Abstract
A multistage cancer model that describes the putative rate-limiting steps in carcinogenesis is developed and used to investigate the potential impact on cumulative lung cancer incidence of the hormesis mechanisms suggested by Feinendegen and Pollycove. In the model, radiation and endogenous processes damage the DNA of target cells in the lung. Some fraction of the misrepaired or unrepaired DNA damage induces genomic instability and, ultimately, leads to the accumulation of malignant cells. The model explicitly accounts for cell birth and death processes, the clonal expansion of initiated cells, malignant conversion, and a lag period for tumor formation. Radioprotective mechanisms are incorporated into the model by postulating dose and dose-rate-dependent radical scavenging. The accuracy of DNA damage repair also depends on dose and dose rate. As currently formulated, the model is most applicable to low-linear-energy-transfer (LET) radiation delivered at low dose rates. Sensitivity studies are conducted to identify critical model inputs and to help define the shapes of the cumulative lung cancer incidence curves that may arise when dose and dose-rate-dependent cellular defense mechanisms are incorporated into a multistage cancer model. For lung cancer, both linear no-threshold (LNT-), and non-LNT-shaped responses can be obtained. If experiments demonstrate that the effects of DNA damage repair and radical scavenging are enhanced at least three-fold under low-dose conditions, our studies would support the existence of U-shaped responses. The overall fidelity of the DNA damage repair process may have a large impact on the cumulative incidence of lung cancer. The reported studies also highlight the need to know whether or not (or to what extent) multiply damaged DNA sites are formed by endogenous processes. Model inputs that give rise to U-shaped responses are consistent with an effective cumulative lung cancer incidence threshold that may be as high as 300 mGy (4 mGy per year for 75 years) for low-LET radiation.
Keywords: radioprotective mechanisms, LNT, U-shaped, threshold, hormesis, endogenous damage
INTRODUCTION
The biological significance of hormesis—the stimulating effect of sub-inhibitory concentrations of any toxic substance on any organism (Dorland, 1974)—is highly controversial in the radiation research community and is the subject of numerous publications. Becker (2002) criticized the conclusion of Report No. 136 of the National Council on Radiation Protection and Measurements (NCRP, 2001) that the linear no-threshold (LNT) model was valid for characterizing low-dose radiation risks. Manifold review articles about chemical hormesis have been published (Calabrese and Baldwin, 2000, 2001a, 2001b, 2001c). However, strong criticism of radiation hormesis and threshold concepts have also been voiced (UNSCEAR 1993, 1994, 2000; Heitzmann and Wilson, 1997; BEIR VI, 1999; Mossman, 2001). The evidence for and against hormesis has been summarized in several recent articles in Science (Kaiser, 2003a, 2003b) and Scientific American (Renner, 2003).
Feinendegen et al. (1987, 1988) and Bond et al. (1987) provided several explanations for the possible existence of radiation hormesis phenomena. They stated that, in the low-dose region, radiation exerts protection against other challenges involving radicals and thus causes a net beneficial effect by temporarily shielding the hit cell against radicals produced through endogenous processes (Feinendegen et al., 1987). Calabrese and Baldwin (2001d) suggest that hormetic effects represent evolutionary-based adaptive responses to environmentally induced disruptions in homeostasis. In contrast to these views, Crump et al. (1976) and Guess et al. (1977) pointed out that many classes of cancer model predict that an incremental increase in dose or dose rate will produce an incremental increase in the incidence of cancer. They argue that, even if a biological event has a threshold or is nonlinear, the existence of background cancers shows that this threshold has already been exceeded because of the presence of pollutants in the environment. These sentiments are echoed by Slob (1999). However, these ideas are premised on the belief that the relevant biological mechanisms are invariant for the doses and dose rates of interest, and evidence casting doubt on invariance of biological mechanisms with dose and dose rate is accumulating.
Fleck et al. (1999) reviewed approaches to incorporate hormesis effects into mathematical models for various in vivo and in vitro endpoints and presented an approach to incorporate inducible repair and antioxidation into an Armitage–Doll-type cancer model. Schöllnberger et al. (1999, 2001c, 2002a, 2002b) demonstrated that cellular defence mechanisms can be incorporated into multistage models for neoplastic transformation and used to explain nonlinear biological responses. Some studies have also been performed to explain hormetic effects with mechanism-based cancer models (Bogen, 1997, 1998, 2001; Andersen and Conolly, 1998; Downs and Frankowski, 1998; Schöllnberger et al., 2001a; Radivoyevitch et al., 2002).
Although uncertainties continue to surround the significance to tumorigenesis of adaptive responses to DNA damage (UNSCEAR, 2000), we are convinced that sufficient evidence has accumulated to warrant the development of cancer models that include adaptive responses. These models can then be used to help identify the conditions necessary to produce nonlinear responses. For example, how big does a change in a cell’s repair or radical scavenging capacity need to be to produce significant deviations from an LNT type of response?
A central aim of this paper is to develop methods of including adaptations in DNA repair and radical scavenging into multistage cancer models. Toward this end, we have formulated a deterministic multistage cancer model that incorporates some of the cellular defense mechanisms proposed by Pollycove and Feinendegen (2001). Multistage cancer models (e.g., Moolgavkar and Knudson, 1981; Leenhouts, 1999) and state-vector models (Scott, 1977; Scott and Ainsworth, 1980; Crawford-Brown and Hofmann, 1990; Schöllnberger et al., 2001b) embody many of the key rate-limiting steps that are believed to be involved in the pathogenesis of cancer. The second goal of this paper is to use the proposed cancer model to investigate the potential impact on lung cancer of radiologically induced changes in a cell’s capacity for radical scavenging and DNA repair. The results of these studies are intended to provide quantitative predictions that can contribute to the ongoing debate about the existence and potential impact of radioprotective mechanisms under low dose and dose-rate exposure conditions. The model is also used to examine the relative contributions that endogenous processes and ionizing radiation may have on the cumulative incidence of cancer.
STUDIES SUPPORTING OR REFUTING TOXICANT-INDUCED ADAPTATIONS IN RADICAL SCAVENGING AND DNA REPAIR
Cells often display an adaptive response to fractionated doses of chemical and/or radiological agents (Raaphorst and Boyden, 1999; Ye et al., 1999; Marples and Joiner, 2000). Transient up-regulation of one or more of the repair pathways involved in the processing of multiply damaged sites [including double-strand breaks (DSBs)] provide one plausible explanation for the observed adaptations in cellular responses (reviewed by Joiner et al., 2001). In vivo experiments have shown that low doses of radiation help protect against radiation-induced myeloid leukemia (Mitchel et al., 1999) and spontaneous cancer in mice (Mitchel et al., 2003), and these phenomena may also be related to adaptations in DNA repair processes.
Le et al. (1998) demonstrated that an exposure of A549 cells (a human lung carcinoma cell line) to a dose of 0.25 Gy 4 h before a dose of 2 Gy enhanced removal of thymine glycols after the higher dose. These data provide evidence for an inducible repair response for radiation-induced damage to DNA bases. Ye et al. (1999) found that the kinetics of nucleotide excision repair (NER) in human cells is transiently enhanced when a chronic dose of quinacrine mustard preceded a dose of ultraviolet radiation. Wolff (1995) showed that human lymphocytes exposed to low doses of ionizing radiation (IR) are less susceptible to the induction of cytogenetic damage (chromatid aberrations) by subsequent high doses of X-rays. This adaptive response to IR, which occurs after exposures that are so low (0.005–0.01 Gy) that they do not induce discernible aberrations themselves, has been attributed to the induction of a repair mechanism that causes the restitution of X-ray-induced chromosome breaks (Wolff, 1998). Variations and the absence of adaptive responses have also been reported (Jacobson-Kram and Williams, 1988; Bauchinger et al., 1989; Bosi and Olivieri, 1989; Sankaranarayanan et al., 1989; Schmid et al., 1989; Hain et al., 1992; Müller et al., 1992; Wojcik et al., 1992, 1993; Shadley, 1994; UNSCEAR, 1994; Vijayalaxmi et al., 1995; Boothman et al., 1996; Raaphorst and Boyden, 1999; Sorensen et al., 2002).
Azzam et al. (1996) exposed quiescent mouse fibroblasts (C3H 10T1/2) cells to doses of 1–100 mGy -rays and observed that the risk of neoplastic transformation was reduced from the spontaneous level to a rate three- to four-fold below that level. They argued that these results demonstrate that low or chronic exposure to radiation can induce processes that protect the cell against naturally occurring as well as radiation-induced alterations leading to cell transformation. Other data show a reduced TF/SC in C3H 10T1/2 cells caused by low-dose, low-dose-rate exposure prior to a large test dose (Azzam et al., 1994a). These data also demonstrate a reducing effect of various adapting doses on radiation-induced micronucleus (MN) formation. The reduction in MN frequency correlates with a reduction in the neoplastic transformation frequency, suggesting that DSB repair may be enhanced by pre-exposure to low-dose-rate irradiation (Azzam et al., 1994a, 1994b).
The increased capacity for DNA DSB repair (as measured by a reduction in MN frequency) has recently been shown to be maximally induced in normal human fibroblasts at a dose of 1 mGy of γ-rays, a dose where many cells do not receive one hit, indicating that bystander effects are involved (Broome et al., 2002). The induced process is likely homologous recombination (HR), a potentially error-free DSB repair mechanism. In yeast, the repair process induced by radiation has unambiguously been shown to be HR (Mitchel and Morrison, 1982). The data of Azzam et al. (1996) can be explained in terms of a state-vector model (Schöllnberger et al., 2002b). In this model, HR is up-regulated by radiation and impacts on the repair of DSBs produced through endogenous processes. The protective effects of low doses of γ-rays delivered at low dose rates, as discovered by Azzam and colleagues, have been confirmed by studies in human (HeLa) cells (Redpath and Antoniono, 1998; Redpath et al., 2001).
Yamaoka et al. (1991) report increased superoxide dismutase (SOD) activities induced by low doses of X-rays in rat organs. SOD activities in the thymus, spleen, and bone marrow show a significant increase starting at very low doses of a few mGy. Yukawa et al. (1999) showed a statistically significant increase of radical scavenging ability of liver cytosol after a whole-body irradiation of rats with 5 cGy X-rays. A small transient increase in radical concentrations above the normal level in the cell can cause a measurable activation of detoxification mechanisms, which will act on chemically and radiologically induced radicals (UNSCEAR, 1994). Various other studies also report on the induction of antioxidants through radiation (Zamboglou et al., 1981; Feinendegen et al., 1984, 1987; Oberley et al., 1987; Summers et al. 1989; Akashi et al., 1995; Wong et al., 1996; Hachiya et al., 1997; Kim et al., 1997; Shimizu et al., 1998; Bravard et al., 1999; Morcillo et al., 2000; Guo et al., 2003).
Stecca and Gerber (1998) reviewed potential mechanisms of adaptive response including the activation of later genes that can promote production of growth factors and cytokines, trigger DNA repair, and regulate progress through the cell cycle. Tuschl et al. (1980, 1983) report an enhanced repair capacity for DNA damage after human occupational exposure to low doses of IR. For a more comprehensive review of studies about low-dose adaptations in biological responses at the cell, tissue, and organism levels, see, for example, Feinendegen and Pollycove (2001).
Although the aforementioned studies provide extensive cellular evidence for low-dose induced adaptations in DNA repair and radical scavenging, the magnitude and potential importance of these phenomena for carcinogenesis in vivo remain open to debate. UNSCEAR (2000), for example, states that knowledge of inducible processes in mammalian cells is fragmentary and, in some cases, controversial.
GENERAL FORMULATION OF THE MODEL
A conceptual view of the model is given in Figure 1. Cells in state 0 are undamaged stem or other critical target cells in the human lung. Cells in state 0 are converted to state 1 cells when they become damaged through endogenous processes or by radiation. The latter comprises all background radiation sources including particles from radon progeny. The parameter k (year–1) describes the rate at which undamaged cells acquire persistent problematic genomic instability (Coleman and Tsongalis, 1995; Scott, 1997; Hanawalt, 1998; Schmutte and Fishel, 1999). Cells in state 2 have a more severe form of genomic instability than cells in state 1. State 2 cells are considered initiated or transformed cells. Cells in state 1 and 2 undergo mitotic cell division at rates kM1 and kM2, respectively. Cells are lost from state 1 and 2 through lethal chromosome aberrations and point mutations, apoptosis, and through terminal differentiation. The rate constants kd1 and kd2 govern the rate of cell loss from states 1 and 2, respectively; kd1 and kd2 comprise necrosis, apoptosis, and cell differentiation. In the model, apoptosis can eliminate cells in states 1 and 2 regardless of whether they were formed through endogenous damage or by radiation. Cells in states 1 and 2 undergo clonal expansion when the rate of cell birth is greater than the rate of cell death/differentiation (e.g., when kM1 ≥ kd1). Cells in state 2 become malignant at rate kmt (year–1). These malignant cells then grow into a detectable tumor after lag period tlag (Leenhouts, 1999).
FIGURE 1.
Conceptual overview of the multistage cancer model. Cells in state 0 are normal somatic (stem) cells. State 0 cells require three critical mutational events to transition to a fully malignant state (state 3). The model explicitly accounts for the formation of initiated cells via rate constant k, cell birth (via mitotic rates kM1 and kM2) and death processes (constant rates kd1 and kd2 that comprise necrosis, apoptosis, and cell differentiation), malignant transformation (kmt ), and a lag period (tlag) for tumor formation. Once initiated, cells cannot return to a normal (undamaged) state. Table 1 summarizes the meaning of the model parameters and gives a range of biologically plausible values.
The following system of coupled, first-order differential equations models the initiation of the critical (stem) cell population, the promotion (clonal expansion) and malignant transformation of cells in the human body:
| (1) |
| (2) |
| (3) |
| (4) |
Equation (1) implies that the total number of undamaged (stem) cells in the human body (state 0) is constant from birth to death. All calculations reported in this work are based on N0 =107 cells in the human lung (Leenhouts and Chadwick, 1994). The initial conditions are N1(0) =N2(0) =N3(0) =0; that is, we assume that the average number of cells in stages N1, N2, and N3 is zero at birth.
A biologically plausible range of values for kM2, the mitotic cell division rate for stage 2 cells, is 1 to 100/year.*For state 1 cells, we assume that the rate of mitotic cell division is approximately the same as the mitotic rate for undamaged target cells in the lung; that is, kM1 =12/year (BEIR VI, 1999). Initiated cells can undergo malignant transformation at rate kmt (year–1). Several lines of evidence suggest that the rate of malignant transformation is not a strong function of dose or dose rate (areviewed by Schöllnberger et al., 2001a), and in this paper we assume the rate of malignant transformation is independent of dose and dose rate, as others have done (Luebeck et al., 1999). In this paper, all calculations are based on kmt =10–5/year (Luebeck et al., 1999).
The expected number of detectable tumors per person at time t is
| (5) |
where tlag is the lag time required for a malignant cell to grow into a detectable tumor. All calculations reported in this paper are based on the representative value tlag =5 years (Leenhouts, 1999). When tumor incidence is low, the distribution of the number of malignant tumors among a population of individuals is reasonably described by a Prison distribution (Leenhouts, 1999), and the probability N5(t ) that an individual in the population has one or more tumors is given by
| (6) |
The probability N5(t ) may be equated to cumulative incidence of cancer at time t (Leenhouts, 1999). For the special case when k, kM1, kM2, kd1, kd2, and kmt are independent of time, closed-form solutions to Eqs. (1)–(6) can be obtained using the variation of constants or eigenvalue methods (see Appendix). Numerical solutions to the system of differential equations are obtained using Microsoft Excel software and the closed-form solutions given in the Appendix.
Models for DNA Repair and the Induction of Genomic Instability
The yield of nonlethal genetic alterations (point mutations and chromosome aberrations) produced in a cell by radiation can be plausibly linked to the activation or inactivation of critical genes, the induction of genome instability, and neoplastic transformation (Coleman and Tsongalis, 1995; Hanawalt, 1998; Schmutte and Fishel, 1999). First, radiation creates a spectrum of randomly located DNA damage configurations. Then, biochemical repair processes convert a portion of the initial damage sites into point mutations and chromosome aberrations. A subset of these genetic alterations alters the function or expression of one or a few critical genes and causes the genome to become unstable. It has been suggested that mutations in the genes responsible for chromosomal segregation may be one of the main targets for the induction of genome instability (Loeb and Loeb, 2000). Mutational events that affect the mismatch repair system may be another major route for the induction of genome instability (Loeb and Loeb, 2000). Events initiated through intercellular signaling also contribute to genome instability (Little, 2000).
To model the induction of genomic instability (i.e., the parameter k in Figure 1), the spectrum of all possible DNA damage configurations is grouped into simple lesions and complex lesions. Simple lesions are a collection of one or more elementary damage sites that are arranged in the DNA such that the template for repair is intact (undamaged). Because the template used in the repair process is undamaged, the probability of misrepair is on the order of 10–6 to 10–9 (Friedberg et al., 1995). Single-strand breaks (SSBs) and other singly damaged sites [e.g., 8-oxoguanine (8-oxoG) or apurinic/apyrimidinic (AP) sites] are examples of simple lesions. Complex lesions are the collection of all DNA damage configurations composed of two or more damage sites that are arranged in the DNA such that the template for repair is damaged in some way. DSBs and some configurations of clustered or multiply damaged DNA sites (Ward, 1988, 1994) are examples of complex lesions.
The rate and fidelity of DNA damage repair may be affected by a large number of factors such as the availability of repair enzymes (i.e., repair saturation; Goodhead, 1985; Wheeler and Nelson, 1987; Dikomey and Lorenzen, 1993), preferential repair of transcriptionally active DNA (Hanawalt, 1994; Friedberg, 1996), and chromatin structure (Oleinick et al., 1984; Friedberg et al., 1995; Wellinger and Thoma, 1997). The overall fidelity of the complex lesion repair process is expected to be much lower than the fidelity of repair for simple lesions (Ward, 1988, 1994; Goodhead et al., 1993; Goodhead, 1994). The probability a complex lesion is misrepaired by nonhomologous end-joining (NHEJ) may approach unity. Excision repair of some multiply damaged site configurations may also have a high probability of incorrect repair. On the other hand, HR of a complex lesion is a potentially error-free repair process (Alberts, et al., 2002; Symington, 2002). In yeast, radiation-induced adaptations in recombinational repair have been shown to process at least some of the lesions produced by the chemical agent MNNG (Mitchel and Morrison, 1986,Mitchel and Morrison, 1987; Boreham and Mitchel, 1991). These studies provide some support for the hypothesis that radiologically induced adaptations in DNA repair may impact on the repair of DNA damage caused by other enviornmental toxicants as well as the DNA damages formed through endogenous processes.
Although the kinetics and accuracy of DNA damage repair are due to the interplay among many factors and processes, DNA damage is often removed with approximately first-order kinetics (Frankenberg-Schwager, 1990). The rate of change in the expected number of simple or complex lesions per cell at time t may thus be written as
| (7) |
where Li (t ) is the expected number of simple or complex lesions per cell at time t, is the expected number of ith type (simple or complex) lesion created by endogenous processes (cell–1 year–1), is the expected number of ith type lesion created by radiation (mGy–1 cell–1), Ḋ is the dose rate (mGy/yr), and λi (year–1) is the rate of lesions removal (correct or incorrect repair). The mutation rate at time t is dM(t)
| (8) |
where φi is the probability the ith type of lesion is misrepaired. The quantity (1 – φi ) is the probability the ith type of lesion is repaired correctly. On biophysical grounds, φi must be in the range [0, 1].
For protracted irradiation conditions, d Li /dt →0 and Eq. (7) can be rearranged to give
| (9) |
After substitution of Eq. (9) into Eq. (8) and rearranging terms, the mutation rate under equilibrium (protracted irradiation) conditions can be expressed as
| (10) |
Equation (10) implies that the mutation rate is independent of the rate of damage repair for protracted exposure conditions (i.e., independent of λi ). The key repair parameter that determines the mutation rate is the probability of lesion misrepair, φi .
To complete the model, assume that the rate at which genomic instability is induced in state 0 and state 1 cells is proportional to the mutation rate; that is,
| (11) |
The parameter Ωis the probability that a mutation formed at a random location in the DNA induces genomic instability by modifying the expression or function of a critical gene. At least 130 human genes are directly involved in the repair of DNA damage (Wood et al., 2001). Hundreds or thousands of other nonrepair genes may indirectly affect damage repair, DNA replication, or chromosomal segregation. A mutational event that alters any one of these genes could induce some form of genomic instability, and the severity of the induced genomic instability most likely depends on the specific gene or combinations of genes damaged through mutation. The most critical gene targets for the induction of genomic instability are not known. Epigenetic effects (i.e., intercellular signaling events) also contribute to genomic instability, although these phenomena are neglected in the current model.
A reasonable range of values of Ωcan be estimated by multiplying the total number of target genes times the average gene size and then dividing by the total genome size. The human genome contains approximately 2.91×109 base pair (bp) and 25,000 to 40,000 genes (Venter et al., 2001). The average size of a human gene is approximately 27,000 bp (Venter et al., 2001). Therefore, if all genes are potential targets for the induction of genomic instability, Ωmay be as high as to 0.232 to 0.371. Damage to noncoding sections of the DNA may also contribute to the induction of genomic instability (increase Ω) by disrupting signaling pathways or altering gene expression. If only a few tens or hudreds of genes are critical to maintaining genome stability, Ωmay be as low as 10–4 to 10–2. The results shown in the Figures of the Results section are based on Ω=2.958 ×10–5.
MODELS FOR RADIATION HORMESIS MECHANISMS
Equation (11) defines the rate at which genomic instability is induced when cellular defense mechanisms remain constant at all radiation exposure levels (i.e., in the absence of toxicant-induced adaptations in the biosys-tems involved in the prevention, repair, or removal of genetic damage). Feinendegen et al. (2004) have suggested that exposure to low doses of acute and also chronic IR with repetitive energy-deposition events in defined micro-masses (e.g., the cell) stimulates cellular defense mechanisms and reduces the cumulative mutational load associated with aging, disease, and cancer. Ultimately, they argue that exposure to low levels of IR tends to reduce mortality from all causes, decrease the cancer mortality, and may even be protective against accidental high-level radiation exposures (Feinendegen et al., 2004). In this section, the genomic instability model is extended to account for putative toxicant-induced adaptations in cellular repair systems as well as for toxicant-induced adaptations in the radical scavenging capacity of a cell. The experimental evidence suggesting the existence of radiopro-tective effects mainly comes from experiments performed with low doses of low-linear-energy-transfer (LET) radiation (e.g., Azzam et al., 1994a, 1996; Redpath and Antoniono, 1998; Redpath et al., 2001, 2003a, 2003b; Mitchel et al., 2003; Pant et al., 2003), and the proposed model may be more appropriate for- and X-rays than for high-LET radiations, such as α-particles from randon progeny.
To incorporate adaptive and protective mechanisms induced by low-LET radiation into the genomic instability model, rewrite Eq. (11) as
| (12) |
where Gi (Ḋ) is a dimensionless function that accounts for changes in φi as a function of dose rate, and Fi (Ḋ) is a dimensionless function that accounts for changes in the radical scavenging capacity of a cell (and hence the initial yield of DNA damage) as a function of dose rate. The subscript i indicates the type of damage (simple or complex). The probability of correct lesion repair, 1 – φi , is enhanced for values of Gi (Ḋ) greater than 1 and reduced for values of Gi (Ḋ) less than 1. Values of Fi (Ḋ) greater than 1 indicate that enhanced radical scavenging reduces the initial yield of DNA damage. For the special case when Gi (Ḋ) = Fi (Ḋ) =1, Eq. (12) reduces to the genomic instability model without toxicant-induced adaptations in cellular defense mechanisms [i.e., Eq. (11)].
Two hypotheses are implicit in Eq. (12). Hypothesis 1 is that cells that are damaged by low-LET radiation alter radical scavenging or DNA repair in a way that reduces the impact of later doses of radiation. Hypothesis 2 is that cells that are damaged by radiation alter radical scavenging or DNA repair in a way that reduces the impact of damage formed through endogenous processes. Either or both of these modes of action may reduce the overall chance that radiation will induce genomic instability and, ultimately, cancer. Micro-dosimetric considerations provide some useful insights into the plausibility of hypothesis 1 for doses and dose rates typical of background radiation. The average annual dose rate from background radiation in the United States is 3.0 mGy/year (NCRP, 1987). In other regions of the world, the dose rate from background radiation may vary as much as 10-fold (UNSCEAR, 2000).
The frequency-mean specific energy per hit (ICRU, 1983), denoted z̄F , is 350 mGy for 4 MeV α-particles and 0.4 mGy for Cs-137 α-rays (Table 1 in Feinendegen et al., 1996). The average time between two consecutive hits to the same cell may be expressed as z̄F Ḋ. For dose rates in the range from 1 to 3 mGy/year (i.e., bracketing the U.S. average), the average time between two consecutive radiation hits is on the order 50 to 150 days for low-LET radiation and from 100 to 350 years (!) for α-particles. Most of the in vitro and in vivo studies suggest that cells return to the baseline (nonadapted) state within a few hours, days, or perhaps weeks. Under low-dose-rate conditions, almost all cells return to the nonadapted state before they are again hit by radiation. As long as the dose rate is sufficiently low, this statement is true even if the total accumulated dose is quite large. Microdosimetric considerations such as these argue against hypothesis 1 for low dose rates. However endogenous processes create a large amount of DNA damage every day (e.g., see Table 1), and therefore microdosimetric arguments do not exclude hypothesis 2.
TABLE 1.
Summary of the Range and Best-Estimate Model Parameters Used for the Reported Studies.a
| Parameter | Description | Range | Best estimate (default) | Comments |
|---|---|---|---|---|
| Ω | Probability that a randomly located mutation induces genomic instability | 10–4 to 0.37 | 2.958 ×10–5 | Best estimate was fitted, see main text |
| φsl | Probability a simple lesion is misrepaired | 10–6 to 10–9 | 10–9 | Friedberg et al., 1995 |
| φcl | Probability a complex lesion is misrepaired | 0 to 1 | 0.3437 | Best estimate was fitted, see main text |
| Expected rate simple lesions are created by endogenous processes | 105 to 106 cell–1 day–1 | 1.92 ×105 cell–1 day–1 | Billen, 1990 | |
| Expected rate complex lesions (DSBs) are created by endogenous processes | 0 to 5.5 cell–1 day–1 | 8.84 ×10–8 cell–1 day–1 | Best estimate was fitted, see main text | |
| Expected number of simple lesions created by radiation | 2,500 to 5,000 cell–1 Gy–1 | 3,700 cell–1 Gy–1 | Ward, 1985, 1988 | |
| Expected number of complex lesions created by radiation | 20 to 60 cell–1 Gy–1 | 40 cell–1 Gy–1 | Ward, 1985, 1988 | |
| Gi (Ḋ) | Dimensionless function that accounts for radiological adaptations in φsl and φcl | 1 to 3 (peak value) | 1.4 (peak value) | Pollycove and Feinendegen, 2001 |
| Fi (Ḋ) | Dimensionless function that accounts for radiological adaptations in radical-scavenging capacity of cell | 1 to 5 (peak value) | 1.4 (peak value) | Pollycove and Feinendegen, 2001 |
| kM1 | Mitotic rate for state 1 cells | — | 12 yr–1 | Schöllnberger et al., 2001a |
| kM2 | Mitotic rate for state 2 cells | 1 to 100 yr–1 | 50 yr–1 | E. G. Luebeck, Fred Hutchinson Cancer Research Center, personal communication |
| kd1 | Cell death and differentiation rate (state 1 cells) | < kM1 | 11.988 yr–1 | = kM1(1–0.1%) |
| kd2 | Cell death and differentiation rate (state 2 cells) | < kM2 | 49.95 yr–1 | = kM2(1–0.1%) |
| kmt | Rate of malignant cell transformation | 10–5 to 5 ×10–5 yr–1 | 1 ×10–5 yr–1 | Luebeck et al., 1999; E. G. Luebeck, Fred Hutchinson Cancer Research Center, personal communication |
| N0 | Expected number of critical cells (stem cells) in the lung | 107 to 5 ×1010 | 107 | Leenhouts and Chadwick, 1994b |
| tlag | Lag time for the first malignant cell to grow into a detectable tumor | 2 to 10 yr | 5 yr | Leenhouts, 1999 |
aExcept where explicitly stated otherwise, all of the reported results are based on the best estimate (default) value.
bThe value of 5 ×1010 target cells in the lung was calculated as follows: In the human lung the target cells for malignant transformation are alveolar type II cells and airway epithelial cells in bronchi and bronchioles (Gazdar and Carbone, 1994; S. Belinsky, personal communication). 15.9% of the 2.3 ×1011 cells in the lung are alveolar type II cells (Crapo et al., 1983), that is 3.7 ×1010 cells. In addition, there are 7.167 ×109 epithelial cells in the human bronchi and 3.287 ×109 epithelial cells in the bronchioles (Mercer et al., 1994). That gives together approximately 5 ×1010 target cells for lung cancer.
Radiation-damaged cells may also generate signals that enhance radical scavenging and DNA repair (Broome et al., 2002; Iyer and Lehnert, 2002a, 2002b) in nearby undamaged cells. These bystander effects would then, in effect, amplify the adaptive response of the tissue beyond the response expected from the relatively few radiation-damaged cells. The dimensionless radioprotective functions G(Ḋ) and F(Ḋ) may be taken to represent the net change in radical scavenging and DNA repair produced in the directly damaged and bystander cells.
To simplify the modeling, assume that adaptations in cellular defense mechanisms are indentical for both simple and complex lesions; that is, Gi (Ḋ) →G(Ḋ) and Fi (Ḋ) →F(Ḋ). Also, although large changes in the radical-scavenging capacity of a cell can modulate the amount of radiation damage (Ward et al., 1985; Milligan et al., 1993), the indirect action of radiation on the DNA is associated with radicals formed within about 1 to 4 nm form the DNA (Brenner and Ward, 1992). For the range of radical-scavenging capacities expected in living cells, the effects of small perturbations in the radical-scavenging capacity of a cell will have a negligible impact on the initial yield of radiation damage (see also Fleck et al., 1999; Schöllnberger et al., 2001a). Thus, Eq. (12) becomes
| (13) |
The shape and magnitude of G(Ḋ) and F(Ḋ) as a function of dose rate is unknown at present. However, Pollycove and Feinendegen (2001) have suggested cellular adaptations in DNA repair processes and in the radical-scavenging capacity of a cell increase with increasing dose up to about 200 mGy and then decrease with increasing dose (see Figure 5 in Pollycove and Feinendegen, 2001). A 200-mGy dose of radiation corresponds to an average lifetime (75-year) dose rate of 2.67 mGy/yr.
FIGURE 5.
Predicted shapes of the cumulative lung-cancer incidence curves when both cellular defence mechanisms are included in the model. Solid line: no protective effects (Af = Ag =1 and Bf = Bg =0); long dashed line: effects of radical scavenging and DNA repair (F and G in the range from 1 to 1.4); short dashed line: combined effects of radical scavenging and DNA repair (F and G in the range from 0.8 to 1.4); dash-dotted line: combined effects of radical scavenging and DNA repair (F and G in the range from 0.8 to 3).
For doses above about 500 mGy, Pollycove and Feinendegen suggest that the cellular defense mechanisms approach the baseline (background radiation) level or may even be suppressed below the baseline level (Pollycove and Feinendegen, 2001), presumably because of radiation damage to regulatory or rate-limiting biomolecules involved in the repair or radical-scavenging process. For doses below at least several or tens of grays, the hypothesis that radiation damage to regulatory or rate-limiting biomolecules has a significant impact on damage repair or radical scavenging is highly debatable. Nevertheless, a central aim of the current article is to explore the potential impact and conditions under which the Pollycove and Feinendegen (2001) hormesis mechanisms give rise to non-LNT-shaped biological responses.
The trends in low-dose cellular defense mechanism suggested by Polly-cove and Feinendegen can be mimicked using a normal distribution with an appropriate mean, variance, and adjustable offset:
| (14) |
and
| (15) |
The parameters Af, Bf, C f, Ag , Bg , and Cg are nonnegative adjustable parameters, and Ḋf and Ḋg are approximately equal to 2.67 mGy/year (i.e., approximately equal to the background dose rate in the United States).
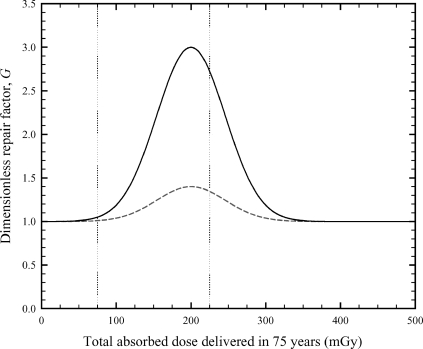
Figure 2 shows two representative examples of the dimensionless DNA repair function, G(Ḋ). The absorbed dose rate in Eq. (15) is the total delivered dose divided by 75 years (e.g., a 75 mGy delivered dose corresponds to an annual dose rate of 1 mGy/year). The parameters Af, Bf, Ag , and Bg determine the magnitude of cellular adaptation (peak value), Cf and Cg determine the width of the peak along the dose axis, and Ḋ and Ḋg determine the location of the peak along the dose axis. When Af =Ag =1 and Bf =Bg =0, DNA repair and radical-scavenging processes become independent of dose and dose rate. The dimensionless radical-scavenger function, F(Ḋ), has the same functional form (overall shape) as the repair function.
FIGURE 2.
Representative examples of the dimensionless DNA repair function, G(Ḋ). The mathematical form of the DNA repair function is given by Eq. (15). The dose rate used in Eq. (15) is the total delivered dose divided by 75 years. Solid line: Ag =1, Bg =2, Cg = 1.333 (mGy/year)–2, and Ḋg =2.67 mGy/year; dashed line: Ag =1, Bg =0.4, Cg =1.333 (mGy/year)–2, and Ḋg =2.67 mGy/year. The vertical dotted lines indicate the typical dose range expected from naturally occurring radiation sources (i.e., background radiation). The lower dose bound is set at 75 mGy (1 mGy/year), and the upper dose bound is set at 225 mGy (3 mGy/year).
In addition to fitting Ωas discussed earlier, the values of parameters Af, Bf, C f, Ag , Bg , and Cg were also determined by data fitting as follows. To produce, for example, an F(D ) function with corresponding F(0 mGy) =F(1000 mGy) =1 and F(200 mGy) =3, the Excel solver was used to optimize the values of Af, Bf, and Cf so that the constraints at 0, 200, and 1,000 mGy were met. Thereafter, these functional forms for F and G were used to produce Figures 4 and 5 (see Results section).
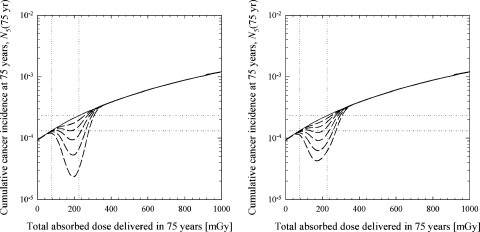
FIGURE 4.
Effects of cellular adaptations in DNA repair and enzymatic radical scavenging. The best-estimate parameter values listed in Table 1 were used for all of the results shown. The vertical dotted lines indicate the typical dose range expected from naturally occurring radiation sources (lower bound corresponds to 1 mGy/year and the upper bound corresponds to 3 mGy/year). Horizontal dotted lines at 1.3×10–4 and 2.3×10–4 indicated the level of cumulative lung-cancer incidence at 1 and 3 mGy/year, respectively. The mathematical form of the radical scavenger function, F, and the DNA repair function, G, are given by Eqs. (14) and (15), respectively. Solid line: no protective effects (Af = Ag = 1 and Bf = Bg =0); dashed lines show the effects of selecting parameters that give peak values for F and G in the range from 1.1 to 5 (refer to Figure 2). Left panel: effects of cellular adaptations in DNA repair (F =1 and 1.1 ≤G ≤3); right panel: effects of cellular adaptations in enzymatic radical scavenging (1.1 ≤ F ≤5 and G =1).
RESULTS
A perennial challenge in mechanism-based modeling of cancer is that the number of adjustable model inputs is often larger than can be directly estimated from the available epidemiological and experimental data. The multistage model formulated in this work requires a total of 14 parameters plus the parameters associated with F(Ḋ) and G(Ḋ). We have attempted to circumvent parameter estimation difficulties by estimating as many of these parameters as possible using secondary data sources. For example, the initial yield of DNA damage induced by radiation is based on information from in vitro studies (Ward, 1985, 1988). Alternatively, estimates of several key model inputs, such as cell birth and death rates and the lag time for a malignant cell to grow into a detectable tumor, are estimated from other published studies (Leenhouts, 1999; Luebeck et al., 1999). Except where explicitly noted otherwise, the parameter values summarized in Table 1, termed the best estimate parameters, are used in all of the reported sensitivity studies (refer also to Figure 1). Table 1 also lists the estimated range and meaning of all model parameters.
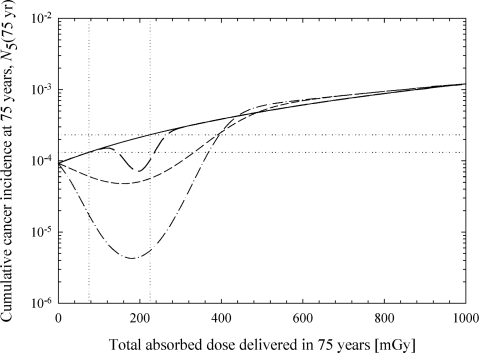
Figure 3 shows the model-predicted cumulative lung-cancer incidence level for the best-estimate parameter values listed in Table 1 (no protective effects) with (solid line) and without (dashed line) the endogenous DNA damage terms (i.e., = =0). Figure 3 (dotted line) also shows the model-predicted cumulative lung-cancer incidence level with = 0.1 cell–1 year–1 instead of the default (Table 1) value of =3.23×10–5 cell–1 year–1 (8.84×10–8 cell–1 day–1). In the model calculations that include the endogenous and radiation DNA damage mechanisms (solid line), the predicted cumulative cancer incidence level at 1 Gy is 1.2 ×10–3. This value was found by fitting the model to three ICRP-derived risk values at 75, 225, and 1,000 mGy. The probability of fatal lung cancer is 0.85×10–2 Sv–1 (ICRP, 1991). With 1 Sv =wT wR Gy and wT =0.12 for the lung and wR =1 we get a risk estimate of 1.02 ×10–3 Gy–1 for lung cancer fatality; wR =1 needs to be taken because, beyond the background dose rates of 1–3 mGy/year, our analysis is restricted to low-LET radiation. The relative 1- and 5-year survival rate for all lung cancer sites is 41 and 15%, respectively (ACS, 2002). The ICRP’s risk estimate thus corresponds to a cumulative lung-cancer incidence level in the range from 1.2 ×10–3 Gy–1 to 1.7 ×10–3 Gy–1. The best estimate values reported in Table 1 for, Ωφcl and were estimated by fitting the model to 1.2 ×10–3 and to linear extrapolations of this value for 75 and 225 mGy.
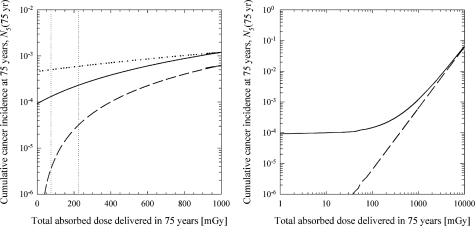
FIGURE 3.
Contribution to cumulative lung-cancer incidence of DNA damage formed by endogenous processes and ionizing radiation. Except where explicitly noted otherwise, calculations are based on the best-estimate parameters listed in Table 1. Protective effects are not included in this set of calculations (i.e., Af =Ag =1 and Bf = Bg =0). Solid line: DNA damage formed by ionizing radiation and endogenous processes; dashed line: endogenous processes do not create any DNA damage ( = =0); dotted line: DNA damage formed by ionizing radiation and endogenous processes with = 0.1/cell/year and adjusted values for Ωand φcl. Left panel: semi-log plot of cumulative incidence versus dose; right panel: log-log plot of cumulative incidence versus dose (expanded dose scale). The vertical dotted lines indicate the typical dose range expected from naturally occurring radiation sources (lower bound corresponds to 1 mGy/year and the upper bound corresponds to 3 mGy/year).
In the absence of radiation (i.e., the 0 mGy delivered dose), the model predicts that DNA damage from endogenous sources results in a cumulative cancer incidence level of 9.2 ×10–5 after 75 years. For total delivered doses in the range from 75 to 225 mGy (i.e., the range of exposure levels arising from environmental sources of radiation), the model predicts a cumulative incidence level in the range from 1.3 ×10–4 to 2.3 ×10–4. For comparison, linear extrapolation of the ICRP risk estimates from 1 Gy to background radiation levels gives cumulative incidence projections in the range from 9.0 ×10–5 to 3.8 ×10–4. The model predictions that use the best-estimate parameters listed in Table 1 are in reasonable agreement with the ICRP values at 1 Gy and at background radiation levels. Differences in the predictions of the ICRP and the multistage cancer model are reasonably attributed to uncertainties in model inputs and to uncertainties associated with the relative survival rate for lung cancer.
As illustrated in Figure 3, endogenous DNA damage has the potential to make a substantial contribution to the cumulative cancer incidence level below a few hundred mGy. In the background radiation dose range (75–225 mGy), endogenous processes are responsible for about 86–97% of the predicted cancers. Even at 1 Gy, the model predicts that endogenous processes are responsible for about 48% of the cancers.
Although the results shown in Figure 3 suggest that endogenous DNA damage may be responsible for a large portion of the lung cancer at background radiation levels, the production of complex multiply damaged sites by endogenous processes, the value of the parameter, is uncertain. The accuracy of the repair process for simple (φsl ) and complex DNA damage sites (φcl ) is also uncertain (see estimated range in Table 1). Theoretical considerations suggest that the rate of DSB formation by endogenous processes may be as large as 5.5 DSB/cell/day (Stewart, 1999). Stewart (1999) calculated a best-estimate rate of DSB formation of 0.1 DSB/cell/day. For a representative 2-h repair half-time, these estimates suggest that the equilibrium number of DSB/cell [Eq. (9) with Ḋ =0] should fall in the range from 0.012 DSB/cell to 0.661 DSB/cell. Recent experimental evidence points to an equilibrium (background) value of approximately 0.05 DSB/cell in primary human fibroblasts from the lung (Rothkamm and Löbrich, 2003), a value in reasonable agreement with the predicted equilibrium value. The “best-estimate” value given in Table 1 was found by fitting the ICRP-derived risk values. For the default value of reported in Table 1, complex lesions (i.e., multiply damaged sites) have a negligible impact on the estimated cumulative cancer incidence level. However, if the value of is increased to 1.14×10–5 DSB/cell/h (0.1/cell/year), a value that is still 380 times lower than the estimate of 4.33 ×10–3 DSB/cell/h (Stewart, 1999), this parameter has a dramatic impact on the predicted cumulative cancer incidence level (dotted line in Figure 3). Additional experimental work is needed to reduce the uncertainties associated with the possible formation of multiply damaged sites (including DSBs) by endogenous processes.
Cellular adaptations in radical scavenging and DNA damage repair are introduced into the multistage cancer model through the dimensionless F and G functions, respectively. Representative examples of the dimensionless repair function, G, are shown in Figure 2. Figure 4 illustrates the effects that radiation-induced adaptations in DNA damage repair (left panel) and radical scavenging (right panel) may have on the cumulative incidence of lung cancer.
As the results in Figure 4 show, the manner in which the cumulative incidence level deviates from the baseline cancer incidence level (no hormetic effects) is reminiscent of the shape of the F and G functions. The exact shape and magnitude of the cumulative incidence curve is sensitive to the numerical value selected for the parameters used in the dimensionless F (radical-scavenging) and G (DNA repair) functions. For all of the studies shown in Figure 4, F and G reach a maximum at 200 mGy (i.e., the dose value that corresponds to dose rate Ḋφ = Ḋg =2.67 mGy/year).
The maximum departure from the predicted baseline cumulative incidence level (no protective effects) occurs around 200 mGy for cellular adaptations in DNA repair (Figure 4, left panel) and about 175 mGy for cellular adaptations in radical scavenging (Figure 4, right panel). Changes in radical-scavenging and DNA repair processes affect the overall shape of the cumulative incidence curve in different ways because repair processes impact on both endogenous and radiation DNA damage whereas small changes in a cell’s radical-scavenging capacity are presumed to impact on the formation of endogenous DNA damage but not the formation of radiation damage [see discussion associated with Eqs. (12) and (13)].
The expected number of lung cancers decreases in approximately linear fashion as the value of F increases. For a lifetime dose of 200 mGy, a 10% increase in the efficiency of radical scavenging (F =1.1) translates into an approximate 10% decrease in the predicted cumulative cancer incidence level. On the other hand, projections of cumulative cancer incidence are approximately proportional to the square of G. For example, increasing the accuracy of DNA repair by 10% (G =1.1) decreases the predicted cumulative incidence level by approximately 20% at 200 mGy. Increasing the accuracy of DNA repair by 50% (G =1.5) decreases the cumulative incidence level at 200 mGy by a factor of 2.25.
Discussions on radiation hormesis often raise the issue of whether the cancer incidence level for some low-dose or dose-rate exposure condition is lower or higher than the background cancer incidence level. In Figure 4, the horizontal dotted lines at 1.3×10–4 and 2.3×10–4 represent the range of cumulative incidence levels expected from endogenous processes that damage the DNA and from radiation sources commonly found in the environment (e.g., cosmic rays, α-rays from the ground and buildings, radon, and 14C and 40K inside the human body). The zero-dose cancer incidence level at 9.2 ×10–5 represents the predicted cumulative incidence level in the complete absence of all radiation, including all environmental sources of radiation. For the analysis of human epidemiological studies, the selection of the zero-dose cancer incidence level is inappropriate because all terrestrial organisms are inevitably exposed to cosmic rays and other sources of radiation in the environment. The results shown in Figure 4 suggest that the background cumulative cancer incidence level may vary as much as 1.8-fold among individuals 75 years old. The range of possible background cancer incidence levels increases with increasing age.
Figure 5 shows the combined effects of cellular adaptations in radical-scavenging and DNA repair processes. The results shown in this curve represent some of the possible non-LNT responses that may reasonably arise from cellular adaptations in DNA repair and radical scavenging. These results, and the results of other sensitivity studies (not shown), suggest that radiation must induce changes in radical scavenging and DNA repair greater than about 30 or 40% (F and G >1.3 to 1.4) of the baseline values to produce cumulative incidence levels outside the range expected for endogenous processes and background radiation (i.e., the horizontal dotted lines 1.3 ×10–4 and 2.3×10–4). For the results given in Figure 5 (short dashed line and dash-dotted line), slightly different values forφcl and were used to anchor the model at the ICRP-derived risk value at 1 Gy and at the model-predicted value at 0 Gy found with G = F =const =1.
DISCUSSION
Multistage models capture some of the putative rate-limiting steps involved in the development of cancer. In this paper, we developed models and methods to incorporate radiation-induced changes in the efficiency of DNA damage repair and radical scavenging into a deterministic multistage cancer model. The hormesis mechanisms incorporated into the model are generally consistent with those proposed by Feinendegen et al. (1987). Model inputs have been identified that correctly predict the cumulative lung-cancer incidence rates reported by the ICRP for high and low doses.
The dose along the x-axis of Figures 2 through 5 arises from two types of radiation sources: (1) environmental sources of radiation delivering 1–3 mGy/yr and (2) an additional “man-made” source of low-LET radiation that contributes extra radiation over the lifetime of the individual. The man-made radiation source component can also be viewed as an additional medical or environmental source of low-LET radiation that may depend on location and/or elevation, such as dental X-rays, nuclear medicine procedures, or airline travel. The plethora of man-made and environmental sources gives rise to a very complicated temporal pattern of mixed low- and high-LET radiation exposure.
In addition to LET effects, two limiting dose delivery patterns are of special interest. The first pattern of interest arises when the man-made component of the radiation field is delivered at low dose rate (scenario 1). The second dose delivery pattern of interest is the one that arises when the man-made component is delivered at high dose rate (scenario 2), such as those that may arise in the workplace. For exposure scenario 1, the dose rates considered in Figures 3 (left panel) through 5 correspond to annual (average or effective) dose rates from (1,000 mGy –225 mGy)/(75 years) =10.33 mGy/year to (1,000 mGy – 75 mGy)/(75 years) =12.33 mGy/year. The total (average) dose rate is the sum of the environmental and man-made radiation terms: 1–3 mGy/year + 10.33 to 12.33 mGy/year. Pollycove and Feinendegen report that a 10-fold increase of background radiation from 1 to 10 mGy/year stimulates the overall DNA damage-control activity by about 20% (Pollycove and Feinendegen, 2001, 2003). In the second exposure scenario, which is not considered in the current series of studies, environmental radiation sources deliver 1–3 mGy/year and the man-made radiation sources deliver an extra dose in a relatively short period of time (e.g., minutes or hours). The extra dose component may be as large as 775–925 mGy.
Doses in the range from 1 to 200 mGy of low-LET radiation delivered at dose rates of a few mGy/min produce experimentally detectable levels of gene induction (Azzam et al., 1996; Redpath and Antoniono, 1998). The dose rates considered in our studies are, however, much lower than the ones used by Azzam et al. (1996) and Redpath and Antoniono (1998). Data on MN formation in human fibroblasts after various priming doses of φ-radition followed by a challenge dose show that any priming dose from 1 to 500 mGy (delivered at 1–3 mGy/min) produced the same drop in MN frequency (Broome et al., 2002). These experiments indicate that a single Co-60 α-ray suffices to cause gene induction.
Considerations such as these suggest that gene induction can occur even for low-dose and/or dose-rate exposure conditions (see also the discussion regarding hypothesis 2 in the section titled Models for Radiation Hormesis Mechanisms). On the other hand, many questions remain about the overall significance of these phenomena. To improve the modeling of low-dose radiation effects, additional information on the spatial and temporal pattern of gene induction is needed. For example, how long does gene induction last after a cell is hit by radiation? How many bystander cells are affected by signals emitted by the radiation-damaged cells? Is the signal strength (signaling distance) the same for low- and high-LET radiation? To the best of our knowledge currently the literature provides only very limited answers to questions such as these.
The results shown in Figure 3 suggest that DNA damage formed through endogenous processes is important for both low and high doses. As in earlier studies (Schöllnberger et al., 2001a), model predictions are sensitive to the net birth rate (kM1 – kd1) and (kM2 – kd2) but not overly sensitive to the specific value selected for kM1 and kM2 (if kd1 and kd2 are chosen appropriately). For the same level of defense induction (i.e., F =G = some constant), our studies suggest that toxicant adaptations in DNA repair processes (i.e., the φsl and φcl parameters) have a larger impact on dose-response relations than toxicant-induced adaptations in radical scavenging. This trend arises because the effects of small toxicant-induced perturbations in a cell’s capacity to scavenge radicals will have a negligible impact on the initial yield of radiation damage (see also Fleck et al., 1999; Schöllnberger et al., 2001a). Sensitivity studies suggest that the model can give rise to classical LNT curves, threshold-like curves and U-shaped curves (Figures 4 and 5). To obtain threshold-like curves or U-shaped responses, the repair of DNA damage must be transiently modified after a cell is damaged by radiation. The production of enzymatic radical scavengers can also be transiently modified after a cell is damaged by radiation, although it is not necessary for both radical scavenging and DNA repair to be altered in the same way or to the same extent to produce non-LNT-type responses.
At least five separate repair pathways are involved in the removal of DNA damage. NHEJ and HR are important pathways for the removal of DSBs (Jeggo, 1998; Lewis and Resnick, 2000). The base excision repair (BER) pathway is adept at repairing damages, such as deaminated, (some) oxidized, alkylated, and AP sites that cause relatively minor disturbances in the helical structure of DNA (Memisoglu and Samson, 2000a, 2000b). The BER pathway also recognizes and attempts to repair some multiply damaged DNA sites (Harrison et al., 1999; David-Cordonnier et al., 2001). BER of an 8-oxoG or an AP site opposite a strand break sometimes results in the creation of a DSB (Harrison et al., 1999). The fourth repair pathway is NER. NER recognizes bulky lesions that cannot be repaired by BER (Friedberg et al., 1995; Nickoloff and Hoekstra, 1998), for example, some oxidation products or UV-induced cyclobutane pyrimidine dimers and pyrimidine (6-4) pyrimidone photodimers. DNA mismatch repair is also important for the removal of DNA damage after IR, as observed by DeWeese et al. (1998) using low-dose rates of IR.
Damage repair kinetics may be affected by pathway crosstalk, sharing of proteins among various repair and transcriptional pathways, and competition for the same kind of damage. For example, Cucinotta et al. (2000) demonstrated that competition between two different DSB rejoining pathways results in a linear-quadratic dose dependence for the creation of simple exchange-type chromosome aberrations. Cell-cycle effects also have the potential to impact DNA repair. Various lines of evidence indicate that NHEJ and HR are regulated during the cell cycle (reviewed by Daboussi et al., 2002). NHEJ predominates in G1 and early S-phase cells whereas HR is important in late S and the G2 phase (Daboussi et al., 2002). The inducibility, or lack thereof, of either of these systems in relation to the cell cycle is unknown.
Rothkamm and Löbrich (2003) irradiated nondividing confluent cell cultures of primary human fibroblasts from the lung and skin with X-ray doses ranging from 1.2 mGy to 80 Gy. DSBs, measured via foci of γ-H2AX, induced by 5 mGy persisted considerably longer than DSBs induced by 20 or 200 mGy. For an exposure with 1.2 mGy, the authors find a total lack of DSB repair in nondividing cells, and if the cells are allowed to divide these cells die by apoptosis. They speculate that the apparent lack of DSB repair at 1 mGy in MRC-5 human fibroblast cells from the lung is a protective mechanism that may reduce the cancer risks of very low radiation doses. That is, instead of rejoining a DSB, which has some nonzero probability of causing genetic alterations, it could be beneficial for an organism to remove the damaged cell and replace it by the division of an undamaged neighboring cell (Rothkamm and Löbrich, 2003). A similar phenomenon was reported for nondividing human lymphocytes exposed to an adapting dose followed by a challenge dose (Cregan et al., 1999). The biological conditions associated with nondividing cell cultures are not unlike the situation in an organ, where most of the cells are nondividing most of the time, and then occasionally are allowed to divide. The paper by Rothkamm and Löbrich suggests that lung cells in vivo may rejoin DSBs very infrequently (or not at all) under low-dose or dose-rate exposure conditions. Although these phenomena are not explicitly considered in the current work, threshold-like responses such as the long dashed line shown in Figure 5 might arise as the result of a repair threshold.
The final outcome of the repair process depends on the overall fidelity of the DNA repair processes involved. Any time- (or dose- or dose-rate-) dependent change in BER, NER, NHEJ, or HR may cause the overall fidelity of damage repair to increase or decrease. For example, transient up-regulation of the potentially error-free HR pathway might increase the rate of damage repair (increase λ) and increase the probability of correct repair [increase (1 – φ)]. On the other hand, transient up-regulation of the potentially error-prone NHEJ pathway could increase λ and decrease (1 – φ). While the relative importance of NHEJ and HR varies during the cell cycle, constitutive processes are frequentely down-regulated under stress conditions, and this may also impact on the overall fidelity of DNA repair. The reduced risk from low doses could have to do with a dominance of HR over NHEJ at low doses. Although for the latter there is no direct evidence, there is another attractive hypothesis to explain the reduced risk: an alteration in the error rate of NHEJ in response to radiation. DNA-PK, a protein required for NHEJ, forms a complex with Tp53, which is then phosphorylated and hence stabilized (Achanta et al., 2001). Tp53, which is inducible at low doses, prevents illegitimate recombination during NHEJ (Akyuz et al., 2002). Therefore, the coordinated action of activated Tp53 and DNA-PK could improve the quality of error-prone NHEJ repair. This was pointed out by Dittmann et al. (2003), who were looking at the effect of the Bowman–Birk protease inhibitor to reduce the dicentric frequency.
In the human lung, the target cells for malignant transformation are alveolar type II cells and airway epithelial cells in bronchi and bronchioles (Gazdar and Carbone, 1994†). In the cell population believed critical for lung cancer in humans, we are not aware of any in vitro or in vivo data that support through direct biochemical evidence the antimutagenic DNA damage-control biosystems proposed by Feinendegen and Pollycove (2001). However, the many references cited in the section Studies Supporting or Refuting Toxicant-Induced Adaptations in Radical Scavenging and DNA Repair are a strong indication that different mechanisms of action may be important under low versus high dose exposure conditions. These studies may also provide support for the hypotheses that DNA repair and radical scavenging are inducible by low doses of IR. Additional research in this area is highly desirable.
Epidemiological evidence for hormetic effects in lung cancer caused by low-LET radiation has been reviewed by Rossi and Zaider (1997). Based on 1,178 lung-cancer deaths in a large fluoroscopy cohort study, Howe (1995) found that there was no evidence of any positive association between risk and dose, with the relative risk at 1 Sv being 1.00 (95% CI =0.94–1.07). Another study of tuberculosis patients who were examined by multiple X-ray fluoroscopy showed that, among the irradiated patients, with an estimated mean radiation dose to the lung of 0.84 Gy, the SMR was 0.8 (95% CI =0.6–1.1) (Davis et al., 1989). Blettner et al. (2003) reported a lung cancer SMR of 0.53 (95% CI =0.44–0.62) for airline cockpit crew. British radiologists registered after 1935 have an SMR for lung cancer smaller than 1 (Berrington et al., 2001).
The 0.3-Gy threshold calculated in the current study seems to be consistent with the values of dose points for transition from protective to detrimental effects actually observed in studies in vitro and in vivo. Redpath and Antoniono (1998) showed that this occurred between 100 and 300 mGy for TF/SC in human cells. Azzam et al. (1996) found that it was above 100 mGy in rodent cells. In vivo, Mitchel et al. (1999) have shown that it is above 100 mGy in mice for myeloid leukemia and about 100 mGy for a variety of tumors in cancer-prone mice (Mitchel et al., 2003).
In addition to the DNA repair and radical-scavenging mechanisms considered in this paper, Pollycove and Feinendegen argue that the immune response could be up-regulated by low doses of IR and cite some experimental evidence to support this possibility (Pollycove and Feinendegen, 2001). Although these phenomena are not considered in the present work, these effects could be included by modifying the rate constant kmt so that it depends on dose and dose rate. Alternatively, these effects could be included in the model by modulating the cell death terms as a function of dose or dose rate and by also allowing N3 cells to be removed.
The literature contains many examples of detrimental bystander effects (e.g., Sawant et al., 2001a; Ballarini et al., 2002; and references given therein) as well as examples of radioprotective bystander effects (Bauer, 1996, 2000; Sawant et al., 2001b; Belyakov et al., 2002a, Iyer and Lehnert, 2002a, 2002b). For example, the analysis of cell transformation data for α-particle microbeam experiments by Brenner et al. (2001) suggest the possibility of supralinear responses for very low doses of high-LET radiation. On the other hand, the selective removal of damaged cells through apoptosis or terminal differentiation are examples of nongenotoxic bystander-induced radioprotective mechanisms (Bauer, 1995, 1996,Bauer, 2002; Belyakov et al., 2001a, 2001b, 2002a, 2002b, 2003). Scott et al. (2003, 2004) use the concept of a radiation-induced bystander effect for apoptosis that protects the cell community from problematic mutations, neoplastic transformation and cancer. The concept of cell killing has also been used by Radivoyevitch et al. (2002) and Bogen (1997, 1998, 2001) to develope hormesis models. Barcellos-Hoff and Brooks (2001) propose that very low doses of IR could stimulate the extracellular signaling that eliminates abnormal cells. Redpath et al. (2003a) recently reported that selective killing of a transformation-sensitive G(2)/M-phase subpopulation as a consequence of low-dose hyperradiosensitivity could account in part for the observed reduction of induced transformation frequencies at low doses to values below that observed spontaneously. The net effects of the interplay among different types of bystander effects are open to debate, and additional research in this area is needed.
The genotoxic endpoint most clearly associated with human tumors are chromosome aberrations. Chromosomal alterations are present in 99.9% of all tumors, and they are both involved in the production of the tumor and produced as a consequence of the carcinogenic and genomic instability process (Preston, 2003). The great majority of structural chromosome aberrations require two DNA lesions in their formation. These lesions, in the case of radiation-induced chromosome alterations, can be produced by a single track of high- or low-LET radiation or by two independent tracks of low-LET radiation. Preston and others state that consequently the dose-response curves for low-LET-induced chromosome aberrations are linear or linear-quadratic with a linear slope at low dose levels (NCRP, 2001; Preston, 2003). The majority of biological dosimetry studies on chromosome aberrations provide broad support for a linear response at low dose levels (NCRP, 2001). From that it was concluded that low-dose responses for cancer are linear at low doses (NCRP, 2001). This line of arguments, however, ignores the possibility that cellular defense mechanisms, induced also by the DNA lesions that will eventually form chromosome aberrations, also impact on the vast amount of endogenously produced reactive oxygen species and lesions; that is, the hormesis concepts examined in the current study.
CONCLUSIONS
Sensitivity studies highlight the potential importance of including endogenously formed DNA damage in calculations of low-dose cumulative cancer incidence levels. For dose levels comparable to background radiation, our studies suggest that endogenous DNA damage may account for 86–97% of the predicted cancers. Even for a lifetime dose of 1 Gy, endogenous processes may account for as much as 48% of the predicted cancers. These predictions are sensitive to the rate at which simple lesions are created through endogenous processes (i.e., parameter). The rate multiply damaged sites are formed through endogenous processes ( parameter) is a potentially important parameter. However, additional experimental work is needed to reduce the uncertainties associated with this parameter. Additional research to better estimate the fidelity of multiply damaged site DNA repair, φcl , could have a substantial impact on uncertainties in model predictions.
Support for the possibility of hormetic effects from low doses of low-LET radiation comes from both in vivo animal carcinogenesis studies (Wang et al., 1998, 2000; Mitchel et al., 1999, 2002, 2003) as well as studies performed using cell cultures irradiated with low-LET radiation at very low doses rates. These in vitro experiments show a reduction of induced transformation frequencies at low doses to values below that observed spontaneously (Azzam et al., 1996; Redpath and Antoniono, 1998; Redpath et al., 2001, 2003a, 2003b). DNA repair mechanisms are highly conserved among all eukaryotes (Mitchel, 1995; Mitchel et al., 1997), and if these phenomena arise as the result of adaptations in DNA repair, it is not unreasonable to expect that some or all of these phenomena will also occur in humans. Nevertheless, environmental radiation sources result in dose rates in the range from 1 to 3 mGy/year, and this range of dose rates is approximately a factor of 106 lower than the dose rates used in the cell culture studies mentioned earlier (2.4–3.3 mGy/min). Additional research is needed to examine how DNA repair and radical scavenging affect in vitro and in vivo responses.
In the current model, distinct U-shaped curves are only produced when both the accuracy of DNA repair and the capacity for radical scavenging are enhanced about three-fold. Although this degree of defence induction is much higher than the 40% proposed by Pollycove and Feinendegen, their hormesis concept comprises other mechanisms that are not included in this modeling effort. If experiments demonstrate that the effects of DNA damage repair and radical scavenging are enhanced at least three-fold under low-dose conditions, our studies would support the existence of U-shaped responses for lung cancer. If repair processes are altered to a lesser degree, U-shaped responses may not arise unless factors other than repair and radical scavenging play a significant role in the pathogenesis of lung cancer. Dose- or dose-rate-dependent modulation of cell-killing, bystander-induced apoptosis and cell differentiation and detrimental bystander effects are processes that may further alter the shape of cancer incidence curves (see, for example, discussions by Pollycove and Feinendegen, 2001, 2003) and that should be addressed in future studies.
The multistage cancer model formulated in this work provides a useful formalism to investigate the potential impact of some cellular defence mechanisms. Future modeling efforts should extend the approach to account for other possible cell and tissue-level defence mechanisms as well account for possible detrimental effects that may arise through cell-to-cell signaling phenomena (i.e., bystander effects). In addition, extended sensitivity analyses applying Monte Carlo techniques with respect to the uncertainty ranges of the model parameters could prove to be a valuable extension of the current study.
Acknowledgments
The authors wish to thank Dr. Marco Brugmans, RIVM; Dr. Ludwig E. Feinendegen, Heinrich-Heine-University Düsseldorf; Dr. Hatim Fakir, University of Salzburg; and Dr. Steve Belinsky, LRRI, for valuable discussions. We also thank the journal referees for their valuable comments. This work was supported by the EU project CEC Contract FIGH-CT-1999-00005, by a Marie Curie Individual Fellowship EC Contract FIGH-CT-2002–50513, and by the Office of Science (BER), U.S. Department of Energy, Grants DE-FG02-03ER63541 and DE-FG02-03ER63665.
APPENDIX: DERIVATION OF THE CLOSED-FORM SOLUTION FOR THE CANCER MODEL
A three-stage clonal expansion model was developed. A conceptual view of the model is given in Figure 1. The model equations, a set of coupled differential equations, Eqs. (1)–(6), were solved with variation of constants as shown.
Equations (2) and (3) can be written as follows: dN1(t)
| (A1) |
| (A2) |
We define the parameters a =kM1 – kd1 – k and b = kM2 – kd2 – kmt. This gives
| (A3) |
| (A4) |
Equations (A3) and (A4) can be solved, for example, with variation of constants or eigenvector methods. We briefly sketch the method that uses variation of constants.
We first deal with Eq. (A3). The homogenous equation is dN1(t)/dt –aN1(t) =0. Ansatz N1(t) =exp α(t ) leads to the general solution of the homogenous equation: N1(t) =c exp(at); c is a constant. A solution of the inhomogenous equation (A3) is called a particular solution and is derived with variation of constants: Ansatz N1part(t) =c(t) exp(at) is used within the inhomogenous equation. This leads to N1part(t) =kN0[exp(at) –1]/a. The general solution of the inhomogenous equation therefore is N1(t) =ceat +kN0[exp(at) –1]/a. The initial condition N1(t =0) =0 applied within this latter equation yields c =0 and gives the final solution for N1(t ):
| (A5) |
Analogously, the solution for N2(t ) was derived as
| (A6) |
where a and b were defined earlier. The dose-rate-dependent rate constant k(Ḋ) was defined in Eq. (13).
It can easily be shown that N1(t ) and N2(t ) fulfill the initial conditions. For N2(t ), for example, we get at t =0
The expression in parentheses on the right-hand side of this equation is also zero. It is also easy to see that N1(t ) fulfills the initial condition.
To calculate N3(t ) [see Eq. (4)], we need to integrate Eq. (A6) over time. This leads to
| (A7) |
As explained earlier, kmt is constant and was therefore moved to the front of the integral. With N3(t =0) =0, one gets
| (A8) |
with
N4(t ) is then given as
| (A9) |
Simulations were performed with Excel software using the closed-form solution for N5(t ) given in Eq. (6).
Footnotes
E. G. Luebeck, Fred Hutchinson Cancer Research Center, personal communication.
S. Belinsky, Lovelace Respiratory Research Institute, personal communication.
REFERENCES
- Achanta G, Pelicano H, Feng L, Plunkett W, Huang P. Interaction of p53 and DNA-PK in response to nucleoside analogues: Potential role as a sensor complex for DNA damage. Cancer Res. 2001;61(24):8723–8729. [PubMed] [Google Scholar]
- ACS (American Cancer Society) Cancer Facts and Figures 2002 New York: American Cancer Society; 2002. (http:www.cancer.org). [Google Scholar]
- Akashi M, Hachiya M, Paquette RL, Osawa Y, Shimizu S, Suzuki G. Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. Possible mechanisms for its accumulation. J. Biol Chem. 1995;270(26):15864–15869. doi: 10.1074/jbc.270.26.15864. [DOI] [PubMed] [Google Scholar]
- Akyuz N, Boehden GS, Susse S, Rimek A, Preuss U, Scheidtmann KH, Wiesmuller L. DNA substrate dependence of p53-mediated regulation of double-strand break repair. Mol Cell Biol. 2002;22(17):6306–6317. doi: 10.1128/MCB.22.17.6306-6317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Publishing (Taylor & Francis); 2002. [Google Scholar]
- Andersen ME, Conolly RB. Mechanistic modeling of rodent liver tumor promotion at low levels of exposure: An example related to dose-response relationships for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Hum Exp Toxicol. 1998;17(12):683–690. doi: 10.1177/096032719801701208. [DOI] [PubMed] [Google Scholar]
- Azzam EI, Raaphorst GP, Mitchel REJ. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat Res. 1994a;138(1 Suppl):S28–31. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Réponse adaptative au rayonnement ionisant des fibroblastes de peau humaine Augmentation de la vitesse de réparation de 1’ADN et variation de l’expression des génes. J Chim Phys. 1994b;91:931–936. [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146(4):369–373. [PubMed] [Google Scholar]
- Ballarini F, Biaggi M, Ottolenghi A, Sapora O. Cellular communication and bystander effects: A critical review for modelling low-dose radiation action. Mutat Res. 2002;501(1–2):1–12. doi: 10.1016/s0027-5107(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Brooks AL.Extracellular signaling through the microenvironment: A hypothesis relating carcinogenesis, bystander effects, and genomic instability Radiat Res 2001156(5 Pt 2):618–627. [DOI] [PubMed] [Google Scholar]
- Bauchinger M, Schmid E, Braselmann H, Nahrstedt U. Absence of adaptive response to low-level irradiation from tritiated thymidine and X-rays in lymphocytes of two individuals examined in serial experiments. Mutat Res. 1989;227(2):103–107. doi: 10.1016/0165-7992(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Bauer G. Resistance to TGF-β-induced elimination of transformed cells is required during tumor progression. Int J Oncol. 1995;6:1227–1229. doi: 10.3892/ijo.6.6.1227. [DOI] [PubMed] [Google Scholar]
- Bauer G. Elimination of transformed cells by normal cells: A novel concept for the control of carcino-genesis. Histol Histopathol. 1996;11(1):237–255. [PubMed] [Google Scholar]
- Bauer G. Reactive oxygen and nitrogen species: Efficient, selective, and interactive signals during inter-cellular induction of apoptosis. Anticancer Res. 2000;20(6B):4115–4139. [PubMed] [Google Scholar]
- Becker K. Book review: Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation, NCRP Report 136. Health Phys. 2002;82(2):257–258. [Google Scholar]
- BEIR VI (Biological Effects of Ionizing Radiation) Health Risks of Exposure to RadonCommittee on the Biological Effects of Ionizing Radiation, Board on Radiation Effects Research, Commission on Life Sciences, National Research Council. Washington, DC: National Academy Press; 1999 [Google Scholar]
- Belyakov OV, Malcolmson AM, Folkard M, Prise KM, Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Brit J Cancer. 2001a;84(5):674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander effect and genomic instability. Challenging the classic paradigm of radiobiology. In: Korogodin VI, Korogodina VL, Dubrovina NI, editors. Modern Problems of Radiobiology, Radioecology and Evolution. Proceedings of the International Conference Dedicated to the Centenary of the Birth of NW Timofeeff-Ressovsky. Dubna; Russia: 2001b. pp. 80–90. [Google Scholar]
- Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation. Radiat Prot Dosim. 2002a;99(1–4):249–251. doi: 10.1093/oxfordjournals.rpd.a006775. [DOI] [PubMed] [Google Scholar]
- Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD.Non-targeted effects of radiation: Applications for radiation protection and contribution to LNT discussion Proceedings of the European IRPA Congress 2002: Towards Harmonization of Radiation Protection in EuropeFlorence; Italy: 8.11October2002b [Google Scholar]
- Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Brit J Cancer. 2003;88(5):767–774. doi: 10.1038/sj.bjc.6600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: Mortality from cancer and other causes 1897–1997. Brit J Radiol. 2001;74(882):507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- Billen D. Spontaneous DNA damage and its significance for the “negligible dose” controversy in radiation protection. Radiat Res. 1990;124(2):242–245. [PubMed] [Google Scholar]
- Blettner M, Zeeb H, Auvinen A, Ballard TJ, Caldora M, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hammer GP, Irvine D, Langner I, Paridou A, Pukkala E, Rafnsson V, Storm H, Tulinius H, Tveten U, Tzonou A. Mortality from cancer and other causes among male airline cockpit crew in Europe. Int J Cancer. 2003;106(6):946–952. doi: 10.1002/ijc.11328. [DOI] [PubMed] [Google Scholar]
- Bogen KT. Do U.S county data disprove linear no-threshold predictions of lung cancer risk for residential radon? A preliminary assessment of biological plausibility. Hum Ecol Risk Assess. 1997;3:157–186. [Google Scholar]
- Bogen KT. Mechanistic model predicts a U-shaped relation of radon exposure to lung cancer risk reflected in combined occupational and US residential data. Hum Exp Toxicol. 1998;17(12):691–696. doi: 10.1177/096032719801701209. [DOI] [PubMed] [Google Scholar]
- Bogen KT. Biologically based prediction of empirical nonlinearity in lung cancer risk vs. residential/ occupational radon exposure. Hum Ecol Risk Assess. 2001;7:811–827. [Google Scholar]
- Bond VP, Feinendegen LE, Sondhaus CA. Microdosimetric concepts applied to hormesis. Health Phys. 1987;52(5):659–661. doi: 10.1097/00004032-198705000-00019. [DOI] [PubMed] [Google Scholar]
- Boothman DA, Meyers M, Odegaard E, Wang M. Altered G1 checkpoint control determines adaptive survival responses to ionizing radiation. Mutat Res. 1996;358(2):143–153. doi: 10.1016/s0027-5107(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Boreham DR, Mitchel REJ. DNA lesions that signal the induction of radioresistance and DNA repair in yeast. Radiat Res. 1991;128:19–28. [PubMed] [Google Scholar]
- Bosi A, Olivieri G. Variability of the adaptive response to ionizing radiations in humans. Mutat Res. 1989;211(1):13–17. doi: 10.1016/0027-5107(89)90102-4. [DOI] [PubMed] [Google Scholar]
- Bravard A, Luccioni C, Moustacchi E, Rigaud O. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int J Radiat Biol. 1999;75(5):639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Ward JF. Constraints on energy deposition and target size of multiply damaged sites associated with DNA double-strand breaks. Int J Radiat Biol. 1992;61(6):737–748. doi: 10.1080/09553009214551591. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Little JB, Sachs RK. The bystander effect in radiation oncogenesis: II A quantitative model. Radiat Res. 2001;155(3):402–408. doi: 10.1667/0033-7587(2001)155[0402:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Broome EJ, Brown DL, Mitchel REJ. Dose responses for adaptation to low doses of 60Co-gamma rays and 3H beta particles in normal human fibroblasts. Radiat Res. 2002;158(2):181–186. doi: 10.1667/0033-7587(2002)158[0181:drfatl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Special issue on hormesis. Hum Exp Toxicol. 2000;19(1):2–97. doi: 10.1191/096032700678815585. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Special issue: Scientific foundations of hormesis. Crit Rev Toxicol. 2001a;31(4–5):353–695. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001b;22(6):285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. U-shaped dose-responses in biology, toxicology, and public health. Annu Rev Publ Health. 2001c;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: A generalizable and unifying hypothesis. Crit Rev Toxicol. 2001d;31(4–5):353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Coleman WB, Tsongalis GJ. Multiple mechanisms account for genomic instability and molecular mutation in neoplastic transformation. Clin Chem. 1995;41(5):644–657. [PubMed] [Google Scholar]
- Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO.Morphometric characteristics of cells in the alveolar region of mammalian lungs Am Rev Respir Dis 1983128(2 Pt 2)S42–S46. [DOI] [PubMed] [Google Scholar]
- Crawford-Brown DJ, Hofmann W. A generalized state-vector model for radiation induced cellular transformation. Int J Radiat Biol. 1990;57:407–423. doi: 10.1080/09553009014552501. [DOI] [PubMed] [Google Scholar]
- Cregan SP, Brown DL, Mitchel REJ. Apoptosis and the adaptive response in human lymphocytes. Int J Radiat Biol. 1999;75:1087–1094. doi: 10.1080/095530099139548. [DOI] [PubMed] [Google Scholar]
- Crump KS, Hoel DG, Langley CH, Peto R.Fundamental carcinogenic processes and their implications for low dose risk assessment Cancer Res 197636(9 Pt 1)2973–2979. [PubMed] [Google Scholar]
- Cucinotta FA, Nikjoo H, O’Neill P, Goodhead DT. Kinetics of DSB rejoining and formation of simple chromosome exchange aberrations. Int J Radiat Biol. 2000;76(11):1463–1474. doi: 10.1080/09553000050176225. [DOI] [PubMed] [Google Scholar]
- Daboussi F, Dumay A, Delacôte F, Lopez BS. DNA double-strand break repair signaling: The case of RAD51 post-translational regulation. Cell Signal. 2002;14:969–975. doi: 10.1016/s0898-6568(02)00052-9. [DOI] [PubMed] [Google Scholar]
- David-Cordonnier MH, Boiteux S, O’Neill P. Excision of 8-oxoguanine within clustered damage by the yeast OGG1 protein. Nucleic Acids Res. 2001;29(5):1107–1113. doi: 10.1093/nar/29.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FG, Boice JD, Jr, Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Mas-sachusetts tuberculosis patients. Cancer Res. 1989;49(21):6130–6136. [PubMed] [Google Scholar]
- DeWeese TL, Shipman JM, Larrier NA, Buckley NM, Kidd LR, Groopman JD, Cutler RG, te Riele H, Nelson WG. Mouse embryonic stem cells carrying one or two defective Msh2 alleles respond abnormally to oxidative stress inflicted by low-level radiation. Proc Nat Acad Sci USA. 1998;95(20):11915–11920. doi: 10.1073/pnas.95.20.11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikomey E, Lorenzen J. Saturated and unsaturated repair of DNA strand breaks in CHO cells after x-irradiation with doses ranging from 3 to 90 Gy. Int J Radiat Biol. 1993;64(6):659–667. doi: 10.1080/09553009314551901. [DOI] [PubMed] [Google Scholar]
- Dittmann K, Virsik-Kopp P, Mayer C, Rave-Frank M, Rodemann HP. Bowman–Birk protease inhibitor activates DNA-dependent protein kinase and reduces formation of radiation-induced dicentric chromosomes. Int J Radiat Biol. 2003;79(10):801–808. doi: 10.1080/09553000310001610277. [DOI] [PubMed] [Google Scholar]
- Dorland . Dorland’s Illustrated Medical Dictionary. 25th ed. Philadelphia, London, Toronto: Saunders WB; 1974. [Google Scholar]
- Downs T, Frankowski R. A cancer risk model with adaptive repair. Hum Exp. Toxicol. 1998;17(12):697–700. doi: 10.1177/096032719801701210. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M. Biologic responses to low doses of ionizing radiation: Detriment versus hormesis Part 1. Dose responses of cells and tissues. J Nucl Med. 2001;42(7):17N–27N. [PubMed] [Google Scholar]
- Feinendegen LE, Mühlensiepen H, Lindberg C, Marx J, Porschen W, Booz J. Acute and temporary inhibition of thymidine kinase in mouse bone marrow cells after low-dose exposure. Int J Radiat Biol Re. 1984;45(3):205–215. doi: 10.1080/09553008414550301. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Mühlensiepen H, Bond VP, Sondhaus CA. Intracellular stimulation of biochemical control mechanisms by low-dose, low-LET irradiation. Health Phys. 1987;52(5):663–669. doi: 10.1097/00004032-198705000-00020. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Bond VP, Booz J, Mühlensiepen H. Biochemical and cellular mechanisms of low-dose effects. Int J Radiat Biol Re. 1988;53(1):23–37. doi: 10.1080/09553008814550391. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Bond VP, Sondhaus CA, Mühlensiepen H. Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Mutat Res. 1996;358(2):199–205. doi: 10.1016/s0027-5107(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Sondhaus CA.Responses to low doses of ionizing radiation in biological systems Nonlin Biol Tox MedIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck CM, Schöllnberger H, Kottbauer MM, Dockal Th, Prüfert U. Modeling radioprotective mechanisms in the dose effect relation for low doses and low dose rates of ionizing radiation. Math Biosci. 1999;155(1):13–44. doi: 10.1016/s0025-5564(98)10053-6. [DOI] [PubMed] [Google Scholar]
- Frankenberg-Schwager M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat Environ Biophys. 1990;29:273–292. doi: 10.1007/BF01210408. [DOI] [PubMed] [Google Scholar]
- Friedberg EC. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:12–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W. DNA Repair and Mutagenesis. Washington, DC: ASM; 1995. [Google Scholar]
- Gazdar AF, Carbone DP. Austin, TX: RG Landes Company; 1994. The biology and molecular genetics of lung cancer. Medical Intelligence Unit. [Google Scholar]
- Goodhead DT. Saturable rapair models of radiation action in mammalian cells. Radiat Res Suppl. 1985;104:S58–S67. [PubMed] [Google Scholar]
- Goodhead DT. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int J Radiat Biol. 1994;65(1):7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- Goodhead DT, Thacker J, Cox R. Weiss lecture. Effects of radiations of different qualities on cells: Molecular mechanisms of damage and repair. Int J Radiat Biol. 1993;63:543–556. doi: 10.1080/09553009314450721. [DOI] [PubMed] [Google Scholar]
- Guess H, Crump K, Peto R. Uncertainty estimates for low-dose-rate extrapolations of animal carcinogenicity data. Cancer Res. 1977;37(10):3475–3483. [PubMed] [Google Scholar]
- Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang T-T, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya M, Shimizu S, Osawa Y, Akashi M.Endogenous production of tumour necrosis factor is required for manganese superoxide dismutase expression by irradiation in the human monocytic cell line THP-1 Biochem J 1997328(Pt 2):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J, Jaussi R, Burkart W. Lack of adaptive response to low doses of ionizing radiation in human lymphocytes from five different donors. Mutat Res. 1992;283(2):137–144. doi: 10.1016/0165-7992(92)90146-9. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC. Transcription-coupled repair and human disease. Science. 1994;266:1457–1458. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC. Genomic instability: Environmental invasion and the enemies within. Mutat Res. 1998;400(1–2):117–125. doi: 10.1016/s0027-5107(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Harrison L, Hatahet Z, Wallace SS. In vitro repair of synthetic ionizing radiation-induced multiply damaged DNA sites. J. Mol Biol. 1999;290(3):667–684. doi: 10.1006/jmbi.1999.2892. [DOI] [PubMed] [Google Scholar]
- Heitzmann M, Wilson R. Low-dose linearity: The rule or the exception? BELLE Newsletter. 1997;6(1) [Google Scholar]
- Howe GR. Lung cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with lung cancer mortality in the Atomic Bomb survivors study. Radiat Res. 1995;142(3):295–304. [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Publication 60, 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991;21(1–3) [PubMed] [Google Scholar]
- ICRU (International Commission on Radiation Units and Measurements) Publication 36.Microdosimetry Bethesda, MD: ICRU; 1983 [Google Scholar]
- Iyer R, Lehnert BE. Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res. 2002a;157(1):3–7. doi: 10.1667/0033-7587(2002)157[0003:apiiit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res. 2002b;503(1–2):1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Jacobson-Kram D, Williams JR. Failure to observe adaptive response to ionising radiation in mouse bone marrow cells in vitro. Environ Mol Mutagen. 1988;2:49–50. [Google Scholar]
- Jeggo PA. Identification of genes involved in repair of DNA double-strand breaks in mammalian cells. Radiat Res. 1998;150(5 Suppl):S80–S91. [PubMed] [Google Scholar]
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol. 2001;49(2):379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Hormesis. A healthful dab of radiation? Science. 2003a;302(5644):378. doi: 10.1126/science.302.5644.378. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Hormesis. Sipping from a poisoned chalice. Science. 2003b;302(5644):376–379. doi: 10.1126/science.302.5644.376. [DOI] [PubMed] [Google Scholar]
- Kim SG, Nam SY, Kim CW, Kim JH, Cho CK, Yoo SY. Enhancement of radiation-inducible hepatic glutathione-S-transferases Ya, Yb1, Yb2, Yc1, and Yc2 gene expression by oltipraz: Possible role in radioprotection. Mol Pharmacol. 1997;51(2):225–233. doi: 10.1124/mol.51.2.225. [DOI] [PubMed] [Google Scholar]
- Le XC, Xing JZ, Lee J, Leadon SA, Weinfeld M. Inducible repair of thymine glycol detected by an ultrasensitive assay for DNA damage. Science. 1998;280:1066–1069. doi: 10.1126/science.280.5366.1066. [DOI] [PubMed] [Google Scholar]
- Leenhouts HP. Radon-induced lung cancer in smokers and non-smokers: Risk implications using a two-mutation carcinogenesis model. Radiat Environ Biophys. 1999;38(1):57–71. doi: 10.1007/s004110050138. [DOI] [PubMed] [Google Scholar]
- Leenhouts HP, Chadwick KH. A two-mutation model of radiation carcinogenesis: Application to lung tumours in rodents and implications for risk evaluation. J Radiol Prot. 1994;14(2):115–130. [Google Scholar]
- Lewis LK, Resnick MA. Tying up loose ends: Nonhomologous end-joining in Saccharomyces cerevisiae. Mutat Res. 2000;451(1–2):71–89. doi: 10.1016/s0027-5107(00)00041-5. [DOI] [PubMed] [Google Scholar]
- Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21(3):397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21(3):379–385. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- Luebeck EG, Heidenreich WF, Hazelton WD, Paretzke HG, Moolgavkar SH. Biologically based analysis of the data for the Colorado Uranium Miners cohort: Age, dose and dose-rate effects. Radiat Res. 1999;152:339–351. [PubMed] [Google Scholar]
- Marples B, Joiner MC. Modification of survival by DNA repair modifiers: A probable explanation for the phenomenon of increased radioresistance. Int J Radiat Biol. 2000;76(3):305–312. doi: 10.1080/095530000138646. [DOI] [PubMed] [Google Scholar]
- Memisoglu A, Samson L. Base excision repair in yeast and mammals. Mutat Res. 2000a;451(1–2):39–51. doi: 10.1016/s0027-5107(00)00039-7. [DOI] [PubMed] [Google Scholar]
- Memisoglu A, Samson L. Contribution of base excision repair, nucleotide excision repair, and DNA recombination to alkylation resistance of the fission yeast. Schizosaccharomyces pombe. J Bacteriol. 2000b;182:2104–2112. doi: 10.1128/jb.182.8.2104-2112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Resp Cell Mol. 1994;10(6):613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Milligan JR, Aguilera JA, Ward JF. Variation of single-strand break yield with scavenger concentration for plasmid DNA irradiated in aqueous solution. Radiat Res. 1993;133(2):151–157. [PubMed] [Google Scholar]
- Mitchel REJ. Mechanisms of the adaptive response in irradiated mammalian cells. Radiat Res. 1995;141:117–118. [Google Scholar]
- Mitchel REJ, Morrison DP. Heat-shock induction of ionizing radiation resistance in Saccharomyces cerevisiae, and correlation with stationary growth phase. Radiat Res. 1982;90:284–291. [PubMed] [Google Scholar]
- Mitchel REJ, Morrison DP. Inducible error-prone repair in yeast. Suppression by heat shock. Mutat Res. 1986;159(1–2):31–39. doi: 10.1016/0027-5107(86)90109-0. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Morrison DP. Inducible DNA-repair systems in yeast: Competition for lesions. Mutat Res. 1987;183(2):149–159. doi: 10.1016/0167-8817(87)90057-5. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Azzam EI, de Toledo SM. Adaptation to ionizing radiation in mammalian cells. In: Koval TM, editor. Stress-Inducible Processes in Higher Eukaryotic Cells. New York: Plenum Press; 1997. pp. 221–243. [Google Scholar]
- Mitchel REJ, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel REJ, Dolling JA, Misonoh J, Boreham DR. Influence of prior exposure to low dose adapting radiation on radiation induced teratogenic effects in fetal mice with varying Trp 53 function. Radiat Res. 2002;158:458–463. doi: 10.1667/0033-7587(2002)158[0458:iopetl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Jakson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Knudson AG., Jr Mutation and cancer: A model for human carcinogenesis. J. Natl Cancer Inst. 1981;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- Mossman KL. Deconstructing radiation hormesis. Health Phys. 2001;80(3):263–269. doi: 10.1097/00004032-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Morcillo MA, Rucandio MI, Santamaria J. Effect of gamma irradiation on liver metallothionein synthesis and lipid peroxidation in rats. Cell Mol Biol. 2000;46(2):435–444. [PubMed] [Google Scholar]
- Müller WU, Streffer C, Niedereichholz F. Adaptive response in mouse embryos? Int J Radiat Biol. 1992;62(2):169–175. doi: 10.1080/09553009214551981. [DOI] [PubMed] [Google Scholar]
- NCRP (National Council on Radiation Protection and Measurements). Ionizing Radiation Exposure of the Population of the United States; 1987. NCRP Report 93, Bethesda, MD.
- NCRP. Evaluation of the Linear-Nonthreshold Dose-Respons Model for Ionizing Radiation, 2001. NCRP Report 136, Bethesda, MD.
- Nickoloff JA, Hoekstra MF, editors. DNA Damage and Repair. Totowa, NJ: Humana Press; 1998. [Google Scholar]
- Oberley LW, St Clair DK, Autor AP, Oberley TD. Increase in manganese superoxide dismutase activity in the mouse heart after X-irradiation. Arch Biochem Biophys. 1987;254:69–80. doi: 10.1016/0003-9861(87)90082-8. [DOI] [PubMed] [Google Scholar]
- Oleinick NL, Chiu S, Friedman LR. Gamma radiation as a probe of chromatin structure: Damage to and repair of active chromatin in the metaphase chromosome. Radiat Res. 1984;98:629–641. [PubMed] [Google Scholar]
- Pant MC, Liao XY, Lu Q, Molloi S, Elmore E, Redpath JL. Mechanisms of suppression of neoplastic transformation in vitro by low doses of low LET radiation. Carcinogenesis. 2003;24(12):1961–1965. doi: 10.1093/carcin/bgg172. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Biologic responses to low doses of ionizing radiation: Detriment versus hormesis. Part 2. Dose responses of organisms. J Nuc Med. 2001;42(9):26N–32N. 37N. [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: Possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22(6):290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Preston RJ. The LNT model is the best we can do—Today. J Radiol Prot. 2003;23(3):263–268. doi: 10.1088/0952-4746/23/3/303. [DOI] [PubMed] [Google Scholar]
- Raaphorst GP, Boyden S. Adaptive response and its variation in human normal and tumour cells. Int J Radiat Biol. 1999;75(7):865–873. doi: 10.1080/095530099139926. [DOI] [PubMed] [Google Scholar]
- Radivoyevitch T, Kozubek S, Sachs RK. The risk of chronic myeloid leukemia: Can the dose-response curve be U-shaped? Radiat Res. 2002;157(1):106–109. doi: 10.1667/0033-7587(2002)157[0106:trocml]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath LJ, Antoniono RJ. Induction of an adaptive response against spontaneous neoplastic transformation in vitro by low-dose gamma radiation. Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, Christie C, Elmore E. The shape of the dose response curve for radiation-induced neoplastic transformation in vitro: Evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156(6):700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Short SC, Woodcock M, Johnston PJ. Low-dose reduction in transformation frequency compared to unirradiated controls: The role of hyper-radiosensitivity to cell death. Radiat Res. 2003a;159(3):433–436. doi: 10.1667/0033-7587(2003)159[0433:ldritf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Lao X, Molloi S, Elmore E. Low doses of diagnostic energy X rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003b;79(4):235–240. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Renner R. Hormesis in vitro. Nietzsche’s toxicology. Sci Am. 2003;289(3):28–30. doi: 10.1038/scientificamerican0903-28. [DOI] [PubMed] [Google Scholar]
- Rossi HH, Zaider M. Radiogenic lung cancer: The effects of low doses of low linear energy transfer (LET) radiation. Radiat Environ Biophys. 1997;36(2):85–88. [PubMed] [Google Scholar]
- Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan K, von Duyn A, Loos MJ, Natarajan AT. Adaptive response of human lymphocytes to low-level radiation from radioisotopes or X-rays. Mutat Res. 1989;211(1):7–12. doi: 10.1016/0027-5107(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncoge-nesis: I Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res. 2001a;155(3):397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Metting NF, Hall EJ. Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat Res. 2001b;156(2):177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schmid E, Bauchinger M, Nahrstedt U. Adaptive response after X-irradiation of human lymphocytes? Mutagenesis. 1989;4(2):87–89. doi: 10.1093/mutage/4.2.87. [DOI] [PubMed] [Google Scholar]
- Schmutte C, Fishel R. Genomic instability: First step to carcinogenesis. Anticancer Res. 1999;19(6A):4665–4696. [PubMed] [Google Scholar]
- Schöllnberger H, Kotecki M, Crawford-Brown DJ, Hofmann W, Eckl PM. Adaptive response and dose-response plateaus for initiation in a state-vector model of carcinogenesis. Int J Radiat Biol. 1999;75(3):351–364. doi: 10.1080/095530099140528. [DOI] [PubMed] [Google Scholar]
- Schöllnberger H, M′enache MM, Hanson TE. A biomathematical modeling approach to explain the phenomenon of radiation hormesis. Hum Ecol Risk Assess. 2001a;7(4):867–890. [Google Scholar]
- Schöllnberger H, Scott BR, Hanson TE. Application of Bayesian inference to characterize risks associated with low doses of low-LET radiation. Bull Math Biol. 2001b;63(5):865–883. doi: 10.1006/bulm.2001.0243. [DOI] [PubMed] [Google Scholar]
- Schöllnberger H, Mebust MR, Crawford-Brown DJ, Eckl PM, Hofmann W. The significance of cell-cycle delay, multiple initiation pathways, misrepair, and replication errors in a model of radiobiological effects. Int J Radiat Biol. 2001c;77(4):519–527. doi: 10.1080/09553000010029132. [DOI] [PubMed] [Google Scholar]
- Schöllnberger H, Mitchel REJ, Crawford-Brown DJ, Hofmann W. Nonlinear dose-response relationships and inducible cellular defence mechanisms. J Radiol Prot. 2002a;22(3A):A21–A25. doi: 10.1088/0952-4746/22/3a/304. [DOI] [PubMed] [Google Scholar]
- Schöllnberger H, Mitchel REJ, Crawford-Brown DJ, Hofmann W. Explanation of protective effects of low doses of -radiation with a mechanistic radiobiological model. Int J Radiat Biol. 2002b;78(12):1159–1173. doi: 10.1080/0955300021000034693. [DOI] [PubMed] [Google Scholar]
- Scott BR. Mechanistic state vector model for cell killing by ionizing radiation. Radiat Environ Biophys. 1977;14:195–211. doi: 10.1007/BF01323939. [DOI] [PubMed] [Google Scholar]
- Scott BR. A mechanistic model for neoplastic transformation of cells by high LET radiation and its implication for low dose, low dose rate risk assessment. Radiat Prot Dosim. 1997;72:105–117. [Google Scholar]
- Scott BR, Ainsworth EJ. State vector model for life shortening in mice after brief exposures to low doses of ionizing radiation. Math Biosci. 1980;49:185–205. [Google Scholar]
- Scott BR, Walker DM, Tesfaigzi Y, Schöllnberger H, Walker V. Mechanistic basis for nonlinear dose-response relationships for low-dose radiation-induced stochastic effects. Nonlin Biol Tox Med. 2003;1(1):93–122. doi: 10.1080/15401420390844492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Walker DM, Walker VE.Low-dose radiation and genotoxic chemicals can protect against stochastic biological effects Nonlin Biol Tox Medin press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadley JD. Chromosomal adaptive response in human lymphocytes. Radiat Res. 1994;138(1 Suppl):S9–12. [PubMed] [Google Scholar]
- Shimizu T, Iwanaga M, Yasunaga A, Urata Y, Goto S, Shibata S, Kondo T. Protective role of glutathione synthesis on radiation-induced DNA damage in rabbit brain. Cell Mol Neurobiol. 1998;18:299–310. doi: 10.1023/a:1022525214871. [DOI] [PubMed] [Google Scholar]
- Slob W. Thresholds in toxicology and risk assessment. Int. J. of Toxicol. 1999;18:259–268. [Google Scholar]
- Sorensen KJ, Attix CM, Christian AT, Wyrobek AJ, Tucker JD. Adaptive response induction and variation in human lymphoblastoid cell lines. Mutat Res. 2002;519(1–2):15–24. doi: 10.1016/s1383-5718(02)00110-9. [DOI] [PubMed] [Google Scholar]
- Stecca C, Gerber GB. Adaptive response to DNA-damaging agents. Biochem Pharmacol. 55:941–951. doi: 10.1016/s0006-2952(97)00448-6. [DOI] [PubMed] [Google Scholar]
- Stewart RD. On the complexity of the DNA damage created by endogenous processes. Radiat Res. 1999;152(1):101–104. [PubMed] [Google Scholar]
- Summers RW, Maves BV, Reeves RD, Arjes LJ, Oberley LW. Irradiation increases superoxide dismutase in rat intestinal smooth muscle. Free Radic Biol Med. 1989;6:261–270. doi: 10.1016/0891-5849(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Symington LS. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev. 2002;66(4):630–670. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl H, Altmann H, Kovac R, Topaloglou A, Egg D, Günther R. Effects of low-dose radiation on repair processes in human lymphocytes. Radiat Res. 1980;81:1–9. [PubMed] [Google Scholar]
- Tuschl H, Kovac R, Altmann H. UDS and SCE in lymphocytes of persons occupationally exposed to low levels of ionizing radiation. Health Phys. 1983;45:1–7. doi: 10.1097/00004032-198307000-00001. [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Sources and Effects of Ionizing Radiation. UNSCEAR 1993 Report to the General Assembly, with Scientific Annexes; 1993.
- UNSCEAR. Sources and Effects of Ionizing Radiation. UNSCEAR 1994 Report to the General Assembly with Scientific Annexes; 1994. [Google Scholar]
- UNSCEAR. Sources and effects of ionizing radiation, Vol II: Effects. UNSCEAR 2000 Report to the General Assembly with Scientific Annexes; 2000. [Google Scholar]
- Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Leal BZ, Deahl TS, Meltz ML. Variability in adaptive response to low dose radiation in human blood lymphocytes: Consistent results from chromosome aberrations and micronuclei. Mutat Res. 1995;348(1):45–50. doi: 10.1016/0165-7992(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Wang B, Ohyama H, Nose T, Itsukaichi H, Nakajima T, Yukawa O, Odaka T, Tanaka K, Kojima E, Yamada T, Hayata I. Adaptive response in embryogenesis: I Dose and timing of radiation for reduction of prenatal death and congenital malformation during the late period of organogenesis. Radiat Res. 1998;150:120–122. [PubMed] [Google Scholar]
- Wang B, Ohyama H, Haginoya K, Odaka T, Itsukaichi H, Yukawa O, Yamada T, Hayata I. Adaptive response in embryogenesis III. Relationship to radiation-induced apoptosis and Trp53 gene status. Radiat Res. 2000;154:277–282. doi: 10.1667/0033-7587(2000)154[0277:arieir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ward JF. Biochemistry of DNA lesions. Radiat Res. 1985;104:S103–S111. [PubMed] [Google Scholar]
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Ward JF. The complexity of DNA damage: Relevance to biological consequences. Int J Radiat Biol. 1994;66(5):427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- Ward JF, Blakely WF, Joner EI. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985;103(3):383–392. [PubMed] [Google Scholar]
- Wellinger RE, Thoma F. Nucleosome structure and positioning modulate nucleotide excision repair in the non-transcribed strand of an active gene. EMBO J. 1997;16:5046–5056. doi: 10.1093/emboj/16.16.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler KT, Nelson GB. Saturation of a DNA repair process in dividing and nondividing mammalian cells. Radiat Res. 1987;109:109–117. [Google Scholar]
- Wojcik A, Bonk K, Müller WU, Streffer C, Weissenborn U, Obe G. Absence of adaptive response to low doses of X-rays in preimplantation embryos and spleen lymphocytes of an inbred mouse strain as compared to human peripheral lymphocytes: A cytogenetic study. Int J Radiat Biol. 1992;62(2):177–186. doi: 10.1080/09553009214551991. [DOI] [PubMed] [Google Scholar]
- Wojcik A, Bonk K, Streffer C. Is there an adaptive response in spleen lymphocytes of C57B1/6 mice as assessed by chromosomal aberrations? Radiat Res. 1993;135(2):249–256. [PubMed] [Google Scholar]
- Wolff S. Adaptation of human and other mammalian cells to low doses of radiation. Radiat Res. 1995;141:115–117. [Google Scholar]
- Wolff S. The adaptive response in radiobiology: Evolving insights and implications. Environ Health Persp. 1998;106(Suppl 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- Wong GH, Kaspar RL, Vehar G. Tumor necrosis factor and lymphotoxin: Protection against oxidative stress through induction of MnSOD. EXS. 1996;77:321–333. doi: 10.1007/978-3-0348-9088-5_21. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Edamatsu R, Mori A. Increased SOD activities and decreased lipid peroxide levels induced by low dose X irradiation in rat organs. Free Radical Biol Med. 1991;11:299–306. doi: 10.1016/0891-5849(91)90127-o. [DOI] [PubMed] [Google Scholar]
- Ye N, Bianchi MS, Bianchi NO, Holmquist GP. Adaptive enhancement and kinetics of nucleotide excision repair in humans. Mutat Res. 1999;435(1):43–61. doi: 10.1016/s0921-8777(99)00022-1. [DOI] [PubMed] [Google Scholar]
- Yukawa O, Nakajima T, Yukawa M, Ozawa T, Yamada T. 1999. Induction of radical scavenging ability and protection against radiation-induced damage to miscrosomal membranes following low-dose irradiation. Int J Radiat Biol. 1999;75(9):1189–1199. doi: 10.1080/095530099139665. [DOI] [PubMed] [Google Scholar]
- Zamboglou N, Porschen W, Mühlensiepen H, Booz J, Feinendegen LE. Low dose effect of ionizing radiation on incorporation of iododeoxyuridine into bone marrow cells. Int J Radiat Biol Re. 1981;39(1):83–93. doi: 10.1080/09553008114550101. [DOI] [PubMed] [Google Scholar]