison of the results obtained by the combined treatment of TBI or HBI and local irradiation with the results obtained by the combined treatment of cancer chemotherapy and local irradiation. The results suggest that the former method is more effective than the latter in achieving long-term patient survival.
FIGURE 17.
Survivals of non-Hodgkin’s lymphoma patients, stage I and II, treated by the combined therapy of TBI and local irradiation compared with patients treated by the combined therapy of local irradiation followed by chemotherapy (Takai et al., 1991; Sakamoto et al., 1997); there are no significant differences in disease-free survivals between two groups.
Table 3 provides data on the change in fundamental subsets of peripheral lymphocytes and the blood cell number, measured before and after TBI or HBI, using the two-color method. Helper T cells, helper-inducer T cells, the ratio of active helper and inducer T cells, and the ratio of active suppressor to cytotoxic T cells all show higher values after TBI or HBI compared to the values measured before TBI or HBI treatments, although the absolute number of lymphocytes decreased by TBI or HBI treatment. The two-color method used in the present study is the only method in Japan to determine immunological background.
TABLE 3.
Alteration of Subsets of Lymphocytes in Patients Treated with the Combined Treatment of TBI and Local Irradiation, as Measured by the Two-Color Method (Takase, 1985, 1986, 1987; Takai et al., 1991; Takai, 1992)
| Ratio in subsets of lymphocytes
|
||
|---|---|---|
| Subsets of lymphocytes | Before TBI or HBI | After TBI or HBI |
| Suppressor-induced T (CD4+2H4+) | 8.9 ± 4.3% | 7.5 ± 5.7% |
| Helper T (CD4+2H4+) | 27.1 ± 7.7% | 33.1 ± 7.7%* |
| Helper-inducer T (CD4+4B4+) | 23.7 ± 6.8% | 28.7 ± 7.2%* |
| Suppressor T (CD8+CD11+) | 9.7 ± 6.6% | 9.3 ± 4.9% |
| Cytotoxic T (CD8+CD11) | 21.0 ± 8.5% | 21.4 ± 6.9% |
| Active helper/inducer T (CD4+HLA-DR+) | 4.1 ± 1.6% | 6.8 ± 2.5%* |
| Active suppressor/cytotoxic T (CD8+HLA-DR+) | 8.3 ± 5.6% | 10.8 ± 7.4% |
| NK activity (++) (CD16+Leu7−) | 5.9 ± 2.4% | 5.4 ± 2.5% |
| NK activity (+) (CD16+Leu7+) | 14.3 ± 9.4% | 14.0 ± 7.6% |
| NK activity (+) (CD16-Leu7+) | 21.0 ± 9.2% | 20.0 ± 8.0% |
| Normalized helper T/suppressor T ratio | 1.0 | 1.42 ± 0.86% |
| Type of blood cell
|
Number of blood cells
|
|
| Blood platelet (× 104) | 22.0 ± 6.0 | 16.6 ± 6.1** |
| WBC | 5395 ± 1239 | 4000 ± 1424** |
| Lymphocyte | 1408 ± 517 | 898 ± 434** |
| Helper T (actual number) | 400 ± 215 | 300 ± 172 |
| Helper-inducer T (actual number) | 343 ± 173 | 256 ± 143 |
| Active helper/inducer T (actual number) | 51 ± 23 | 53 ± 29 |
* p ≤ 0.05;
** p ≤ 0.01.
There were no clear indications of any serious change in hematological conditions due to TBI or HBI treatment. Lymphocytopenia was observed in some of the patients, though not very severe, and almost all of the patients were able to finish treatment as initially planned. The patients that showed signs of lymphocytopenia recovered within 2 or 3 months after their treatment. And thereafter, their hematological findings remained within normal levels.
DISCUSSION
Generally, TBI is considered to cause an immunosuppressive effect, even if the TBI dose is very low. Actually, TBI is used in animal experiments to suppress the immunological response with a sublethal radiation dose. And TBI is often used in tissue transplantation experiments to prevent rejection by destruction of the immunological response. However, it was not known whether TBI causes immunosuppressive effects when the radiation dose is very small.
In fundamental studies, suppressor T cells are known to be relatively radiosensitive compared with other cells related to the immunological response in animal experimental systems. Therefore, the percentage of suppressor T cells decreases following TBI compared with the other cells related to the immunological response. The use of this phenomenon to achieve enhanced tumor control was reported by several investigators (Hellstrom and Hellstrom, 1978; Tilkin et al., 1981; Anderson et al., 1982), although the optimal TBI doses to cause synergistic effects in these studies ranged from 20 cGy to 4 Gy. Anderson et al. reported that growth control effects on tumors were observed if the recipient mice were exposed to 5 or 25 cGy of TBI before or just after tumor cell transplantation (Anderson et al., 1986). However, in our study, the effect of TBI was maintained during a 12-h period, before or after tumor-cell transplantation. Anderson et al. considered that the effect of low doses of TBI was due to the high radiosensitivity of suppressor T cells or its precursor cells, although there were no experimental data to support this assumption.
In our experimental results, a higher tumor control rate is demonstrated in tumors that received local irradiation between 5 and 15 h after a TBI treatment of 10 or 15 cGy compared with tumors that received local irradiation therapy only.
In the experiments that examined the effect of TBI on artificial metastasis in murine experimental systems, TBI was observed to suppress distant metastases; that is, lung-colony formation induced by tumor-cell inoculation into the tail vein was disturbed. These effects may be attributed to an immunopotentiating effect produced by a low dose of TBI.
To investigate the immunological background of the TBI effect, Con-A, PHA, IL-2 and MLR responses were examined. All of the observations suggest TBI enhancement of their responsiveness; that is, TBI reactivates tumor immunological ability in tumor-bearing mice.
The combined treatment of TBI and local irradiation shows an enhancing effect for tumor-cell killing over a large dose range of local irradiation, even though no cell killing was detected by 10 cGy of TBI alone. The enhancing effect on cell killing may derive from reoxygenation of anoxic tumor cells, induced by low dose TBI. However, reoxygenation does not seem to be caused by a dose as low as 10 cGy—especially as it is necessary to wait at least 24 h after irradiation to find reoxygenation, except in a few tumors. And the murine tumor used in the present study certainly did not show reoxygenation within 24 h of 10 cGy irradiation. Therefore, the enhancing effect on tumor-cell killing in combined therapy is difficult to attribute to reoxygenation. In any case, the cell-killing effect is not observed following 10 cGy of TBI alone, as mentioned earlier, indicating that the enhancing effect caused by TBI may be due to some change in the host’s radiosensitivities.
Gerber et al. reported that an exposure dose of 15 cGy or more to the spleen of tumor-bearing mice brought about a decrease in the activity of killer T cells, but in a dose range between 5 and 12 cGy the activity of killer T cells was increased (Gerber et al., 1981a, Gerber et al., 1981). These results suggest that opposite effects are caused by small differences in dose to the spleen. In our experiments, low values of TD50 were observed in mice exposed to more than 25 cGy of TBI, but only high values of TD50 were seen in mice that received 10 cGy of TBI or in mice that received 10 cGy to spleen.
Tumor growth delay was observed in tumors that received local irradiation after 10 cGy of TBI compared with tumors that received local irradiation only, consistent with the prolongation of life span of tumor-bearing mice treated with combined irradiation.
In the present study, the effects of TBI were examined only on the basis of tumor immunology. Other factors, which may bring about such effects, were not investigated. Therefore, we are unable to attribute the effect of TBI solely to an enhancement of tumor immunological ability. However, the change in immunological abilities following a low dose of TBI seems to be strongly related to the enhancing effect.
In a clinical study, Chaffey et al. (1976) reported that TBI doses smaller than 25 cGy were effective for lymphosarcoma tumor control, and Choi et al. (1979) also reported that it is effective to use low doses of TBI in the treatment of advanced non-Hodgkin’s lymphoma. Holder (1965) also recognized that multiple myeloma can be treated successfully by low doses of TBI.
The remarkable results of the combined treatment of TBI and local irradiation, or even TBI alone, employed in the clinical studies for malignant lymphoma described in the present paper suggest that favorable results, like those shown in Figures 13–17, can be expected. It is not clear whether or not the immunological mechanisms work in humans as well as they do in murine experimental systems. However, the results of the measurements, using the two-color method, suggest enhanced immunological abilities in humans following TBI or HBI.
We have tried to treat solid tumors such as tongue cancer, lung cancer, esophagus cancer, and uterine cervical cancers. Unfortunately, the results were not statistically significant because of the small number of patients that were recruited to the trial.
CONCLUSIONS
Experimental and clinical studies of TBI or HBI treatment are presented in this paper.
In the experimental studies, low doses of TBI (10 or 15 cGy) resulted in synergistic effects on tumor-cell killing by local high-dose irradiation, when the local irradiation was given at 6–15 h after the TBI or HBI treatment. TBI or HBI suppressed lung-colony formation of tumor cells induced by tumor cells injected into murine tail veins (artificial metastasis). TBI or HBI also suppressed lung colonies formed spontaneously (spontaneous metastasis) in mice. These effects are considered to indicate a reactivation of tumor immunological abilities, which were reduced in tumor-bearing mice. All kinds of investigations concerning immunological response suggest they are diminished in tumor-bearing mice.
In the clinical studies, malignant lymphoma was chosen to be the first tumor type to be tried and good results were obtained. We expect that the combined treatment of TBI or HBI and local irradiation therapy will bring about better tumor cure rate results in the radiotherapy regime by the enhancement of local control rate and the suppression of distant metastases.
However, TBI, HBI, or the combined therapy of TBI or HBI and local irradiation were not effective for advanced tumors, recurrent cases, or aged patients. The reasons may be that, in these patients, some sites (receptors) for receiving some change produced in the body by TBI or HBI have been destroyed. Tables 4A and 4B show the prognosis of non-Hodgkin’s lymphoma cases treated with the combined method of TBI or HBI and local irradiation, and Table 5 shows the prognosis of patients with advanced and recurrent cases treated with combined methods of TBI or HBI and local irradiation for reference, though the prognoses of all patients are not shown in these tables.
TABLE 4A.
Prognosis of Non-Hodgkin’s Lymphoma Patients Treated by Combined Method of TBI Followed by Local Irradiation (Takai et al., 1991; Takai, 1992)
| Age/sex | Stage | Primary site | Treatment method | Response | Survival, months | Present status | Recurrence |
|---|---|---|---|---|---|---|---|
| 27/M | II | Neck | TBI+Local-Chemo | CR | 51 | Alive | No |
| 64/M | III | Tonsil | TBI-Chemo | CR | 49 | Alive | No |
| 60/M | II | Tonsil | TBI+Local-Chemo | CR | 46 | Alive | No |
| 70/F | I | Tonsil | TBI+Local-Chemo | CR | 28 | Alive | No |
| 65/F | II | Tonsil | TBI+Local-Chemo | CR | 29 | Alive | No |
| 55/M | III | Tonsil | TBI+Local-Chemo | CR | 14 | Dead | Yes |
| 73/F | IV | Neck | TBI+Local-Chemo | CR | 13 | Dead | Yes |
| 62/M | II | Neck | TBI+Local-Chemo | CR | 25 | Alive | No |
| 32/M | IV | Neck | TBI-Chemo | PR | 22 | Alive | No |
| 61/F | I | Nasal cavity | TBI+Local-Chemo | CR | 8 | Alive | No |
| 54/M | III | Tonsil | TBI+Local-Chemo | CR | 2 | Alive | No |
Chemotherapy followed a few months later as a boost therapy after completing radiotherapy. CR: complete response, PR: partial response. Survival results observed in 1995.
TABLE 4B.
Prognosis of Patients Receiving Local Irradiation on the Same Day after TBI or HBI (Takai et al., 1991; Takai, 1992)
| Age/sex | Stage | Primary site | Treatment method | Response | Survival, months | Present status | Recurrence |
|---|---|---|---|---|---|---|---|
| 72/M | II | Eye | HBI+Local-Chemo | CR | 11 | Dead* | No |
| 63/M | I | Tonsil | TBI+Local-Chemo | CR | 50 | Alive | No |
| 49/F | II | Tonsil | TBI+Local-Chemo | CR | 49 | Alive | No |
| 59/M | II | Tonsil | TBI+Local-Chemo | CR | 10 | Dead | Yes |
| 55/M | II | Neck | HBI+Local-Chemo | CR | 30 | Alive | No |
| 54/M | I | Tonsil | HBI+Local-Chemo | CR | 20 | Alive | No |
| 60/F | I | Maxilla | HBI+Local-Chemo | CR | 22 | Alive | No |
| 72/M | I | Neck | HBI+Local-Chemo | CR | 22 | Alive | No |
| 63/F | I | Tonsil | TBI+Local-Chemo | CR | 23 | Alive | No |
| 64/M | II | Tonsil | TBI+Local-Chemo | CR | 24 | Alive | No |
| 59/M | I | Neck | Oper+TBI-Chemo | CR | 26 | Alive | No |
*Heart failure.
TABLE 5.
Prognosis of Recurrent and Advanced Cases of Non-Hodgkin’s Lymphoma (Takai et al., 1991; Takai, 1992)
| Age/sex | Stage | Primary site | Treatment method | Response | Survival, months | Present status | Cause of death |
|---|---|---|---|---|---|---|---|
| 36/M | IV | Axilla | TBI+Local | PD | 1 | Dead | Pancytopenia |
| 69/F | IV | Neck | TBI+Local | PD | 1 | Dead | Pancytopenia |
| 57/M | III | Neck | TBI+Local | PD | 2 | Dead | Leukenic change |
| 56/M | IV | Cecum | TBI only | PD | 1 | Dead | Thrombocytopenia |
| 61/F | III | Neck | TBI+Local | PD | 1 | Dead | Leukenic change |
| 45/M | IV | Smallintestine | TBI+Local | PD | 1 | Dead | Cancerous peritonitis |
| 52/F | IV | Inguinal | TBI+Local | PD | 2 | Dead | Pancytopenia |
| 62/M | II | Neck | TBI+Local | NC | 2 | Alive | |
| 46/M | III | Neck | TBI+Local | PR | 3 | Alive | |
| 83/M | II | Skin | TBI-Chemo | CR | 7 | Alive | |
| 55/M | II | Tonsil | TBI+Local-Chemo | CR | 8 | Alive |
Chemotherapy followed a few months later as a boost therapy after completing radiotherapy. PD: progression of disease, NC: no change, PR: partial response, CR: complete response. Survival results observed in 1995.
LIMITATIONS OF THIS THERAPY AND FUTURE PROSPECTS
As mentioned earlier, the treatment demonstrated in this review paper did not bring about good results for the patients with advanced and/or recurrent non-Hodgkin’s lymphoma and for aged patients.
A vexing problem is that low-dose TBI or HBI therapy may not easily receive the approval of the ethics committee in many cancer treatment facilities without submission of convincing data from fundamental and preliminary studies. The procedure of the ethics committee may be troublesome in many facilities.
However, we have not identified any severe acute or chronic hematological disturbances due to low-dose TBI or HBI. If studies concerning the effects of low-dose levels on humans are developed, and if data clarifying the lack of harmful damage are collected in the near future, it will be very easy to do radiotherapy using TBI or HBI.
TBI or HBI will suppress local and distant metastases, and TBI or HBI will increase the effect of local irradiation. These capabilities of TBI or HBI are quite important. The side effects of this therapy are negligible compared to the side effects of cancer chemotherapy. We believe that the combined therapy of TBI or HBI and local irradiation is a very useful therapeutic method for controlling tumors.
Footnotes
The study introduced in this review paper was performed in cooperation with Drs. M. Myojin, Y. Hosoi, Y. Takai, and my colleagues belonging to the Radiotherapy Department of the Tohoku University. I am indebted to all of them, and I am very grateful for the assistance from Dr. Jerry M. Cuttler in the preparation of the manuscript. The study was supported by grants provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan, and Tohoku Electric Power Co. Ltd.
The number of cells required for a tumor incidence of 50%.
†Cause-specific survivals exclude the patients who died from other causes (e.g., heart disease).
‡Disease-free survivals are patients who are living without any recurrence or metastases.
REFERENCES
- Anderson RE, Tokuda S, Williams WL, Warner NL. Radiation-induced augumentation of the response of A/J mice to Sa I tumor cells. Am J Pathol. 1982;108:24–37. [PMC free article] [PubMed] [Google Scholar]
- Anderson RE, Tokuda S, Williams WL, Spellman CW. Low dose irradiation permits immunization of A/J mice with subimmunogenic numbers of Sa I cells. Brit J Cancer. 1986;54:505–509. doi: 10.1038/bjc.1986.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey JT, Rosenthal DS, Moloney WC, Hellman S. Total body irradiation as treatment for lymphosarcoma. Int J Radiat Oncol. 1976;1:399–405. doi: 10.1016/0360-3016(76)90004-3. [DOI] [PubMed] [Google Scholar]
- Choi NC, Timothy AR, Kaufman SD, Carsey RW, Aisenberg AC. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin’s lymphoma. Cancer. 1979;43:1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gerber M, Dobis JB, Pioch Y, Serrou B. Effect of localized radiotherapy upon the cellular immune response. Radiat Res. 1981a;85:390–398. [PubMed] [Google Scholar]
- Gerber M, Dubois JB, Serrou B. Effect of low doses of irradiation on T cell meditated cytotoxic response: Immunopharmacologic Effect of Radiation Therapy. Raven Press. 1981:53–74. [Google Scholar]
- Hellstrom KE, Hellstrom J. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978;148:799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt HB. The effect on cell survival of inhalation of oxygen under high pressure during irradiation in vivo of a solid mouse sarcoma. Brit J Radiol. 1966;39:19–24. doi: 10.1259/0007-1285-39-457-19. [DOI] [PubMed] [Google Scholar]
- Hewitt HB, Sakamoto K. The comparative survival of clonogenic cells of a murine epithelioma after irradiation in mice breathing air, oxygen and carbon dioxide or hyperbaric oxygen. Brit J Radiol. 1971;44:457–463. doi: 10.1259/0007-1285-44-522-457. [DOI] [PubMed] [Google Scholar]
- Hewitt HB, Chan DP, Blake ER. Survival curves for clonogenic cells of murine keratinizing squamous cell carcinoma irradiated in vivo or under hypoxic conditions. Int J Radiat Biol. 1967;12:535–549. doi: 10.1080/09553006714551151. [DOI] [PubMed] [Google Scholar]
- Holder DL. Total body irradiation in multiple myeloma. Radiology. 1965;84:83–86. [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: Dose dependency and effective period. Radiother Oncol. 1993;26:177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Kabawat SE, Bast RC, Bhan AK, Welch WR, Clovin RB. Tissue distribution of a coelomic epithelium-related antigen recognized by the monoclonal antibody OC 125. Lab Invest. 1983a;48:24A. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- Kabawat SE, Bast RC, Welch WR, Knapp RC, Colvin RB. Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serious, endometrioid and clear cell types. Am J Clin Pathol. 1983b;79:98–104. doi: 10.1093/ajcp/79.1.98. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose effect experiments. J Pharmacol Exp Ther. 1949;96:99–133. [PubMed] [Google Scholar]
- Miyamoto M, Sakamoto K. Anti-tumor effect of total body irradiation of low doses on WHT/Ht mice. Jpn J Cancer Clin. 1987;33:1211–1220. [PubMed] [Google Scholar]
- Sakamoto K, Miyamoto M. Potentiating effect of low dose total body irradiation on tumor control. Oncologia. 1987;28:86–88. [PubMed] [Google Scholar]
- Sakamoto K, Miyamoto M, Watabe N.The effect of low dose total body irradiation on tumor control Jpn J Cancer Chemother 1987a14(Part II):1545–1549. [PubMed] [Google Scholar]
- Sakamoto K, Miyamoto M, Watabe M, Takai Y. Fundamental studies of low dose total dody irradiation on tumor control. Jap J Cancer Clin. 1987b;33:1633–1638. [PubMed] [Google Scholar]
- Sakamoto K, Myojin M, Hosoi Y, Ogawa Y, Nemoto K, Takai Y, Kakuto Y, Yamada S, Watabe N. Fundamental and clinical studies on cancer control with total or half body irradiation. J Jpn Soc Ther Radiol. 1997;9:161–175. [Google Scholar]
- Takai Y. Anti-tumor effect of low dose total (or half) body irradiation and changes of the functional subset of peripheral blood lymphocytes in non-Hodgikin’s lymphoma patients after TBI(HBI) Jpn J Cancer Clin. 1992;38:1305–1311. [Google Scholar]
- Takase K. Simultaneous detection of dual surface marker. Clin Oncol. 1985;17:914–925. [Google Scholar]
- Takase K. Two color analysis with cytometry. Clin Immunol. 1986;18:253–260. [Google Scholar]
- Takase K. FCM (flow cytometry) technique for the lymphocytic surface antigens. Clin Immunol. 1987;19(Suppl 12):19–27. [Google Scholar]
- Tilkin AF, Schaaf-Lafontaine N, Acker AV, Boccadoro M, Urbain J. Reduced tumor growth after low-dose irradiation or immunization against blastic suppressor T cells. Proc Natl Acad Sci USA. 1981;78:1809–1812. doi: 10.1073/pnas.78.3.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]