Abstract
Dehydroepiandrosterone sulfate (DHEAS) is a steroid hornone that is synthesized, de novo, in the brain. Endogenous DHEAS levels correlate with the quality of mental and physical health, where the highest levels of DHEAS occur in healthy young adults and reduced levels of DHEAS are found with advanced age, disease, or extreme stress. DHEAS supplementation, therefore, may serve as a therapeutic agent against a broad range of maladies. This paper summarizes laboratory findings on dose-response relationships between DHEAS and cognitive and electrophysiological measures of hippocampal functioning. It was found that a low, but not a high, dose of DHEAS enhanced hippocampal primed burst potentiation (a physiological model of memory) as well as spatial (hippocampal-dependent) memory in rats. This complex dose-response function of DHEAS effects on the brain and memory may contribute toward the inconsistent findings that have been obtained by other investigators in studies on DHEAS administration in people.
Keywords: neurosteroid, dehydroepiandrosterone, DHEA, hippocampus, long-term potentiation, memory
INTRODUCTION
Dehydroepiandrosterone sulfate (DHEAS) is in a category of hormones referred to as “neurosteroids” because they are synthesized, de novo, in the nervous system as well as in the periphery (Baulieu, 1998; Baulieu et al., 2001). DHEAS is the most abundant adrenal steroid found in young healthy adult primates, but its concentrations decline dramatically with advanced age and disease (Parker and Schuster, 1991; Ferrari et al., 2001). For example, very old primates, including rhesus monkeys and people, have only about 10% of the levels of DHEAS normally found in young adults (Lane et al., 1997; Mattison et al., 2003). Moreover, a great reduction of DHEAS concentration is found under conditions of extreme physical stress, such as with severe burns (Dolecek, 1989; Araneo et al., 1993). These findings of diminished DHEAS levels consistently occurring under adverse health conditions have led to the suggestion that supplementation with DHEAS may serve a preventive role in ameliorating the adverse symptoms of aging, disease, and stress. DHEAS administration, therefore, has been evaluated as a potential therapeutic agent in the treatment of a broad range of maladies, including AIDS (Clerici et al., 1997; Araghi-Niknam et al., 1998), Alzheimer’s disease (Wolkowitz et al., 2003), and depression (Reus et al., 1997; van Broekhoven and Verkes, 2003). For these reasons, there is a large market for DHEAS, which is sold without a prescription as a dietary supplement. However, whereas the literature on the evidence of low endogenous DHEAS levels in various disease states is extensive, the evidence that DHEAS supplementation is of therapeutic value is inconsistent (Nippoldt and Nair, 1998; Wolf and Kirschbaum, 1999; Kuiper-Geertsma and Derksen, 2003). The purpose of this paper is to review and summarize work from my laboratory on the effects of DHEAS supplementation on memory and the brain in rats. Specifically, we have investigated the effects of DHEAS administration on cognitive and electrophysiological aspects of the functioning of the hippocampus, a brain structure that is important for learning and memory processes (Eichenbaum, 2001; Burgess et al., 2002; Sanders et al., 2003). The findings may prove to be relevant to the inconsistent effects of DHEAS on mental and physical health in people.
EFFECTS OF DHEAS ON HIPPOCAMPAL SYNAPTIC PLASTICITY
There is extensive evidence to indicate that DHEAS has potent modulatory effects on the nervous system. DHEAS increases synaptic excitability by reducing GABAA-mediated inhibition (Baulieu, 1998; Meyer et al., 1999; Park-Chung et al., 1999). This increase in excitability is particularly important because neurophysiological models of memory have shown that a reduction of GABAergic inhibition can enhance the physiological processes that underlie memory (Chapman et al., 1998; Grover and Yan, 1999; Levkovitz et al., 1999). Moreover, NMDA receptor activation is a critical component of the physiology of memory (Nicoll and Malenka, 1999; Huang and Hsu, 2001). Here again, DHEAS exerts an effect that is consistent with its role as a memory facilitator (Roberts et al., 1987; Flood and Roberts, 1988; Flood et al., 1988; Vallee et al., 2001). DHEAS enhances NMDA channel activity and increases the number of NMDA receptors in the hippocampus (Bergeron et al., 1996; Baulieu, 1998; Wen et al., 2001). Finally, there is evidence of neurogenesis in the adult hippocampus across a broad range of species, including rats and people, and the neurogenesis process appears to be important for new learning in adults (Gould et al., 2000; Seri et al., 2001; Shors et al., 2001). DHEAS appears to facilitate this process by stimulating additional neurogenesis and promoting the survival of newly formed cells (Karishma and Herbert, 2002). Taken together, there is strong evidence that DHEAS supplementation exerts a profound facilitating effect on hippocampal functioning in the adult.
On this background of evidence of DHEAS as a positive modulator of hippocampal functioning, my colleagues and I studied the effects of DHEAS administration on an electrophysiological model of memory, which is referred to as primed burst (PB) potentiation (Rose and Dunwiddie, 1986; Diamond et al., 1988). The idea in this area of research is that electrical stimulation of the hippocampus mimics the changes in synapses that occur during memory formation. Thus, a DHEAS-induced modulation of PB potentiation may provide insight into how DHEAS affects learning and memory.
In recordings from adult male Sprague Dawley (Charles Rivers) rats, we found a U-shaped dose-response function between DHEAS and PB potentiation. That is, whereas intermediate doses (24 and 48 mg/kg) of DHEAS given intraperitoneally increased both the magnitude and incidence of PB potentiation, the highest (96 mg/kg) and lowest (6 mg/kg) doses had no effect (Diamond et al., 1996). This work provided the first evidence of nonlinear dose-response characteristics of DHEAS actions on hippocampal synaptic plasticity.
EFFECTS OF CHRONIC ORAL ADMINISTRATION OF DHEAS ON MEMORY AND HIPPOCAMPAL PLASTICITY
In the next phase of the research, we administered DHEAS chronically to rats in their drinking water and then tested their spatial learning and memory in the Morris water maze. In this task, rats learn to escape from water immersion by learning and then remembering the location of a fixed platform, which is hidden just beneath the surface of the water (Brandeis et al., 1989). Rats learn spatial information rapidly and are highly motivated to escape from the water. Thus, a reduction in the time to escape across training trials indicates that a rat has the motivation to escape the water and has learned the location of the hidden platform.
Rats were given the vehicle (lemonade-flavored water) or one of two doses of DHEAS mixed in the their drinking water (100 or 400 μg/100 ml) for 1 week prior to water maze training. The rats were given five training trials on day 1 in which they had up to 2 min/trial to learn the location of the hidden platform. On the second day of training all rats were given a single memory test trial. Rats that remembered the platform location took less time to find the platform than rats that had impaired memory of the platform location.
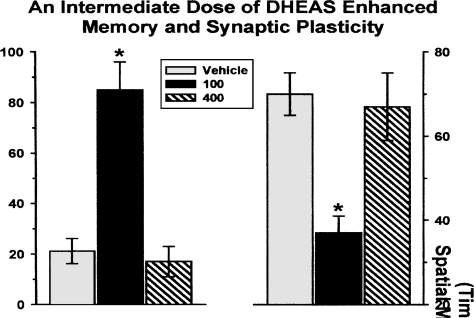
On the first day of training, all three groups reduced their time to find the hidden platform equally over the course of the five training trials, indicating that they had equivalent learning abilities and that they were all motivated to escape from the water. A difference in performance was observed on the 24-h memory test trial. As can be seen from the right-hand side of Figure 1, rats given 100 μg/100 ml of DHEAS-treated water, but not those given 400 μg/100 ml, were superior (that is, had lower escape latencies) to rats treated with the vehicle or 400 μg/100 ml of DHEAS. Thus, the intermediate dose of DHEAS produced superior spatial memory performance compared to the other two groups (Diamond and Fleshner, 1999).
FIGURE 1.
A lower, but not a higher, dose of DHEAS enhanced spatial memory and hippocampal PB potentiation. Rats administered 100 μg/100 ml of DHEAS in their drinking water exhibited superior spatial memory (i.e., less time to swim to the hidden platform) to the control (vehicle) and the high-dose (400 μg/100 ml) groups (right-hand side). The group of rats with superior memory also exhibited significantly greater PB potentiation (left-hand side) than the controls and the group treated with 400 μg/ 100 ml. Overall, the group chronically treated with 100 μg/100 ml of DHEAS in their drinking water exhibited superior memory and greater hippocampal plasticity.
What might be the basis of the DHEAS-induced enhancement of memory? To address this question, we recorded PB potentiation in the same animals that had been trained in the water maze. Once water maze training had been completed, the rats continued to receive DHEAS in their drinking water until the day in which the electrophysiological recordings took place. On that day, the rats were anesthetized with urethane (1.5 g/kg, ip) and PB was recorded in the CA1 region of the hippocampus according to methods described previously (Bennett et al., 1991; Diamond et al., 1992, 1996, 1999). The left-hand side of Figure 1 shows the magnitude of PB in the vehicle- and DHEAS-treated groups. The dose of DHEAS that enhanced spatial memory (l00 μg/100 ml) also increased the magnitude of PB potentiation. The magnitude of PB potentiation was relatively small in the control and high-dose groups (21 and 18% increases, respectively) and significantly greater in the group that received the intermediate dose (Diamond and Fleshner, 1999). Thus, the enhanced memory observed in the rats that received the 100 μg/100 ml dose may have resulted from the enhanced expression of hippocampal synaptic plasticity (PB potentiation) occurring selectively at this dose.
SUMMARY
I have presented findings on the complex dose-response functions of DHEAS on cognitive and electrophysiological aspects of hippocampal functioning in rats. My group reported that there is an inverted U-shaped relationship between acute administration of DHEAS and the magnitude of synaptic plasticity (PB potentiation) in the CA1 region of the hippocampus. In addition, chronic oral administration of DHEAS produced a complex dose-response function, in which the lower dose of DHEAS enhanced both memory and PB potentiation and the higher dose had no effect on either measure. These findings of complex nonlinear dose-response functions between DHEAS and hippocampal functioning may be relevant to the inconsistent effects of DHEAS administration on brain, behavior, and pathological conditions in people.
Footnotes
The work described here was accomplished with the assistance of Berrilyn Branch and Greg Rose. The research was funded by grants to the author from the Office of Naval Research and the Veterans Administration.
REFERENCES
- Araghi-Niknam M, Ardestani SK, Molitor M, Inserra P, Eskelson CD, Watson RR. Dehydroepiandrosterone (DHEA) sulfate prevents reduction in tissue vitamin E and increased lipid peroxidation due to murine retrovirus infection of aged mice. Proc Soc Exp Biol Med. 1998;218:210–217. doi: 10.3181/00379727-218-44288. [DOI] [PubMed] [Google Scholar]
- Araneo BA, Shelby J, Li GZ, Ku W, Daynes RA. Administration of dehydroepiandrosterone to burned mice preserves normal immunologic competence. Arch Surg. 1993;128:318–325. doi: 10.1001/archsurg.1993.01420150074014. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: A novel function of the brain. Psychoneuroendocrinol. 1998;I23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: Beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Bennett MC, Diamond DM, Fleshner M, Rose GM. Serum corticosterone level predicts the magnitude of hippocampal primed burst potentiation and depression in urethane-anesthetized rats. Psychobiol. 1991;19:301–307. [Google Scholar]
- Bergeron R, De Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Perez Y, Lacaille JC. Effects of OABA(A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8:289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Clerici M, Trabattoni D, Piconi S, Fusi ML, Ruzzante S, Clerici C, Villa ML. A possible role for the cortisol/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinol. 1997;22(Suppl1):S27–S31. doi: 10.1016/s0306-4530(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M. Constraints on the DHEAS-induced enhancement of hippocampal function: Non-linear dose-response functions and stress-DHEAS interactions. Kalimi M, Regelson W, editors. Dehydroepicandrosterone (DHEA): Biochemical, Physiological and Clinical Aspects. 1999;II:261–270. [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose OM. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose OM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Branch BJ, Fleshner M. The neurosteroid dehydroepiandrosterone sulfate (DHEAS) enhances hippocampal primed burst, but not long-term, potentiation. Neurosci Lett. 1996;202:204–208. doi: 10.1016/0304-3940(95)12233-8. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Rose OM. The enhancement of hippocampal primed burst potentiation by dehydroepiandrosterone sulfate (DHEAS) is blocked by psychological stress. Stress. 1999;3:107–121. doi: 10.3109/10253899909001116. [DOI] [PubMed] [Google Scholar]
- Dolecek R. Endocrine changes after burn trauma—A review. Keio J Med. 1989;38:262–276. doi: 10.2302/kjm.38.262. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, Fioravanti M, Cuzzoni G, Pontiggia B, Magri F. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. Eur J Endocrinol. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- Flood IF, Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- Flood IF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Grover LM, Yan C. Blockade of GABAA receptors facilitates induction of NMDA receptor-independent long-term potentiation. J Neurophysiol. 1999;81:2814–2822. doi: 10.1152/jn.1999.81.6.2814. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long- term potentiation. Rev Neurosci. 2001;12:51–68. doi: 10.1515/revneuro.2001.12.1.51. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kuiper-Geertsma DG, Derksen RH. Newer drugs for the treatment of lupus nephritis. Drugs. 2003;63:167–180. doi: 10.2165/00003495-200363020-00004. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: A biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Avignone E, Groner Y, Segal M. Upregulation of GABA neurotransmission suppresses hippocampal excitability and prevents long-term potentiation in transgenic superoxide dismutaseoverexpressing mice. J Neurosci. 1999;19:10977–10984. doi: 10.1523/JNEUROSCI.19-24-10977.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Lee S, Wittenberg GF, Randall RD, Gruol DL. Neurosteroid regulation of inhibitory synaptic transmission in the rat hippocampus in vitro. Neuroscience. 1999;90:1177–1183. doi: 10.1016/s0306-4522(98)00543-0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann NY Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Nippoldt TB, Nair KS. Is there a case for DHEA replacement? Bailliere Clin Endocrinol Metab. 1998;12:507–520. doi: 10.1016/s0950-351x(98)80286-3. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acid A receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Schuster MW. Effects of syphilis infection on adrenocortical function in men. Proc Soc Exp Biol Med. 1991;197:165–167. doi: 10.3181/00379727-197-43239. [DOI] [PubMed] [Google Scholar]
- Reus VI, Wolkowitz OM, Frederick S. Antiglucocorticoid treatments in psychiatry. Psychoneuroendocrinol. 1997;22(Suppl 1):S121–S124. doi: 10.1016/s0306-4530(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Roberts E, Bologa L, Flood IF, Smith GE. Effects of dehydroepiandrosterone and its sulfate on brain tissue in culture and on memory in mice. Brain Res. 1987;406:357–362. doi: 10.1016/0006-8993(87)90807-9. [DOI] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BI, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TI, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Rev. 2001;37:301–312. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- van Broekhoven F, Verkes RJ.Neurosteroids in depression: A review Psychopharmacol (Berl). 200316597–110. [DOI] [PubMed] [Google Scholar]
- Wen S, Dong K, Onolfo JP, Vincens M. Treatment with dehydroepiandrosterone sulfate increases NMDA receptors in hippocampus and cortex. Eur J Pharmacol. 2001;430:373–374. doi: 10.1016/s0014-2999(01)01383-8. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of del dehydroepiandrosterone and its sulfate in the central nervous system: Effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, De Souza E, Sadowsky C, Roberts E. DHEA treatment of Alzheimer’s disease: A randomized, double-blind, placebo-controlled study. Neurology. 2003;60:1071–1076. doi: 10.1212/01.wnl.0000052994.54660.58. [DOI] [PubMed] [Google Scholar]