Abstract
A 6-year-old, spayed female dog was evaluated for a history of chronic coughing, excessive panting, and lethargy. Iatrogenic hyperadrenocorticism was diagnosed, and pulmonary mineralization was documented with a 99mTechnitium-methylene diphosphonate (99mTc-MDP) scan. Blood gas analysis showed hypoxia. Clinical signs resolved and blood gas values returned to normal when corticosteroid therapy was discontinued.
Résumé
Diagnostic et devenir d’un chien avec de l’hypercorticisme et une minéralisation pulmonaire secondaire. Une chienne châtrée âgée de 6 ans est évaluée pour des antécédents de toux chronique, d’halètements excessifs et de léthargie. L’hypercorticisme iatrogénique est diagnostiqué et une minéralisation pulmonaire est documentée avec un échogramme au 99m Technétium méthylène disphosphonate (99mTc-MDP). L’analyse des gaz sanguins a montré de l’hypoxie. Les signes cliniques se sont résorbés et les valeurs de gaz sanguins sont retournées à la normale lorsque la thérapie aux corticostéroïdes a été discontinuée.
(Traduit par Isabelle Vallières)
A 6-year-old, spayed female, bichon frisé cross, weighing 8 kg, was referred to the Ontario Veterinary College (OVC) for evaluation of excessive panting, intermittent coughing, and exercise intolerance. The referring veterinarian had radiographed the thorax and observed a diffuse alveolar pattern throughout the lung fields. Treatment with furosemide did not improve the radiographic appearance of the lungs. Theophylline (Apotex, Toronto, Ontario), 6 mg/kg bodyweight (BW), PO, q8h for 2 mo resulted in minimal improvement of the cough. The dog had been treated for pruritus with a combination of trimeprazine tartrate and prednisolone (Vanectyl-P; Pfizer, Kirkland, Quebec), 1.6 mg/kg BW and 0.25 mg/kg BW, respectively, PO, q12h since she was 4 mo of age.
Case description
Upon examination at the OVC, the dog was moderately overweight, with a thin hair coat and rat-tailed appearance. The abdomen was distended, and mild hepatomegaly was palpated. The skin over the abdomen appeared thin. Mildly increased bronchovesicular sounds were ausculted diffusely throughout the lung fields. The patient became tachypneic and cyanotic when stressed.
A complete blood (cell) count (CBC) revealed a mild leukocytosis (leukocytes 20.5 × 109/L; reference range: 4.9 to 15.4 × 109/L) characterized by a mild mature neutrophilia (segmented neutrophils 18.45 × 109/L; reference range: 2.9 to 10.6 × 109/L), and a mild thrombocytosis (platelets 567 × 109/L; reference range: 117 to 418 × 109/L). Results from a serum biochemical profile and a urinalysis were unremarkable, but a urine culture from a sample collected via cystocentesis yielded growth of an Enterococcus sp. Amoxicillin (Amoxil; Pfizer), 25 mg/kg BW, PO, q12h for 2 wk, was prescribed to treat the urinary tract infection. Iatrogenic hyperadrenocorticism was suspected because of the dog’s alopecia and hepatomegaly, as well as its history of chronic prednisolone therapy. An adrenocorticotropic hormone (ACTH) stimulation test was performed, 48 h after the dog had received a combination dose of trimeprazine tartrate and prednisolone (1.6 mg/kg BW and 0.25 mg/kg BW, respectively, PO), by administering corticotropin (Bexco ACTH; Bexco Pharma, Mississauga, Ontario), 2.2 U/kg BW, IM, once. Pre and 1-hour post-ACTH stimulation cortisol levels were < 28 nmol/L (pre-ACTH reference range, 30 to 300; Immulite cortisol assay, Siemens Medical Solutions Diagnostics, Loa Angeles, California, USA), thus confirming that the dog had iatrogenic hyperadrenocorticism. The endogenous ACTH concentration was low (3.3 pmol/L; reference range: 7 to 40 pmol/L).
Results from an arterial blood gas analysis were consistent with mild to moderate hypoxia. The arterial partial pressure of oxygen (PaO2) was 55.1 mmHg (reference range: 80 to 112 mmHg), and the arterial partial pressure of carbon dioxide (PaO2) was 30.2 mmHg (reference range: 23 to 42 mmHg). Oxygen saturation in the sample was 86.7% (reference range: > 95%). An alveolar-arterial oxygen gradient was calculated and found to be elevated (56.1 mmHg, reference range: < 15 mm Hg).
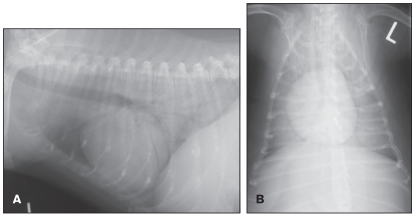
Thoracic radiographs revealed a diffuse alveolar pattern (Figures 1A and 1B). Given the lack of evidence for cardiac disease and the static nature of the radiographic lesions, despite empirical treatment of the dog with furosemide and prednisolone, mineralization, fibrosis, and neoplasia were the primary radiographic differential diagnoses. A thoracic ultrasonograph showed mild irregularity of the pleural surface, but it did not demonstrate fluid or cellular infiltration in the pulmonary parenchyma. Abdominal ultrasonography revealed a diffuse hyperechogenic hepatopathy and adrenal glands that were considered small bilaterally (0.32 cm at the thickest point for the right adrenal gland, 0.29 cm for the left), although this latter finding was considered subjective, as the lower size limit for the canine adrenal gland has not been published. The ultrasonographic changes were consistent with those expected with chronic administration of exogenous steroid. Bronchoscopy and bronchoalveolar lavage were not performed due to the potential risk of oxygen desaturation during anesthesia. Transtracheal wash was declined by the owners due to the cost involved.
Figure 1.
Left lateral (A) and dorsoventral (B) thoracic radiographs showing diffuse alveolar pulmonary changes, most prominent in the caudodorsal lung field.
Based on the suspicion that there was pulmonary mineralization, a nuclear scintigraphic examination of the thorax was performed. Two hours post IV injection of 141 megabecquerel (MBq) of 99mTechnitium-methylene diphosphonate (99mTc-MDP), whole body static images were obtained with a gamma camera (Technicare Omega 500; Technicare, Cleveland, Ohio, USA) and a low-energy, all-purpose, collimator. The images were then processed with a dedicated imaging computer and the appropriate software (Mirage-Vets, Link Medical, Bramshill, Hampshire, UK). Uptake of the radiopharmaceutical could be seen throughout the pulmonary fields (Figures 2A and 2B), so pulmonary mineralization, suspected to be secondary to iatrogenic hyperadrenocorticism, was diagnosed.
Figure 2.
Left lateral (A) and dorsal (B) images of the 99mTc-methylene diphosphonate (MDP) scan showing uptake of the radioisotope by pulmonary parenchyma. Note that the uptake of the isotope by the lungs (asterix) is similar to the uptake observed in the bones.
A recheck examination was performed 5 mo after the initial diagnostic tests. The dose of the combined trimeprazine tartrate and prednisolone had been gradually tapered to 0.8 mg/kg BW and 0.125 mg/kg BW, respectively, PO, q72h by the time of the recheck. According to the client, there had been resolution of the cough and the panting when the dose was decreased, but the exercise intolerance had only partially improved. Upon the decreased dose, the client had also observed a recrudescence of the pruritus, which was mild and intermittent at the time of the recheck. The theophylline therapy had been discontinued 5 mo prior to the recheck. On physical examination, the dog had a normal hair coat, the hepatomegaly and abdominal distention were no longer discernable, and the dog was in good body condition with a body weight of 6 kg. Comedones were noted over the ventral abdominal wall. Mildly increased bronchovesicular sounds were ausculted diffusely throughout the lung fields.
Thoracic radiographs showed changes that were identical to those observed in the radiographs performed 5 mo previously. Results from an arterial blood gas analysis were normal (PaO2 of 90.1 mmHg; PaCO2 of 38.0 mmHg; oxygen saturation of 98.6%). The alveolar-arterial gradient was normal (11.4 mmHg). The combined trimeprazine tartrate and prednisolone therapy was discontinued. Subsequently, the pruritus and comedones were controlled successfully with a hypoallergenic diet.
Discussion
Dystrophic mineralization commonly occurs in association with canine hyperadrenocorticism and can cause calcinosis cutis, as well as mineralization of tracheal rings and bronchial walls, kidneys, gastric mucosa, liver, skeletal muscle, and branches of the abdominal aorta (1,2). Thoracic radiographic features of canine hyperadrenocorticism include a moderate to severe generalized interstitial lung pattern and ectopic calcification of tracheal rings and bronchial walls (3–5). These changes are not specific for hyperadrenocorticism, as they may be age-related (2,5,6). Mineralization in dogs with hyperadrenocorticism is likely due to the protein catabolic actions of cortisol. Protein damage can then lead to calcium and phosphorus deposition in the organic matrix of the abnormal protein, despite normal calcium and phosphorus concentrations in serum (1,2).
Plain thoracic radiographs are considered to lack sensitivity for the diagnosis of pulmonary mineralization (7). Pulmonary mineralization in this case was diagnosed by using a 99mTc-MDP scan. In human medicine, nuclear scintigraphy or high-resolution computed tomography are considered the tests of choice for establishing a definitive diagnosis of pulmonary mineralization (7,8). The calcific nature of the pulmonary interstitial lesions is easily identified with scintigraphy (7). After its IV injection, 99mTc-MDP diffuses into the extracellular space and then rapidly binds to hydroxyapatite crystals in bone or soft tissue. Bone uptake of 99mTc-MDP is proportional to its osteoblastic activity (9). Pulmonary uptake of 99mTc-MDP in canine hyperadrenocorticism has been documented previously (3,10): a study of dogs with pituitary-dependent hyperadrenocorticism revealed that 7 of 21 dogs were hypoxic, but only 2 of those dogs showed pulmonary mineralization through uptake of 99mTc-MDP (3). Six other dogs in the study had an abnormal pulmonary interstitial pattern, and 5 of these dogs were hypoxic (3). Postmortem studies of dogs with hyperadrenocorticism revealed that microscopic pulmonary interstitial mineralization was present in over 90% of cases (2). Nonskeletal uptake of 99mTc-MDP has also been reported in calcinosis cutis; renal infarction with dystrophic mineralization; acute rhabdomyolysis; regional lymph nodes, following extravasation of the radiopharmaceutical; osteosarcoma metastasis; and dystrophic mineralization of soft tissues. Normal uptake of 99mTc-MDP is evident in lactating mammary tissue, the pregnant uterus, and the urinary tract (11).
Blood gas analysis performed on this patient initially revealed hypoxia. An elevated alveolar-arterial oxygen gradient can reflect a ventilation-perfusion inequality, such as pulmonary thromboembolism or pulmonary diffusion impairment (12). Interstitial mineralization may present a diffusion impairment, leading to ineffective gas exchange in the lungs.
Development of pulmonary thromboembolism is a reported, and often fatal, complication of canine hyperadrenocorticism. Dogs with pulmonary thromboembolism may have acute respiratory distress (2). Pulmonary thromboembolism was not ruled out in this patient; however, due to the patient’s chronic respiratory signs, it was believed to be less likely. In previously reported cases of dogs with pulmonary mineralization, the results of perfusion studies were normal (10). Radiographic features of pulmonary thromboembolism are inconsistent. They may be absent or include pulmonary arterial changes, such as blunting or truncation; pulmonary parenchymal changes, such as alveolar pattern or oligemia; cardiac changes, such as right heart or main pulmonary artery enlargement; or pleural effusion (13,14).
Hyperadrenocorticism can lead to respiratory impairment in patients without pulmonary mineralization. Mechanical hypoventilation leading to hypercarbia may be due to a decrease in respiratory excursion effort, secondary to increased fat deposition in the thoracic wall, muscle wasting and weakness of the muscles involved in respiration, and increased pressure on the diaphragm from hepatomegaly and abdominal distention (2). In the case reported herein, resolution of the iatrogenic hyperadrenocorticism may have been partly responsible for the normal blood gas values on follow-up examination, although the normal PaCO2 was not suggestive of hypoventilation.
Coughing is not a typical clinical sign associated with hyperadrenocorticism, and it has not been reported, in previously documented cases of pulmonary mineralization; in the case being reported, it was a historical complaint and its cessation coincided with the tapering of the combined dose of trimeprazine tartrate and prednisolone (2,10,15). Pulmonary mineralization is a pathologic process affecting the interstitium, and is not considered an airway disease. No signs of airway disease were observed on the thoracic radiographs in this case, although the extensive interstitial lung pattern may have made it difficult to fully evaluate the patient’s airways and the absence of radiographic changes do not rule out airway disease. Mineralization of the trachea and bronchi was not observed in this case and usually is not associated with coughing or other clinical signs (2). The reported cough in this case was considered chronic but intermittent by the owners, and the risk involved with bronchoscopy and airway wash was considered too significant to pursue these diagnostic procedures. A transtracheal wash was declined by the owners, so concurrent airway disease cannot be ruled out as the cause of the coughing. The source of the coughing is unknown, as is why the coughing resolved in conjunction with tapering the dose of the steroid therapy.
A poor prognosis has been associated with hyperadrenocorticism and secondary pulmonary mineralization. In a previous case report, a dog with iatrogenic hyperadrenocorticism and dyspnea treated with supportive care died soon after onset of treatment: examination of postmortem lung biopsy revealed mineralization but a complete postmortem examination was not performed, so other underlying diseases related to the patient’s death could not be ruled out (15). However, Berry et al (10) reported that 2 of 4 dogs with pulmonary mineralization and hyperadrenocorticism were euthanized due to persistent signs of hypoxia and lack of response to empirical therapy. The other 2 dogs were treated with mitotane and showed persistent pulmonary mineralization on radiographs taken 6 mo after the initial diagnosis. One of these 2 dogs remained hypoxic on re-examination but did not show signs of respiratory distress (10).
This case of iatrogenic hyperadrenocorticism and pulmonary mineralization highlights 2 clinically important points. Unlike previously reported cases, the dog in this report experienced clinical improvement and resolution of clinical signs as the combined dose of trimeprazine tartrate and prednisolone was first tapered and then discontinued; she continues to be clinically normal 12 mo after her condition was first diagnosed. The improvement observed in clinical signs and blood gas values occurred despite a lack of radiographic improvement. While partial resolution of the pulmonary mineralization upon discontinuation of the corticosteroid therapy may have been responsible for the resolution of the hypoxia, a follow-up scintigraphic pulmonary examination was not performed. Although results of the blood gas analysis were not supportive of hypoventilation, the patient’s weight loss and presumed improvement of respiratory function associated with resolution of the iatrogenic hyperadrenocorticism likely contributed to the resolution of panting and the improvement in exercise tolerance observed.
It is also clinically relevant to note that this case of iatrogenic hyperadrenocorticism and pulmonary mineralization occurred after chronic use of a low dose (0.5 mg/kg BW/d) of prednisolone. Two previously reported cases of iatrogenic hyperadrenocorticism and pulmonary mineralization exist (10,15): one dog developed respiratory compromise after receiving a high dose (2.2 mg/kg BW/d) of prednisone for 4 y (15). The report on other case did not include information regarding the dose or the duration of corticosteroid treatment responsible for inducing the iatrogenic hyperadrenocorticism (10).
Pulmonary mineralization is an important differential diagnosis for the hypoxic or dyspneic patient with hyperadrenocorticism. While previous cases of pulmonary mineralization associated with hyperadrenocorticism have been associated with a poor prognosis, in this patient, clinical resolution of the hyperadrenocorticism led to improvement of clinical signs and results of blood gas analysis. Clinicians should be aware of this potential side-effect of chronic corticosteroid treatment, even at doses that are considered low.
Figure 3.
Left lateral (A) and dorsal (B) images of a 99mTc-MDP scan in a dog without pulmonary mineralization. These images have been provided for reference, and are not directly related to the case herein.
Footnotes
Authors’ contributions
Dr. Blois was responsible for the primary care of the case. Dr. Caron was the supervising clinician. Dr. Mitchell conducted the 99mTc-MDP scan and interpreted the radiologic findings. All the authors were involved in the writing and subsequent preparation of the manuscript for publication. CVJ
References
- 1.La Perle K, Capen C. Endocrine organs. In: McGavin M, Zachary J, editors. Pathologic Basis of Veterinary Disease. 4th ed. St Louis: Mosby Elsevier; 2007. pp. 693–741. [Google Scholar]
- 2.Feldman E, Nelson R. Canine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis: Saunders; 2004. pp. 252–393. [Google Scholar]
- 3.Berry CR, Hawkins EC, Hurley KJ, Monce K. Frequency of pulmonary mineralization and hypoxemia in 21 dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med. 2000;14:151–156. doi: 10.1892/0891-6640(2000)014<0151:fopmah>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Penninck DG, Feldman EC, Nyland TG. Radiographic features of canine hyperadrenocorticism caused by autonomously functioning adrenocortical tumors: 23 cases (1978–1986) J Am Vet Med Assoc. 1988;192:1604–1608. [PubMed] [Google Scholar]
- 5.Huntley K, Frazer J, Gibbs C, Gaskell CJ. The radiological features of canine Cushing’s syndrome: A review of forty-eight cases. J Small Anim Pract. 1982;23:369–380. [Google Scholar]
- 6.Widmer WR, Guptill L. Imaging techniques for facilitating diagnosis of hyperadrenocorticism in dogs and cats. J Am Vet Med Assoc. 1995;206:1857–1864. [PubMed] [Google Scholar]
- 7.Chung MJ, Lee KS, Franquet T, Muller NL, Han J, Kwon OJ. Metabolic lung disease: Imaging and histopathologic findings. Eur J Radiol. 2005;54:233–245. doi: 10.1016/j.ejrad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165:1654–1669. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- 9.Daniel GB, Brawner WR. Thyroid scintigraphy. In: Daniel GB, Berry CR, editors. Textbook of Veterinary Nuclear Medicine. 2nd ed. Raleigh, North Carolina: North Carolina State Univ; 2006. pp. 181–198. [Google Scholar]
- 10.Berry C, Ackerman N, Monce K. Pulmonary mineralization in four dogs with Cushing’s syndrome. Vet Radiol Ultrasound. 1994;35:10–16. [Google Scholar]
- 11.Lamb C. Non-skeletal distribution of bone-seeking radiopharmaceuticals. Vet Radiol. 1990;31:246–253. [Google Scholar]
- 12.West J. Respiratory Physiology — the Essentials. 7th ed. Baltimore: Lippincott Williams & Wilkins; 2004. pp. 55–74. [Google Scholar]
- 13.Burns MG, Kelly AB, Hornof WJ, Howerth EW. Pulmonary artery thrombosis in three dogs with hyperadrenocorticism. J Am Vet Med Assoc. 1981;178:388–393. [PubMed] [Google Scholar]
- 14.Johnson LR, Lappin MR, Baker DC. Pulmonary thromboembolism in 29 dogs: 1985–1995. J Vet Intern Med. 1999;13:338–345. doi: 10.1892/0891-6640(1999)013<0338:ptid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Crawford M, Robertson S, Miller R. Pulmonary complications of Cushing’s syndrome: Metastatic mineralization in a dog with high dose chronic corticosteroid therapy. J Am Anim Hosp Assoc. 1987;23:85–87. [Google Scholar]