Abstract
Objectives:
To correlate the oxidative state of postabsorptive blood plasma after consumption of one or three drinks of different beverages with known J-shaped epidemiological risk curves.
Design, interventions, and main outcome measures:
Red wine, lager beer, stout (alcoholic and alcohol-free), with antioxidant activity, and an aqueous solution of alcohol were compared for the plasma antioxidant or pro-oxidant activity in human volunteers following consumption of one or three typical drinks containing equivalent amounts of alcohol (except for an alcohol-free stout used as a control for stout).
Results:
One drink of red wine, lager beer, or stout (5% alcohol v/v, and alcohol-free) significantly increased the average antioxidant activity in plasma samples obtained from volunteers averaged over 240 min. Three drinks of red wine, lager beer, or stout (5% alcohol v/v, and alcohol-free) significantly increased the average pro-oxidant activity in plasma samples obtained from volunteers averaged over 360 min. For a solution of alcohol, three drinks resulted in pro-oxidant plasma on average, whereas while one drink did not significantly affect the plasma oxidative status. A preliminary experiment in which two volunteers showed a significantly increased time to metabolize ethanol after ingestion resulted in elevated antioxidant activity in plasma for lager beer and red wine.
Conclusions:
One drink of red wine, beer, or stout provided equivalent increases in plasma antioxidant activity. Three drinks of red wine, beer, or stout provided equivalent increases in plasma pro-oxidant activity. This may explain, at least in part, the decreased risk of cataract and atherosclerosis from daily consumption of one drink of different types of alcoholic beverages as well as the increased risk from daily consumption of three drinks of alcoholic beverages. The plasma pro-oxidant activity appears to be due to ethanol metabolism, whereas the antioxidant activity may be due to the absorption of polyphenols in the beverages.
Keywords: alcoholic beverages, ingestion, plasma antioxidant activity, polyphenols, cataract, atherosclerosis, risk reduction, hormesis
INTRODUCTION
Human consumption of alcoholic beverages has resulted in conflicting effects (Clayton et al., 1982; Klatsky, 1994). Excessive or chronic overconsumption has resulted in hydroxyethyl radical formation, lipid peroxidation, and oxidative stress, leading to liver necrosis (Albano et al., 1999; McKim et al., 2002), neuropathy (Tenkova et al., 2003), cataract (Clayton et al., 1982; Ritter et al., 1993; Katoh et al., 2001), and macular degeneration (Klein et al., 2002). At the cellular level, mitochondrial damage can result in initiation of apoptosis by release of cytochrome C (Robertson and Orrenius, 2002) or calcium (Kilic and Trevithick, 1998) activation of calpain by the damaged mitochondria. Such apoptosis has been observed in experimental fetal alcohol syndrome (Tenkova et al., 2003), accounting for a loss of visual neurons after a brief period of alcohol intoxication. Although most of these effects have been attributed to the metabolism of alcohol, Halliwell’s group has also suggested that polyphenols in solution may produce hydrogen peroxide (Long et al., 1999; Evans and Halliwell, 2001; Chai et al., 2003; Halliwell, 2003). In contrast, Folts has shown that red wine flavonoids can inhibit platelet activity and thrombosis (Folts, 2002).
Beer, wine, and matured spirits (rum, whisky, sherry, and port), which extract tannins from the oak casks they are matured or stored in, all contain significant concentrations of polyphenolic substances which vary depending on the plant source. In plants, many of these compounds are precursors of the lignin component of the plant cell wall, which provides a plastic matrix for the long cellulose fibers that give strength to the plant cell wall.
Moderate consumption of alcoholic beverages, by comparison with excessive consumption or alcoholism, results in beneficial effects, reducing the risk of many aging diseases in which oxidative stress plays an important role (Kiechl et al., 1994). Our initial study showed that human volunteers who consumed one typical drink of beer, stout, alcohol-free stout, or wine had significantly increased average plasma antioxidant activity during a 4-h period after beverage ingestion. Although ethanol had antioxidant activity, an aqueous solution containing an equivalent amount of ethanol did not change the average plasma oxidative state. Several aging diseases, including cataract and atherosclerosis, have been reported to show a J-shaped risk response to daily beverage consumption: for people consuming one drink per day, there is 50% risk reduction in both cataracts (Clayton et al., 1982) and atherosclerosis (Kiechl et al., 1994; Klatsky, 1994), and the risk rises from this minimum until it becomes significantly increased at three drinks per day.
Such J-shaped curves of relative risk versus dose have been reported to be typical of the phenomenon of hormesis, in which small amounts of a poisonous substance have beneficial effects whereas at higher concentrations the substance is toxic (Calabrese et al., 1987; Calabrese and Baldwin, 1998; Calabrese and Baldwin, 2001). For this reason we felt it was important to use our luminescent assay (Trevithick and Dzialoszynski, 1994; Trevithick et al., 1999a, 1999b) to examine the plasma oxidative status of people after they consumed one or three typical drinks of beer, stout and nonalcoholic stout, wine, or a solution of an equal amount of ethanol.
METHODS
The beverages used were red wine (Piat D’Or, 11% alcohol v/v), lager beer (malt lager prepared with corn as adjuvant, Labatt Brewing Co Ltd., London, Ontario, Canada, 5% alcohol v/v), stout (provided by Labatt Brewing Co. Ltd., London, Ontario, Canada, 5% alcohol v/v), alcohol-free stout (reconstituted with water after drying to remove the alcohol), and alcohol in bottled spring water (5% v/v, prepared from DeKuyper 40% alcohol v/v).
ASSAY
The luminescent assay was described previously (Trevithick and Dzialoszynski, 1994; Trevithick et al., 1999a, 1999b) using a luminometer (Lumac 1010) to measure the photons counted following addition of a standard amount of peroxide to the assay. For plasma samples, data are normalized to the initial samples, taken before the consumption of the beverages.
To obtain comparative abilities of the drinks to act as antioxidants, aliquots were assayed to see whether they could alter the luminescence resulting from the presence of a standard amount of hydrogen peroxide (37.8 mM) reacting with luminol bound to bovine albumin, as described previously (Trevithick and Dzialoszynski, 1994; Trevithick et al., 1999a, 1999b). Antioxidant activity was calculated as the decrease in counts after addition of the beverage samples, as a percent of the control. If the counts measured following addition of peroxide to the assay mixture containing the beverage or plasma sample were greater than the peroxide itself produced, the sample was judged to be “pro-oxidant.” This signifies that greater oxidative activity was measured in the sample than that generated by the added peroxide. We have previously reported such pro-oxidant activity for the well-known tranquilizer chlorpromazine (Trevithick and Dzialoszynski, 1994).
Following studies with the undiluted drinks, the antioxidant activities of serial ten-fold dilutions of the various beverages were examined. From these data the IC50 values (dilutions that suppressed luminescence by one-half) were obtained for each beverage.
A second set of data was obtained for the drinks, namely a comparative assessment of their polyphenol content. To this end, the concentrations of polyphenols in the beverages were determined using Folin Ciocalteau reagent standardized with catechin (Trevithick et al., 1999a; Vinson et al., 2003). Using these values, an IC50 expressed as catachin equivalent concentration could be calculated to give an idea of the antioxidant availability for each beverage. The antioxidant activities of stout itself on superoxide and hydroxyl radicals were examined by electron spin resonance (ESR) spin trapping, using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO; Trevithick and Dzialoszynski, 1994) in the presence of hypoxanthine and xanthine oxidase as previously described for ethanol (Trevithick et al., 1999b).
Because the initial study showed that high dilutions of beverages could produce discernable decreases in peroxide-induced luminescence and another showed that the method of assessing antioxidant activity could be adapted to plasma, volunteers were recruited to explore the question of whether the consumption of the alcoholic drinks could alter the plasma antioxidant level from pre-ingestion levels. Volunteers recruited for two study sessions (by posters placed on bulletin boards throughout the University of Western Ontario for the first session, previous volunteers from the first session, and word of mouth for the second session) gave written consent to participate in the two studies, which had been approved by the Human Studies Committee of the University of Western Ontario Faculty of Medicine and Dentistry. For the study of the effects of consuming one drink, the volunteers (6 M, 6 F) ranged in age between 20 and 48 years [26.5 ∀ 3.0 (standard error, SE) years]. Their weights were between 55 and 109 kg [76.6 ∀ 4.3 (SE) kg], and their basal metabolic indices were between 21 and 29 kg/m2 [24.4 ∀ 0.8 (SE)]. For the study of effects of three drinks, the volunteers (4 M, 4 F) were between 22 and 49 years of age [29.5 ∀ 3.7 (SE) years]. Their weights were between 43 and 91 kg [62 ∀ 6.5 (SE) kg]. Their basal metabolic indices were between 17 and 27 kg/m2 [21.3 ∀ 1 (SE)]. The numbers of volunteers were modeled on published studies by the groups of Duthie (Duthie et al., 1998) and Rice-Evans (Paganga et al., 1999; Bourne et al., 2000). Although the three-drink group was originally ten volunteers, two volunteers later indicated that they were heavy drinkers: their data were not included with the main group of volunteers, and instead their data were processed as a preliminary experiment on a separate group, which might offer interesting leads to explore further in future tests. Volunteers were not asked whether they were smokers, but it is the impression of the investigators that none were smokers.
After an overnight fast from midnight the previous night until the beginning of the test at about 8 to 9 am the following morning, blood samples were taken from volunteers into heparinized tubes 5 min before ingestion of the one drink of the above beverages (1 × 341 mL), or the first of three drinks of the following beverages: red wine (3 × 155 mL), lager beer (3 × 341 mL), stout (3 × 341 mL), alcohol-free stout (3 × 341 mL), and 5% water in alcohol (3 × 341 mL). The drinks with ethanol each contained the same quantity of ethanol, 13.4 g, equal to that contained in a standard drink in Canada. For one drink, the volunteers generally consumed the beverage in less than 10 min. Following the pre-ingestion control, blood samples were collected, and the experimental blood samples were obtained at 15, 30, 60, 120, and 240 min after consumption commenced. For three drinks, the volunteers finished consuming the three drinks by 1 h after beginning the first drink. For both groups, the drinks were consumed so that each person consumed each beverage with an interval of one week between each period of consumption. Beverages were given to the different volunteers according to a Latin Square design so that two or three different members of the group of volunteers received the same beverage on the same day, but by the end of the test period of approximately five weeks, each person had consumed each of the different beverages. For the three-drink group, following the pre-ingestion controls, blood samples were collected at 30, 60, 90, 120, 240, and 360 min after the first drink. After the 2-h sample for the one-drink group, or the 3-h sample for the three-drink group, volunteers were offered a small breakfast of a plain bagel along with their choice of clear tea or coffee or spring water. Plasma was obtained from blood by centrifugation and then stored under nitrogen at − 70°C until analyzed for antioxidant potential, or for ethanol concentration using a kit available from Sigma [Alcohol (ethanol) #333A, Mississauga, Ontario].
Changes in the plasma measures of oxidative activity at the different times after beverage ingestion were normalized for each volunteer to the preconsumption value; each volunteer then served as his/her own control. Thus, the antioxidant or pro-oxidant activities measured at different time points after ingestion of the beverage could be compared for all volunteers on a similar basis. The antioxidant activity was measured in sextuplicate (six times) for each plasma sample and there are six time points for each of 12 participants, and five beverages in the one-drink study, and seven points for each of 8 participants and five beverages tested in the three-drink study, resulting in 2,160 measurements for the one-drink study and 1680 for the three-drink study (excluding the two anomalous participants). For the two heavy drinkers, the number of measurements on which their antioxidant results were based was 420. Although the number of people studied is only two, the similarities in analytical course for these two participants is reported as an interesting observation that is consistent with and supports the other data suggesting that ethanol is the source of the oxidative metabolites.
The ethanol time-course determinations were performed in triplicate for each time point for each participant and for each beverage.
The antioxidant activity averages for each beverage were used to draw curves (Figure 2, for one drink) of antioxidant activity as a function of time. To calculate the relative effectiveness of the different beverages, the average antioxidant activities of the different beverages were determined and expressed as the ability to destroy peroxide under our standard assay conditions (Table 1). The normalized luminescent assays measured at different time points after ingestion of the beverage were averaged for the volunteers.
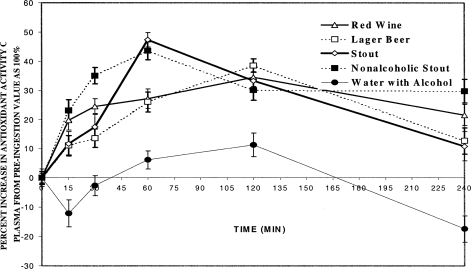
FIGURE 2.
Plasma antioxidant activities as a function of time after consumption of one alcoholic beverage. The counts per minute were obtained with the Lumac Biocounter M2010. Hydrogen peroxide was incubated with the luminol bound to albumin, described as follows. The sample consisted of an aqueous portion (0.3 mL) and a dimethyl sulfoxide portion (0.4 mL). The aqueous portion contained luminol and albumin (0.02 mL, 10 mg/mL of each) prepared as described (Trevithick et al., 1999a, 1999b), plasma (0.07 mL) and phosphate-buffered saline (PBS, 0.04 mL), alcohol (0.07 mL) at appropriate dilution or distilled water (for controls), and hydrogen peroxide (0.1 mL of 0.9%) added after the addition of DMSO (0.4 mL), just before the tube was placed in the counter to begin counting of the emitted light. The counts were recorded for a 10-s time period. The counts were normalized by dividing the counts/min obtained at the different times after beverage consumption by the average of six or more determinations of the count rate of the plasma taken immediately prior to beverage consumption. The abscissa shows the time after beverage consumption. The graph shows plots of the antioxidant activity (percent decrease in a minimum of six normalized counts at each time) versus the time after beverage consumption. The beverages consumed are described in the text: red wine, lager beer, stout, alcohol-free stout, and water containing 5% alcohol.
TABLE 1.
Antioxidant Activities and Polyphenol Concentrations of Beverages
| Beverage tested | Antioxidant activity % of counts remaining | ±Standard error | Polyphenol content in Catechin Equivalents, μmol/mL | ±Standard error |
|---|---|---|---|---|
| Red wine | 99.7 | 0.06 | 6.55 | 0.002 |
| Lager | 97.4 | 0.92 | 0.72 | 0.007 |
| Stout | 99.2 | 0.15 | 1.4 | 0.0002 |
| Alcohol-free stout | 99.8 | 0.04 | 0.39 | 0.0007 |
| Alcohol 5% solution | 20.7 | 2.4 | — | NA |
The average values at the beginning of the period were compared for men and women.
To test, in a preliminary fashion, whether changes in antioxidant values in plasma corresponded to a physiologically important effect, the lag time (Trevithick et al., 1999b), a measure of the low-density lipoprotein (LDL) oxidizability, was determined on the combined LDL + very low-density lipoprotein (VLDL) fraction obtained from the plasma of six volunteers (4 M, 2 F) who consumed one drink of stout (Vinson et al., 2001). The percent increase from the initial sample to the 30-min value was calculated for each person.
The plasma antioxidant measures at different times after ingestion of each beverage were compared using one-way analysis of variance (SPSS program), with the Student–Newman–Keuls test being used for post hoc evaluations. Data were considered significantly different if alpha <0.05. Student’s t-test was used to calculate the percent increase in lag times. The IC50 values for dilutions of individual beverages were obtained graphically using the Excel program for graphical analysis.
RESULTS
The undiluted beverages had high antioxidant activities (Table 1). Although the aqueous alcohol solution had a significant antioxidant activity, it was far less effective than the other beverages. Red wine proved to contain substantially more reacting polyphenols than the stout, beer, or alcohol-free stout (Table 1).
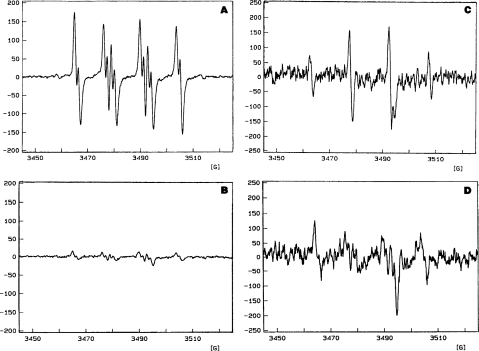
The IC50 values indicated that red wine was more potent as an antioxidant than were the other beverages (Table 2). This was also true when the IC50 values of the beverages were converted into their catechin equivalent concentrations (Table 2). The results obtained from the ESR study (Figure 1) demonstrated that stout virtually destroyed the superoxide radical signal, although a reduction of the spin-trapped signal for hydroxyl radical was not as apparent.
TABLE 2.
Comparison of IC50 Values for Beverages and Folin Catechin Equivalent IC50 Valuesa
| Beverage | IC50 dilution from beverage | ±Standard error | IC50 catechin equivalents, μM | ±Standard error, μM |
|---|---|---|---|---|
| Red wine | 14.9 × 10−6 | 12.9 × 10−6 | 98 × 10−3 | 85 × 10−3 |
| Lager | 3.12 × 10−3 | 6.82 × 10−3 | 2.3 | 4.9 |
| Stout | 5.66 × 10−3 | 4.79 × 10−3 | 7.9 | 6.7 |
| Alcohol-Free stout | 2.8 × 10−3 | 7.0 × 10−3 | 1.1 | 2.7 |
| Catechin (1 mM ) | 0.54 × 10−3 | 1.95 × 10−3 | 0.54 | 1.95 |
aData taken 15 July 2003.
FIGURE 1.
ESR spin-trapping analysis of radical type (superoxide or hydroxyl radical) scavenged by stout. ESR spectra of DMPO-spin-trapped free radicals in the reaction mixture containing xanthine oxidase, in 57% DMSO or aqueous media. All spectra were run on a Bruker ESP 300 with the following parameters: centerfield, 348.005 mT (3480.05 G); sweep width, 8.0 mT (80 G); scan time, 10.49 s; number of scans, 10; microwave frequency, 9.74 GHz; microwave power, 20 mW; modulation amplitude, 0.68 G; gain, 3.2 × 104. The parameters of the spectra shown in the figure are as follows: DMPO/OH: aN = = 15.0 G; DMPO/OOH: aN = 14.2 G, = 11.2 G, = 1.3 G; DMPO/(C?): aN = 16.4 G, = 23.6 G. The DMPO/(C?) is an unknown carbon-centered radical, which approximately fits the parameters described by Buettner (1987) for CH3 or CH2C6H4. The reaction mixtures were as follows, with a description of the spectrum in brackets: (A) xanthine oxidase 0.1 dilution from 25 units per 1.2 ml (0.02 ml), hypoxanthine, 0.5 mM (0.28 ml), DMPO (5 Φl), DMSO 1.2 ml (superoxide), phosphate-buffered saline (0.3 ml), water (0.3 ml), final volume 2.1 ml; (B) same ingredients as A, with the addition of ethanol (0.3 ml of stout, replacing the water in A (superoxide signal strongly reduced); (C) xanthine oxidase, 0.1 dilution from 25 units per 1.2 ml (0.02 ml), hypoxanthine, 0.5 mM (0.28 ml), DMPO (5 Φl), phosphate-buffered saline (0.3 ml), water to 2.1 ml. After 7 min the superoxide radical dismutation had produced peroxide which combined with superoxide to give a typical hydroxyl radical spin-trapped spectrum; (D) same ingredients as C, with the substitution of stout (0.3 ml) for 0.3 ml of the water (hydroxyl radical signal strongly reduced from C).
Examination of data from the plasma samples taken before ingestion of the beverages showed that the baseline plasma antioxidant activities varied widely both between volunteers and in the same volunteer at different times. This finding led in turn to the use of percentage changes in plasma antioxidant activities following consumption. Nonetheless, it was of some interest that, for the one-drink group, the initial antioxidant activities in the plasma of the females were higher than in males (antioxidant activity: percent of counts destroyed by initial plasma sample): for males, 2.6 ± 6.3 μmol/mL peroxide equivalents versus, for females, 17.2 ± 2.3 μmol/mL peroxide-destroying equivalents (p < 0.03). The luminescent assay of plasma samples of the three-drink group prior to ingestion of beverages did not show a significant difference in luminescent counts between male and female participants who drank moderately. For two participants who admitted to being heavy drinkers, their plasma also showed similar pre-ingestion average counts.
To correct for the differences between individuals, counts for each individual during the period of plasma sampling were normalized to the count rate of plasma just prior to beverage ingestion. The average values at the time points were estimated by averaging the normalized values for all 12 participants in the one-drink group, or 8 participants in the three-drink group formed by excluding two heavy drinkers from the 10 volunteers who consumed three drinks. The results from antioxidant analyses of the plasma of volunteers after consumption of one drink showed that the maximum activity occurred in samples collected at 60 and 120 min. For the one-drink group, the samples taken at 4 h still exhibited antioxidant activity when compared to those taken before ingestion, except for the aqueous alcohol solution.
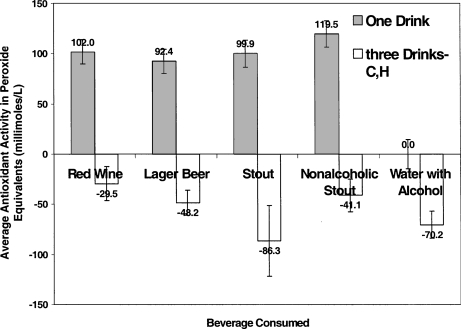
The average antioxidant capacity was calculated by dividing the areas under the curves by the duration of the experiment: except for the aqueous alcohol solution, similar average plasma antioxidant capacities were found after all beverages in the one-drink group (in hydrogen peroxide equivalents destroyed; red wine: 102 ± 12 μmol/mL; lager beer: 92.4 ∀ 12 μmol/mL; stout: 100 ± 14 μmol/mL; alcohol-free stout: 120 ± 13 μmol/mL). In contrast, the average antioxidant capacity of plasma was virtually unchanged following consumption of the aqueous alcohol solution: 0.04 ± 15 μmol/mL hydrogen peroxide equivalents destroyed. The alcohol concentrations in the plasma of volunteers at various times after beverage consumption are shown in Figure 3. Peak levels occurred 30 min postingestion of one drink, and over 30–180 min after three drinks, the aqueous solution of ethanol in water affording a peak concentration slightly higher than levels produced by the other alcohol-containing drinks, which did not differ among themselves.
FIGURE 3.
Comparison of antioxidative status of plasma after one and three drinks. Average plasma antioxidant activities as a function of time after consumption of one or three alcoholic beverages. The counts per minute were obtained with the Lumac Biocounter M2010. Hydrogen peroxide was incubated with the luminol bound to albumin, as described earlier (Figure 2). The sample consisted of an aqueous portion (0.3 mL) and a dimethyl sulfoxide portion (0.4 mL). The aqueous portion contained luminol and albumin (0.02 mL, 10 mg/mL of each) prepared as described (Trevithick et al., 1999a, 1999b), plasma (0.07 mL), phosphate-buffered saline (0.04 mL), alcohol (0.07 mL) at appropriate dilution or distilled water (for controls), and hydrogen peroxide (0.1 mL of 0.9%) added after the addition of DMSO (0.4 mL) just before the tube was placed in the counter to begin counting of the emitted light. The counts were recorded for a 10-s time period. The counts were normalized by dividing the counts/min obtained at the different times after beverage consumption by the average of six or more determinations of the count rate of the plasma taken immediately prior to beverage consumption. The graph shows plots of the average antioxidant or pro-oxidant activity (percent increase from a minimum of six normalized counts at each time, as a time-weighted average) for the different beverages consumed. The beverages consumed are described in the text: red wine, lager beer, stout, alcohol-free stout, and water containing 5% alcohol.
Surprisingly, after the ingestion of three drinks, the plasma became pro-oxidant when averaged over the complete period of assay: the assays with peroxide and plasma after beverage consumption showed higher luminescent counts on the average than those for peroxide and preconsumption plasma that were used to normalize the data. When the pro-oxidant activity was integrated over the 360-min period, all beverages showed similar time-averaged plasma pro-oxidant activities (in equivalents of peroxide—red wine: 29.5 ± 17 μmol/mL; lager beer: 48.2 ± 12.5 μmol/mL; stout: 86.3 ± 35.5 μmol/mL; alcohol-free stout: 41.1 ± 16.0 μmol/mL; solution of alcohol: 70.2 ± 13.3 μmol/mL). Although they were not statistically significantly different, the plasma pro-oxidant activities were highest after consumption of stout and aqueous alcohol. Plasma obtained after consumption of lager beer, or stout with alcohol removed, had only about half the pro-oxidant activity of plasma after stout. Ingestion of red wine resulted in the lowest plasma pro-oxidant activity, although it was not significantly different from lager beer (Figure 3).
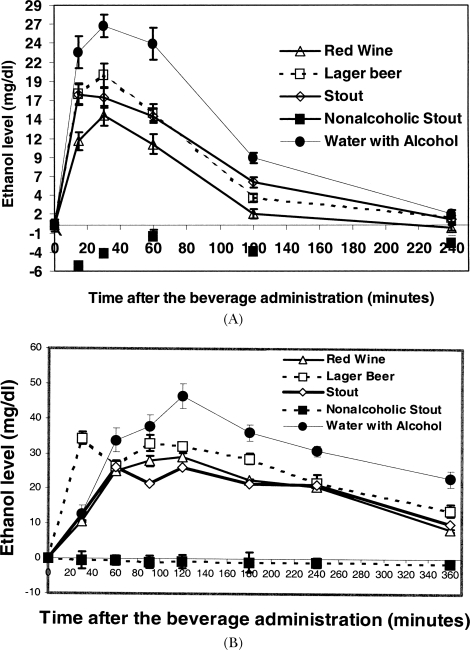
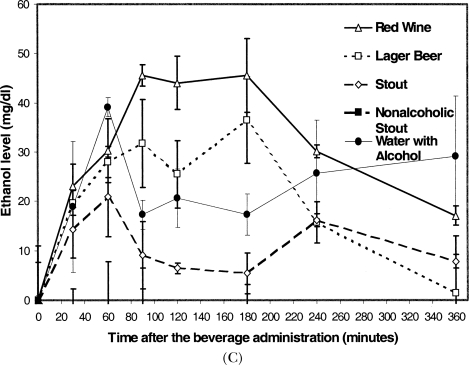
The alcohol concentrations in the plasma of volunteers at various times after beverage consumption are shown in Figure 4. After one drink, peak levels occurred 30 min postingestion, the solution of ethanol in water affording a peak concentration slightly higher than levels produced by the other alcohol-containing drinks, which did not differ among themselves (Figure 4A). For three drinks of alcoholic beverages, lager beer plasma ethanol levels rose earlier, and all remained high for longer than the one-drink study (Figure 4B). For three drinks of red wine or lager beer consumed by the heavy drinkers, plasma ethanol levels remained high after rising later than observed for the other participants and the other beverages (Figure 4C).
FIGURE 4.
Plasma alcohol concentrations after beverage consumption. Plasma samples were deproteinized using 5% trichloroacetic acid and the alcohol concentration determined using a micromodification based on a kit (Sigma Alcohol (ethanol) #333A, Mississauga). The readings in triplicate on an Elisa reader were converted to alcohol concentration and averaged. Ethanol level in blood plasma after (A) one drink, (B) three drinks, and (C) three drinks by heavy drinkers, of various beverages.
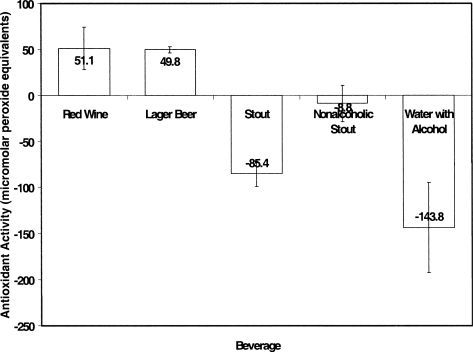
Because the two volunteers who self-identified as heavy drinkers showed delayed metabolism of alcohol for lager beer and red wine, it was of interest to compare the average plasma redox state after consumption of these beverages to the value after the other beverages for which alcohol metabolism was normal. Unlike the other participants, these two volunteers demonstrated an average antioxidant activity after consuming two beverages, lager beer and red wine (Figure 5) which were not significantly different from each other (for lager beer, 49.8 ± 3.3 μmol/mL, and for wine, 51.1 ± 23.2 μmol/mL). For the two drinks for which elevated plasma antioxidant activities were observed, the plasma ethanol concentrations of these participants were elevated to higher levels for much longer than the other beverages, suggesting delayed metabolism of the ethanol. For the main group consuming three drinks, when ethanol metabolism occurred normally, resulting in a decreased ethanol concentration in plasma after the initial 30-min peak, the plasma was pro-oxidant. Both water with alcohol and stout resulted in a rapid pro-oxidant effect on plasma, which remained strongly pro-oxidant throughout the 360-min period of monitoring.
FIGURE 5.
Average antioxidation activity(-c,h) during 6 h after consumption of bevrages by heavy drinkers. Plasma antioxidant activities as a function of time after consumption of three alcoholic beverages for two heavy drinkers. The counts per minute were obtained with the Lumac Biocounter M2010. Hydrogen peroxide was incubated with the luminol bound to albumin, as described in Figure 1. The counts were normalized by dividing the counts/min obtained at the different times after beverage consumption by the average of six or more determinations of the count rate of the plasma taken immediately prior to beverage consumption. The graph shows plots of the average pro-oxidant activity (percent increase from a minimum of six normalized counts at each time, as a time-weighted average) for the different beverages consumed. The beverages consumed are described in the text: red wine, lager beer, stout, alcohol-free stout, and water containing 5% alcohol.
As noted above, LDL plus VLDL was purified (Trevithick et al., 1999b) from combined plasma samples taken prior to any consumption and 30 min after stout ingestion from six subjects. A prolongation of the lag time was found in the postconsumption plasmas. The prolongation averaged 14.0% (SE 4.9%, p < 0.02). The average lag time for copper-induced oxidation of the lipoprotein mixture, from controls before stout consumption, was 172.8 min.
DISCUSSION
We hypothesized that the plasma would have a higher antioxidant activity following three drinks because of the increased concentration of polyphenols absorbed from the beverages (except the solution of alcohol) by volunteers. The beverages tested except for the aqueous alcohol solution all had significant antioxidant activity. In the case of stout, ESR spin-trapping confirmed that it could scavenge superoxide radical. This antioxidant activity was also found in plasma after consumption of one typical drink of these beverages. To our surprise, for the volunteers who consumed three drinks, the average plasma activities over the 6-h period studied were actually strongly pro-oxidant. Unlike the effect of one drink, in which ethanol itself had no effect on increasing the average plasma antioxidant activity, for three drinks ethanol seemed to increase the pro-oxidant activity to the same extent as the other beverages, including the alcohol-free stout. Consistent with this role for ethanol, two heavy drinkers demonstrating delayed metabolism of ethanol for two beverages showed antioxidant plasma for these beverages, although after consuming the other beverages for which ethanol metabolism was normal, their plasma was pro-oxidant.
The in vivo study of one drink shows that the consumption of any of the drinks, with the exception of the solution of alcohol, affords a rapid and sustained increase in the antioxidant potenial of plasma. Duthie’s group found that the greatest increase in antioxidant potential occurred 30 min after a drink of red wine or aged whisky, coincident with the change in the peak concentration of phenolic substances in plasma (Duthie et al., 1998). It is likely, therefore, that the changes in antioxidant potentials observed in the present study are associated with the absorption of some of the polyphenols occurring in the drinks. Consistent with this, Folts et al. (Folts et al., 1997; Folts, 2002) found that the flavonoids in red wine inhibited platelet aggregation and thrombosis (Freedman et al., 2001). Packer’s group(Kilic et al., 1998) reported reduction of cataract risk by the antioxidant ∀-lipoic acid, and we have shown that a variety of antioxidants can reduce the risk of cataracts(Creighton and Trevithick, 1979; Ross et al., 1982; Creighton et al., 1983; Kilic et al., 1999; Mitton et al., 1999).
The data presented support the hypothesis that the J-shaped risk curve for association of cataract (Clayton et al., 1982) and atherosclerosis (Klatsky, 1994) with alcoholic beverage consumption is related to the oxidative status of plasma. The present study shows that the antioxidant activity of plasma is significantly enhanced by the consumption of a single “standard” drink, be it of red wine, lager beer, or stout. For people who wish to avoid alcohol, non-alcoholic stout also provides in vivo antioxidants. Furthermore, the antioxidant contributions from a single drink are most likely related to absorption of phenolic substances, for the alcohol it contains does not have an overall effect on the antioxidant measure. The spin-trapping data indicate that alcoholic beverages (Trevithick et al., 1999b) could destroy superoxide anion, thus reducing the likelihood of hydroxyl radical formation by Haber–Weiss and Fenton reactions. Consumption of three drinks results in increased pro-oxidant activity in plasma, which appears to result from ethanol metabolism; this is consistent with the increased risk of cataract and atherosclerosis associated with this intake on a daily basis. The hormetic J-shaped curve for risk is correlated with the antioxidant activity or alternatively, the pro-oxidant state of blood plasma, suggesting a possible relationship that should be studied further. These findings again emphasize the difference between alcohol and other natural components in fermented beverages, because ethanol is a scavenger of hydroxyl radical but does not interact with superoxide anion (Trevithick et al., 1999b) as we found for stout (Figure 1). Preliminary tests of the plasma using Fox assay and thiobarbituric acid tests for oxidative metabolites are under way to see if they are consistent with these suggestions.
As part of this investigation, an allied experiment was conducted to see whether consumption of beverages might modify oxidizable plasma lipoproteins. These studies were performed using stout only and more extensive investigations are warranted. This study, in which a preconsumption sample was compared to one obtained 30 min after ingestion of stout, suggests that an increase in plasma antioxidant activity may protect the LDL + VLDL fraction of plasma from oxidation. This finding is of particular interest because the oxidized LDL has been shown to be taken up preferentially by foam cells and may play an important role in the pathogenesis of atherosclerosis(Sacco et al., 1999; Trevithick et al., 1999b).
The consumption of three drinks of alcoholic beverages, including alcohol itself, by normal volunteers resulted in the plasma becoming pro-oxidant for a significant period of time (6 h) after beverage ingestion. Three beverages showed similar pro-oxidant activities: beer, red wine, and alcohol-free stout, whereas the alcohol solution and regular stout were more pro-oxidant, although the differences were not statistically significant. The fact that alcohol itself resulted in pro-oxidant plasma supports the idea that alcohol metabolism is responsible for the generation of oxidative species. This cannot be the only source of reactive oxygen species, because alcohol-free stout also resulted in pro-oxidant plasma. In fact, the addition of the pro-oxidant activities of the alcohol and the alcohol-free stout combined gave a pro-oxidant plasma activity approximately equal to that of the alcoholic stout. This is consistent with the suggestion from Halliwell’s laboratory that polyphenols can result in the production of peroxide in biological fluids (Long et al., 1999; Evans and Halliwell, 2001; Chai et al., 2003; Halliwell, 2003).
Averaging the antioxidant potential across the experimental period indicates that there are substantial similarities in antioxidant contributions arising from the different fermented beverages used in the study. It is apparent that a glass of red wine affords the same change in antioxidant potential as that produced by a bottle of stout or lager beer. Because all of the drinks with alcohol contained the same quantity of alcohol, and the diluted solution of alcohol itself caused minimal overall changes in the antioxidant potential in plasma, it is unlikely that the alcohol content of a “standard” drink, 13.4 g, has any meaningful effect on this measure. Indeed, the drink of alcohol-free stout proved to be a highly effective contributor to plasma antioxidant potential, underlining the importance of constituents in the drinks other than ethanol (Trevithick et al., 1999b). The similarities in effect between the fermented drinks suggest that there may well be differences in absorption, metabolism, and excretion of the various phenolic substances they contain. Red wine contains catechins, procyanidins, and anthocyanins (Vinson et al., 2001), whereas beer is endowed with ferulic acid, gallic acid, caffeic acid, catechin (Bourne et al., 2000), and so on. These may well have different bioavailabilities as well as differing intrinsic antioxidant properties. Vinson suggested that beer and wine show similar protective effects in an animal model of atherosclerosis, even though wine contains approximately ten-fold higher concentrations of polyphenols (Vinson et al., 2003). The interactions between pharmacokinetic factors and antioxidant potentials may explain why the alcohol-free stout was so able to elevate the plasma antioxidant potential even after 4 h. Perhaps the partially oxidized products arising from removal of ethanol were better able to be absorbed and were less affected by first-pass metabolism than phenolic ingredients in the parent stout. Although, in the group of eight normal volunteers consuming three drinks, red wine has a profile indicating rapid alcohol metabolism, its polyphenols (mainly resveratrol) may be better able to destroy the reactive oxygen species, resulting in the observed lower average pro-oxidant activity in plasma, perhaps because of a higher concentration in Folin phenol equivalents in the wine, and/or because of the greater intrinsic activity of resveratrol as an antioxidant.
The present study has examined, in vitro and in vivo, the antioxidant potentials inherent in various beverages—red wine, lager beer, stout, and a control alcohol solution—using a luminescent assay. The in vitro data show that the undiluted drinks are able, with the exception of alcohol itself, to react with hydrogen peroxide quite effectively—indeed to similar degrees to that reported for martinis (Trevithick et al., 1999a). Moreover, appreciable dilutions of the drinks are still able to neutralize some measure of the hydrogen peroxide used in the assay: red wine, with the highest concentration of catechin-like polyphenols (Vinson et al., 2001), was substantially more potent in this regard than any of the other drinks. As expected, the stout proved to contain a higher concentration of phenolic components than the lager beer. Of some interest, the phenolic content of the alcohol-free stout used in the study was less than a third that of the parent beverage. This may well reflect some oxidation of the phenols during drying and reconstitution.
Acknowledgments
We thank Labatt Brewing Co Ltd., London, Ontario, Canada and Guinness Ltd for funding and for supplying the lager beer, stout, and nonalcoholic stout consumed by the volunteers. Colleen Trevithick performed preliminary experiments on the antioxidant activity of beers, and with John Trevithick, and Alexandra and Mahela Stefan, performed the ESR spin-trapping experiments. Michelle Chartrand, Judit Wahlman, and Claire Prickett performed the Folin Ciocalteau and ethanol assays on the beverages. Judit Wahlman, Erin Lister, Lai Dinh, Michelle Collins, and Dat Le performed the luminescent assay of antioxidant activity. Erin Lister performed most of the experiments on the antioxidant activity of human plasma, and Judit Wahlman and Claire Prickett coordinated the data entry and statistical analysis. Jan Brindley, assisted by Marilyn Webb and Louise Buchanan, performed the venipuncture, organized the beverage preparation, and prepared the plasma. Maurice Hirst, Earl Noble, and John Trevithick supervised the recruitment of volunteers, the study, data analysis, and participated in the writing of this report. Joe Vinson and John Proch performed the copper-catalyzed LDL lag-time measurements.
REFERENCES
- Albano E, French SW, Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci. 1999;4:D533–D540. doi: 10.2741/albano. [DOI] [PubMed] [Google Scholar]
- Bourne L, Paganga G, Baxter D, Hughes P, Rice-Evans C. Absorption of ferulic acid from low-alcohol beer. Free Radic Res. 2000;32:273–280. doi: 10.1080/10715760000300281. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis as a biological hypothesis. Environ. Health Persp. 1998;106(Suppl 1):357–362. doi: 10.1289/ehp.98106s1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. U-shaped dose-responses in biology, toxicology, and public health. Annu Rev Publ Health. 2001;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, McCarthy ME, Kenyon E. The occurrence of chemically induced hormesis. Health Phys. 1987;52:531–541. doi: 10.1097/00004032-198705000-00002. [DOI] [PubMed] [Google Scholar]
- Chai PC, Long LH, Halliwell B. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem Biophys Res Commun. 2003;304:650–654. doi: 10.1016/s0006-291x(03)00655-7. [DOI] [PubMed] [Google Scholar]
- Clayton RM, Cuthbert J, Duffy J, Seth J, Phillips CI, Bartholomew RS, Reid JM.Some risk factors associated with cataract in S.E. Scotland: A pilot study Trans Ophthal Soc UK 1982102(Pt 3):331–336. [PubMed] [Google Scholar]
- Creighton MO, Trevithick JR. Cortical cataract formation prevented by vitamin E and glutathione. Exp Eye Res. 1979;29:689–693. doi: 10.1016/0014-4835(79)90025-3. [DOI] [PubMed] [Google Scholar]
- Creighton MO, Sanwal M, Stewart-DeHaan PJ, Trevithick JR. Modeling cortical cataractogenesis. V. Steroid cataracts induced by solumedrol partially prevented by vitamin E in vitro. Exp Eye Res. 1983;37:65–76. doi: 10.1016/0014-4835(83)90150-1. [DOI] [PubMed] [Google Scholar]
- Duthie GG, Pedersen MW, Gardner PT, Morrice PC, Jenkinson AM, McPhail DB, Steele GM. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur J Clin Nutr. 1998;52:733–736. doi: 10.1038/sj.ejcn.1600635. [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Micronutrients: Oxidant/antioxidant status. Brit J Nutr. 2001;85(Suppl 2):S67–S74. [PubMed] [Google Scholar]
- Folts JD. Potential health benefits from the flavonoids in grape products on vascular disease. Adv Exp Med Biol. 2002;505:95–111. doi: 10.1007/978-1-4757-5235-9_9. [DOI] [PubMed] [Google Scholar]
- Folts JD, Begolli B, Shanmuganayagam D, Osman H, Maalej N. Inhibition of platelet activity with red wine and grape products. Biofactors. 1997;6:411–414. doi: 10.1002/biof.5520060407. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Parker C, III, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress in cell culture: An underappreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- Katoh N, Jonasson F, Sasaki H, Kojima M, Ono M, Takahashi N, Sasaki K. Cortical lens opacification in Iceland. Risk factor analysis—Reykjavik Eye Study. Acta Ophthal Scand. 2001;79:154–159. doi: 10.1034/j.1600-0420.2001.079002154.x. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Egger G, Oberhollenzer M, Aichner F. Alcohol consumption and carotid atherosclerosis: Evidence of dose-dependent atherogenic and antiatherogenic effects. Results from the Bruneck Study. Stroke. 1994;25:1593–1598. doi: 10.1161/01.str.25.8.1593. [DOI] [PubMed] [Google Scholar]
- Kilic F, Trevithick JR. Modelling cortical cataractogenesis. XXIX. Calpain proteolysis of lens fodrin in cataract. Biochem Mol Biol Int. 1998;45:963–978. doi: 10.1002/iub.7510450514. [DOI] [PubMed] [Google Scholar]
- Kilic F, Handelman GJ, Traber K, Tsang K, Packer L, Trevithick JR. Modelling cortical cataractogenesis XX. In vitro effect of alpha-lipoic acid on glutathione concentrations in lens in model diabetic cataractogenesis. Biochem Mol Biol Int. 1998;46:585–595. doi: 10.1080/15216549800204112. [DOI] [PubMed] [Google Scholar]
- Kilic F, Bhardwaj R, Caulfeild J, Trevithick JR. Modelling cortical cataractogenesis 22: Is in vitro reduction of damage in model diabetic rat cataract by taurine due to its antioxidant activity? Exp Eye Res. 1999;69:291–300. doi: 10.1006/exer.1999.0697. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Epidemiology of coronary heart disease—Influence of alcohol. Alcohol Clin Exp Res. 1994;18:88–96. doi: 10.1111/j.1530-0277.1994.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Tomany SC, Moss SE. Ten-year incidence of age-related maculopathy and smoking and drinking: The Beaver Dam Eye Study. Am J Epidemiol. 2002;156:589–598. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- Long LH, Lan AN, Hsuan FT, Halliwell B. Generation of hydrogen peroxide by “antioxidant” beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic Res. 1999;31:67–71. doi: 10.1080/10715769900300611. [DOI] [PubMed] [Google Scholar]
- McKim SE, Konno A, Gabele E, Uesugi T, Froh M, Sies H, Thurman RG, Arteel GE. Cocoa extract protects against early alcohol-induced liver injury in the rat. Arch Biochem Biophys. 2002;406:40–46. doi: 10.1016/s0003-9861(02)00425-3. [DOI] [PubMed] [Google Scholar]
- Mitton KP, Linklater HA, Dzialoszynski T, Sanford SE, Starkey K, Trevithick JR. Modelling cortical cataractogenesis 21: In diabetic rat lenses taurine supplementation partially reduces damage resulting from osmotic compensation leading to osmolyte loss and antioxidant depletion. Exp Eye Res. 1999;69:279–289. doi: 10.1006/exer.1999.0696. [DOI] [PubMed] [Google Scholar]
- Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res. 1999;30:153–162. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- Ritter LL, Klein BE, Klein R, Mares-Perlman JA. Alcohol use and lens opacities in the Beaver Dam Eye Study. Arch Ophthal. 1993;111:113–117. doi: 10.1001/archopht.1993.01090010117037. [DOI] [PubMed] [Google Scholar]
- Robertson JD, Orrenius S. Role of mitochondria in toxic cell death. Toxicology. 2002:181–182. 491–496. doi: 10.1016/s0300-483x(02)00464-x. [DOI] [PubMed] [Google Scholar]
- Ross WM, Creighton MO, Stewart-DeHaan PJ, Sanwal M, Hirst M, Trevithick JR. Modelling cortical cataractogenesis: 3. In vivo effects of vitamin E on cataractogenesis in diabetic rats. Can J Ophthal. 1982;17:61–66. [PubMed] [Google Scholar]
- Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. J Am Med Assoc. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- Tenkova T, Young C, Dikranian K, Labruyere J, Olney JW. Ethanol-induced apoptosis in the developing visual system during synaptogenesis. Invest Ophthal Vis Sci. 2003;44:2809–2817. doi: 10.1167/iovs.02-0982. [DOI] [PubMed] [Google Scholar]
- Trevithick JR, Dzialoszynski T. A new technique for enhancing luminol luminescent detection of free radicals and reactive oxygen species. Biochem Mol Biol Int. 1994;33:1179–1190. [PubMed] [Google Scholar]
- Trevithick CC, Chartrand MM, Wahlman J, Rahman F, Hirst M, Trevithick JR. Shaken, not stirred: Bioanalytical study of the antioxidant activities of martinis. Brit Med J. 1999a;319:1600–1602. doi: 10.1136/bmj.319.7225.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick CC, Vinson JA, Caulfeild J, Rahman F, Derksen T, Bocksch L, Hong S, Stefan A, Teufel K, Wu N, Hirst M, Trevithick JR. Is ethanol an important antioxidant in alcoholic beverages associated with risk reduction of cataract and atherosclerosis? Redox Rep. 1999b;4:89–93. doi: 10.1179/135100099101534765. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis. 2001;156:67–72. doi: 10.1016/s0021-9150(00)00625-0. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Mandarano M, Hirst M, Trevithick JR, Bose P. Phenol antioxidant quantity and quality in foods: Beers and the effect of two types of beer on an animal model of atherosclerosis. J Agric Food Chem. 2003;51:5528–5533. doi: 10.1021/jf034189k. [DOI] [PubMed] [Google Scholar]