Abstract
Although many studies have investigated the metabolism of selenium and arsenic in hyperaccumulating plants for phytoremediation purposes, few have explored non-hyperaccumulating plants as a model for general contaminant exposure to plants. In addition, the result of simultaneous supplementation with selenium and arsenic has not been investigated in plants. In this study, Chlorophytum comosum, commonly known as the spider plant, was used to investigate the metabolism of selenium and arsenic after single and simultaneous supplementation. Size exclusion and ion-pairing reversed phase liquid chromatography were coupled to an inductively coupled plasma mass spectrometer to obtain putative metabolic information of the selenium and arsenic species in C. comosum after a mild aqueous extraction. The chromatographic results depict that selenium and arsenic species were sequestered in the roots and generally conserved upon translocation to the leaves. The data suggest that selenium was directly absorbed by C. comosum roots when supplemented with SeVI, but a combination of passive and direct absorption occurred when supplemented with SeIV due to the partial oxidation of SeIV to SeVI in the rhizosphere. Higher molecular weight selenium species were more prevalent in the roots of plants supplemented with SeIV, but in the leaves of plants supplemented with SeVI due to an increased translocation rate. When supplemented as AsIII, arsenic is proposed to be passively absorbed as AsIII and partially oxidized to AsV in the plant root. Although total elemental analysis demonstrates a selenium and arsenic antagonism, a compound containing selenium and arsenic was not present in the general aqueous extract of the plant.
Keywords: Arsenic, Chlorophytum comosum, HPLC-ICPMS, selenium, speciation
Introduction
In addition to the natural geological release of arsenic into groundwater and soil, anthropogenic activities such as the industrial production of pesticides, herbicides, wood preservatives, and mining have increased arsenic levels beyond natural concentrations, causing worldwide environmental concern (Bhattacharya et al., 2007). Arsenic present in soil can enter the food chain via plant accumulation. Some of the most common arsenic species in the environment include arsenite (AsIII), arsenate (AsV), monomethlyarsonate (MMA), and dimethylarsinate (DMA), in order of decreasing toxicities (Wang and Mulligan, 2006). General phytoremediation efforts, utilizing plants to remove toxins from the environment, have focused on hyperaccumulating plants for the depletion of arsenic (Ma et al., 2001). Arsenic metabolism should be studied in a variety of plants in order to assess environmental risk accurately and to continue developing more effective phytoremediation strategies using alternative plants.
Selenium is considered to be one of the most widely distributed elements on Earth, having an average soil abundance of 0.09 mg kg−1. Further, considerable concentration variability exists from one location to another, such as high selenium concentrations occurring in a few localized regions (Kopsell and Kopsell, 2007). As with arsenic, selenium contained in the soil environment can enter the food chain through plant accumulation. Although selenium has been identified as a necessary element to animal life and possesses cancer chemopreventive properties from clinical trials, (Combs et al., 2001), its narrow range between deficiency and toxicity deem the uptake and accumulation of selenium worthy of extensive investigation (Brown and Arthur, 2001). While essential to mammalian health, the question of selenium necessity as a micronutrient in plants remains unanswered (Terry et al., 2000). In order properly to assess environmental danger and continue to develop more effective phytoremediation strategies using alternate plants, the metabolism of selenium should be studied in a variety of plants.
In past studies, selenium has been shown to have an antagonistic affect on toxic elements in plants (He et al., 2004). Investigations over half a century ago provided evidence for a detoxifying or protective effect after toxic concentrations of selenium and arsenic were simultaneously administered to rats (Dubois et al., 1940). More recently, the structure elucidated for the interaction of selenium and arsenic in a mammalian system was described as seleno-bis(S-glutathionyl) arsinium ion [(GS)2AsSe]– (Gailer et al., 2000). Although the effects of selenium and arsenic have independently been studied in various plant matrices, little research has been devoted to provide information on a potential selenium and arsenic interaction at the molecular level within plants. If observed, an antagonism between selenium and arsenic may prove useful for further phytoremediation studies.
In general, extensive effort has been put forth to understand the metabolic pathways of contaminants such as arsenic (Fayiga et al., 2008) and selenium (Freeman et al., 2006) in hyperaccumulating plants. However, few studies have investigated the metabolism of such contaminants in non-hyperaccumulating plants, which could act as a model for general environmental exposure. When considering the potential of contaminant remediation by genetically modified or native plants (wild type), the metabolism pathways and any variation in metabolism should be fully understood for accumulating and non-accumulating plants, as investigated in a previous study (Mounicou et al., 2006b). Considering the increasing level of global contamination, studies on the metabolism of selenium and arsenic in non-hyperaccumulating plants are imperative to provide vital information about general environmental effects.
Size exclusion chromatography (SEC) provides a general molecular weight range of the varying species in the soluble portion of a plant matrix, such as extracted proteins (Navaza et al., 2006). SEC has previously been used to monitor selenium and arsenic in various matrices such as Allium schoenoprasum (chives) and Antarctic krill (Li et al., 2005; Kapolna et al., 2006). While SEC can provide information on possible interactions between molecules, poor analyte resolution causes the technique to be unsuitable for small molecule speciation. In the past, the two most frequently employed techniques to speciate and thus identify different selenium and arsenic species have been ion exchange and ion-pairing reversed phase chromatography (IPRP) (B'Hymer and Caruso, 2004, 2006). The most common arsenic and selenium species previously found in plants and soil were AsIII, AsV, MMA, DMA, selenite (SeIV), selenate (SeVI), selenomethione (SeMet), and selenocystine (SeCys2) (Bujdos et al., 2005; Wang and Mulligan, 2006). A recent method displayed the ability to separate all eight species in a timely and sensitive manner using ion-pairing reversed phase chromatography with inductively coupled plasma mass spectrometry (IPRP-ICPMS) for online detection (Afton et al., 2008). In addition, the fast, multi-elemental detection at trace levels allowing for the sensitivity and selectivity provided by ICPMS has previously been used for selenium and arsenic speciation in plant matrices (Pedrero et al., 2007; Bluemlein et al., 2008).
In this study, the selected plant species is the Chlorophytum comosum, commonly known as the spider plant. C. comosum is generally known to be robust in varying cultivation conditions allowing for ease of care and possesses an extensive root system beneficial for nutrient and contaminant absorption. Further, earlier studies in this laboratory have shown preferential segregation of metal toxins in the plant roots (Mounicou et al., 2006a; Yathavakilla and Caruso, 2007). The two main plant compartments, leaves and roots, were monitored for the absorption and translocation of selenium and arsenic metabolites. This study probes the potential effects of single and simultaneous addition of selenium and arsenic within C. comosum plants.
Materials and methods
Instrumentation
High-performance liquid chromatography:
Chromatographic separations were accomplished with an Agilent 1100 liquid chromatograph by Agilent Technologies (Santa Clara, CA) equipped with a vacuum de-gasser system, a binary HPLC pump, an autosampler, and a thermostated column compartment. The column used for SEC was a Superdex Peptide 10/300 GL (10 mm×300 mm×13 μm) from Amersham Pharmacia Biotech AB (Uppsala, Sweden) and was calibrated with the following standards: cytochrome C, 12.5 kDa; insulin chain B oxidized, 3.5 kDa; and vitamin B12, 1.4 kDa obtained from Sigma-Aldrich Co. (St Louis, MO). Reversed phase chromatography was carried out with a ZORBAX Eclipse XDB-C18 column (5 μm×4.6 mm id×250 mm) from Agilent Technologies (Santa Clara, CA).
Inductively coupled plasma mass spectrometry:
The ICPMS used for specific element detection was an Agilent 7500ce by Agilent Technologies (Santa Clara, CA). The instrument was equipped with a microconcentric nebulizer made by Glass Expansion (Pocasset, MA), a Scott double channel spray chamber (cooled to 2 °C), a shielded torch, an octopole collision/reaction cell with hydrogen gas pressurization (purity of 99.999%), a quadrupole mass analyser and an electron multiplier for detection.
Lyophilization and digestion:
A Flexi-Dry MP lyophilizer (Stoneridge, NY) was used for freeze-drying purposes. The microwave system used for digestion was an Intelligent Explorer/Discover system produced by the CEM Corporation (Mathews, NC). The microwave system was programmable for time, temperature, power, and pressure, and equipped with a 24 vial autosampler and a self contained microwave chamber.
A summary of all instrumental conditions can be found in Table 1.
Table 1.
Instrumental conditions in this study
| ICP-MS | |
| Forward power | 1500 W |
| Plasma gas flow | 15.0 l min−1 |
| Carrier gas flow | 0.96 l min−1 |
| Makeup gas flow | 0.14 l min−1 |
| Collision gas | 3.5 ml min−1 H2 |
| Quadrupole bias | –16.0 V |
| Octopole bias | –18.0 V |
| Monitored isotopes | 75As, 77Se, 78Se, 80Se, 82Se |
| Dwell time | 100 ms per isotope |
| HPLC | |
| SEC | |
| Mobile phase | 100 mmol l−1 TRIS-HCl (pH 7.5) |
| Flow rate | 0.60 ml min−1 |
| Injection volume | 100 μl |
| IPRP | |
| Mobile phase (A) | 5 mmol l−1 TBAH in 2.5 mmol l−1 (NH4)3PO4 (pH 6.0) |
| Mobile phase (B) | 10 mmol l−1 (NH4)2SO4 (pH 6.0) |
| Flow rate | 1.0 ml min−1 |
| Injection volume | 100 μl |
| Gradient programme | ||||||
| Time (min) | 0 | 0.5 | 1.5 | 5 | 6 | 18 |
| % A | 100 | 100 | 0 | 0 | 100 | 100 |
| % B | 0 | 0 | 100 | 100 | 0 | 0 |
| Microwave | Stage 1 | Stage 2 | Stage 3 |
| Power (W) | 125 | 125 | 150 |
| Ramp (min) | 1:00 | 1:00 | 1:00 |
| Hold (min) | 1:00 | 2:00 | 2:00 |
| Temperature (°C) | 120 | 175 | 170 |
Reagents and standards
All the solutions were prepared in 18 MΩ cm−1 doubly deionized water (DDW) processed by Sybron/Barnstead (Boston, MA). Standards used for supplementation and identification were the following: disodium methyl arsonate hexahydrate (MMA) purchased from Chem Service (West Chester, PA); L(+)-selenomethionine (SeMet), the form commonly found within biological samples such as plants (Iwaoka et al., 2008), obtained from Acros Organics (Morris Plains, NJ); sodium (meta)arsenite (AsIII), cacodylic acid (DMA), and seleno-L-cystine (SeCys2) acquired from Fluka (Milwaukee, WI); potassium arsenate (AsV), potassium selenate (SeVI), and sodium selenite (SeIV) purchased from Sigma-Aldrich (St Louis, MO).
For total elemental analysis, digestion of plant biomass was accomplished using nitric acid (HNO3) obtained from Pharmco Products Inc. (Brookfield, CT) and hydrogen peroxide (30%) from Fisher Scientific (Fair Lawn, NJ). Claritas PPT selenium and arsenic elemental standards used for quantification were acquired from SpexCertiPrep (Metuchen, NJ). Calibration standards of 1.0 μg l−1 to 500 μg l−1 were prepared through dilution from a stock solution with 2% v/v HNO3.
The following depicts the preparation of mobile phases used for plant extraction and chromatographic separation. The mobile phase for SEC and general plant biomass extraction was made by dissolving tris(hydroxymethyl) aminomethane hydrochloride (TRIS-HCl) from Fisher Scientific (Fair Lawn, NJ) in DDW and adjusting the pH with hydrochloric acid. For IPRP-ICPMS, mobile phase A contained 5 mmol l−1 tetrabutylammonium hydroxide (TBAH) from Fluka (Milwaukee, WI) and 2.5 mmol l−1 ammonium phosphate from Sigma-Aldrich Co. (St Louis, MO) at pH 6.0. Mobile phase B contained 10 mmol l−1 ammonium sulphate from Sigma-Aldrich Co. (St Louis, MO) at pH 6.0. The pH was adjusted with phosphoric acid for mobile phase A and ammonium hydroxide for mobile phase B. A summary of the mobile phase conditions are depicted in Table 1. All samples were filtered through a 0.2 μm membrane syringe filter by Econofilters from Agilent Technologies, Inc. (Santa Clara, CA) before being injected into the HPLC-ICPMS.
Plant growth and supplementation
The C. comosum was cultivated from seed at the University of Cincinnati greenhouse, Department of Biological Sciences, Cincinnati, OH. The general purpose potting soil used to cultivate the plants was Premier Pro-Mix (Riviere-du-Loup, Quebec, Canada). During the growth period, plants were fertilized with 25% Hoagland solution as needed (Hoagland and Arnon, 1938). After 9 months of growth, the plants were split into six groups and supplemented with varying combinations of NaAsO2, K2SeO4, and Na2SeO3 at 25 ml d−1 for 4 d as depicted: Group I, 30 mg l−1 SeIV; Group II, 30 mg l−1 SeVI; Group III, 20 mg l−1 AsIII; Group IV, 30 mg l−1 SeIV and 20 mg l−1 AsIII; Group V, 30 mg l−1 SeVI and 20 mg l−1 AsIII; Group VI, control. AsIII was chosen for supplementation based on prior studies depicting the formation of a selenium and arsenic complex within a mammalian system after simultaneous supplementation with selenium (Gailer et al., 2000). Subsequently, the plants were allowed to mature for one additional week before harvesting. The health of each plant was visually indifferent to the supplementation type given. During the process of harvesting, the plants were separated into roots and leaves, washed with DDW, and lyophilized. Finally, the plants were homogenized into a powder and stored at −20 °C to prevent any further enzymatic activity leading to interspecies conversion, therefore changing the native distribution.
Total selenium and arsenic determination
For the determination of total selenium and arsenic in C. comosum, a closed vessel microwave digestion system was used. Three replicates of lyophilized plant biomass for each supplementation type were subjected to the following three stage digestion programme, which is summarized in Table 1. Briefly, 1 ml of HNO3 was added to approximately 50 mg of plant biomass and digested by Stage 1 and Stage 2 conditions. Subsequently, 0.2 ml of 30% H2O2 were added to the solution and digested by Stage 3 conditions. Following the microwave digestion sequence, the resulting solutions were diluted with DDW to 50 ml and analysed by ICPMS in continuous flow sample introduction mode. Of the selenium isotopes monitored, 78Se was found to give the lowest limits of detection.
Extraction procedures for plant tissues
A mild extraction procedure was incorporated in order to preserve the labile compounds in C. comosum plant tissue. In summary, 30 mg of homogenized plant biomass from the root or leaf were combined with 1.5 ml of 20 mmol l−1 TRIS-HCl (pH 7.5) and stirred at room temperature for 1.5 h. The solution was then centrifuged at 5000 rpm for 15 min. The supernatant was decanted, filtered through a 0.2 μm filter and 100 μl were injected into the SEC-ICPMS and IPRP-ICPMS. The chromatographic mobile phase conditions can be found in Table 1. In addition, total elemental analysis of the supernatant via ICPMS was performed. Extraction efficiencies were calculated as a percentage of the total elemental analysis of the lyophilized plant tissue. A similar treatment was used for all plant supplementation types.
Results and discussion
Total element accumulation
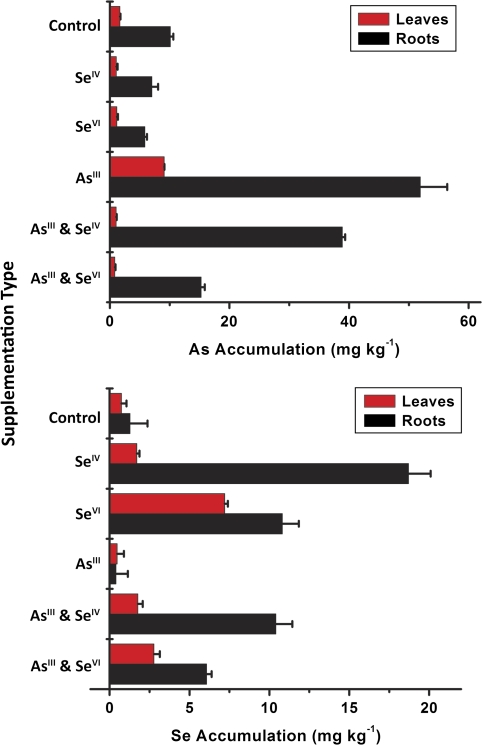
Total C. comosum accumulation of selenium and arsenic was determined via microwave digestion and subsequent analysis by continuous flow ICPMS. The resulting selenium and arsenic concentrations of the leaves and roots for the varying supplementation types are depicted in Fig. 1. The error bars represent one standard deviation of three replicates for each supplementation type. Overall, the results show a sequestering of selenium and arsenic species in the C. comosum roots, which agrees with previous studies demonstrating species sequestering in the roots after supplementation of selenium in Brassica oleracea (Pedrero et al., 2007) and arsenic in Brassica juncea (Pickering et al., 2000).
Fig. 1.
C. comosum accumulation of arsenic and selenium for the varying supplementation types administered during the cultivation process shown as the mean of three independent experiments.
The total concentration of selenium in the roots of the SeIV supplemented plants was 18.7 μg g−1, which displays the inability of C. comosum to accumulate large concentrations of selenium. The difference in accumulation and translocation of selenium between different supplementation types was ascertained by the total selenium concentrations of 1.7 μg g−1 for the leaves and 18.7 μg g−1 for the roots after SeIV supplementation, whereas after SeVI supplementation, concentrations were 7.2 μg g−1 for the leaves and 10.8 μg g−1 for the roots. These findings suggest an increased rate of selenium translocation from roots to leaves in C. comosum after supplementation with SeVI versus SeIV, which is in agreement with previous plant studies (Shrift, 1969). General consensus defines plants as non-accumulators that accumulate less than 25 μg g−1 of environmental contaminants, which classifies C. comosum as a selenium non-accumulator. In contrast to selenium uptake, greater arsenic accumulation was observed. The total concentration of arsenic in roots of the AsIII supplemented plants was 51.9 μg g−1, which demonstrates the capability of C. comosum for arsenic accumulation. In the leaves of the AsIII supplemented plants, the total concentration of arsenic was 9.1 μg g−1, therefore showing a considerable resistance to arsenic translocation. General consensus defines plants that accumulate 25–100 μg g−1 of environmental contaminants as secondary absorbers, which is the case for C. comosum.
For SeIV and AsIII supplemented plants, 10.4 μg g−1 of selenium and 38.9 μg g−1 of arsenic were observed in the roots, exhibiting a 44.4% and 25.0% decrease in accumulation, respectively, compared to single elemental supplementation. For SeVI and AsIII supplemented plants, 6.1 μg g−1 of selenium and 15.2 μg g−1 of arsenic were detected in the roots showing a 43.5% and 70.7% decrease, respectively, compared to single element supplementation. These findings suggest a mutual antagonism between selenium and arsenic upon simultaneous C. comosum supplementation. In accordance with individual supplementation, the degree of accumulation in the roots or leaves of C. comosum varied according to the form of selenium supplemented to the soil.
Overall, selenium and arsenic antagonism may occur by several pathways. The selenium and arsenic species may bind and form an insoluble complex, such as orpiment (As2Se3), resulting in a biologically unavailable selenium and arsenic species. Bacteria have been shown to reduce selenium and sulphur from selenate and sulphate to selenide and sulphide, respectively (Nelson et al., 1996; Zehr and Oremland, 1987). It has also been demonstrated that sulphide, when produced abiotically or microbially, can chemically reduce arsenic resulting in the formation of As2S3 (Stolz and Oremland, 1999). These findings support a possible formation of As2Se3 in the soil environment after simultaneous supplementation of selenium and arsenic. Another possibility allowing for mutual detoxification of the two environmental contaminants may be through the formation of an arsenic–selenium complex similar to that observed in the mammalian system: seleno-bis(S-glutathionyl) arsinium ion [(GS)2AsSe]– (Gailer et al., 2000). In order to investigate further a possible selenium and arsenic-containing species in C. comosum, SEC-ICPMS and IPRP-ICPMS were utilized.
Root extract characterization of selenium and arsenic species
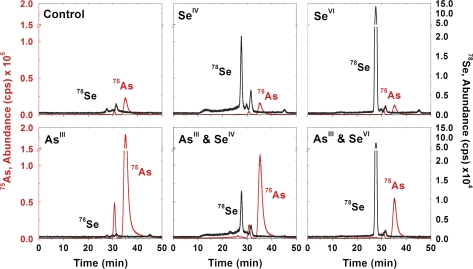
The utilization of SEC-ICPMS provided an overall molecular weight distribution of the selenium and arsenic containing compounds in C. comosum. Plant roots from varying supplementation combinations were analysed after a general extraction at near physiological pH. An example of the extraction efficiencies for the plant roots were calculated as 91±6% (75As) and 31±4% (78Se) with SeIV and AsIII supplemented plants (n=3). Although these results display a near complete arsenic extraction, a large amount of selenium remained in the unextracted fraction of the root. The resulting chromatograms after injecting 100 μl of the water-soluble plant supernatant from the TRIS-HCl extraction into the SEC-ICPMS are represented in Fig. 2. The SEC column recovery was calculated as 108±17% (75As) and 102±3% (78Se) for SeIV and AsIII supplemented plants (n=3) indicating negligible loss from analyte adsorption to the stationary phase. The predominant selenium and arsenic species eluted after the 1.4 kDa standard in all chromatograms, which indicates small molecules such as peptides or inorganic species. High molecular weight species were more prevalent in plants supplemented with SeIV than SeVI, which suggests an alteration in the selenium metabolism depending on the supplementation form. The lack of a void volume peak in the arsenic profiles illustrates arsenic exclusion from macromolecules such as proteins. Overall, the profile consistency demonstrates a general conservation of selenium and arsenic species, whether singly or simultaneously supplemented.
Fig. 2.
78Se and 75As SEC-ICPMS chromatograms of the root extracts from C. comosum after varying supplementation combinations; the profiles shown are highly similar to several other chromatograms collected from identically treated plant material.
While the overall selenium accumulation was reduced when arsenic was supplemented simultaneously, the consistency of the profile suggests that the metabolic pathway remains predominantly unaltered. This same phenomenon is also observed in comparing the arsenic profile of the root extract from plants supplemented with arsenic including or excluding selenium. After investigation of the selenium and arsenic chromatograms, a lack of profile overlap demonstrates that a selenium and arsenic-containing molecule was not present in the plant roots regardless of the supplementation type. Whereas total elemental analysis provides evidence of a selenium and arsenic antagonism, the metabolic pathway of interaction did not result in a water-soluble selenium and arsenic-containing molecule in C. comosum.
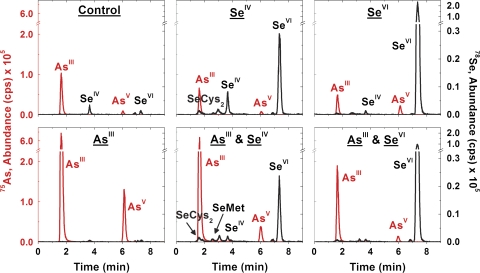
To characterize further the selenium and arsenic-containing compounds in C. comosum root extracts after varying supplementation combinations, IPRP-ICPMS was incorporated and the resulting chromatograms are shown in Fig. 3. The calculated column recovery was 87±3% (75As) and 55±1% (78Se) for SeIV and AsIII supplemented plants (n=3) indicating a minimal loss of arsenic from analyte adsorption to the stationary phase; however, the selenium loss may be caused by non-eluting selenium macromolecular compounds. Although the amount of selenium and arsenic in the soil was not quantified, the control plants provide insight into the low molecular weight species metabolized after long-term exposure to selenium and arsenic concentrations naturally found in commercial soil over the 9 month cultivation period. Only inorganic selenium and arsenic species were observed in C. comosum control roots. In addition, plants supplemented with selenium or arsenic singly showed a decrease in abundance for arsenic or selenium species, respectively, which supports the proposed antagonistic effect between the two.
Fig. 3.
78Se and 75As IPRP-ICPMS chromatograms of the root extracts from C. comosum after varying supplementation combinations; the profiles shown are highly similar to several other chromatograms collected from identically treated plant material.
Inorganic selenium species were predominately observed in the selenium supplemented C. comosum roots. Specifically in plants supplemented with SeIV, the concentration of SeIV and SeVI in root extracts was 14.3% and 74.6% of the total, respectively. The specific percentages reported in the manuscript for IPRP-ICPMS chromatograms are qualitative and used to aid visual interpretation. While the chromatograms were reproducible, no statistical analysis was performed. The conversion of the selenium species to a more oxidized form than originally supplemented is contradictory to the suggested metabolic pathway of selenium in a plant (Terry et al., 2000). This finding suggests that oxidation occurred in the rhizosphere, the dynamic microenvironment immediately surrounding the plant roots, and may provide conditions significantly different from the adjacent bulk soil (Wenzel et al., 1999). The difference in bulk soil pH may be described by the pH values for the solutions administered during supplementation: NaAsO2 (9.15), K2SeO4 (7.17), and Na2SeO3 (8.77). In order to acquire the necessary anions for biological processes, mmols of OH– can be released from the plant roots creating a potential difference between the root–soil interface, which allows for the absorption of anions such as , Cl–, and , to maintain the charge balance. The overall process generates rhizosphere alkalinity (Nye, 1981; Hedley et al., 1982). In addition, a prior study found SeVI to be the major form of selenium in environmental water sources at higher pH values (Bujdos et al., 2005).
After the initial supplementation with SeIV, the selenium species may have oxidized to SeVI due to an alkaline pH shift during nutrient uptake, which would allow for direct absorption of selenium into the plant root through the sulphate pathway. Plants supplemented with SeVI revealed a similar selenium chromatographic profile in general; however, SeIV and SeVI made up 0.5% and 98.8% of the total concentration, respectively, which provides evidence for the storage of inorganic selenium to favour SeVI. The lack of SeIV observed after SeVI supplementation suggests a passive induction of SeIV into C. comosum roots after SeIV supplementation instead of through a reduction pathway in the plant root. The findings suggest a direct absorption of selenium if C. comosum is supplemented with SeVI, but a combination of passive and direct absorption of selenium if C. comosum is supplemented with SeIV.
In the root extract of AsIII-supplemented plants, AsIII and AsV made up 82.1% and 17.9% of the total concentration, respectively. These data suggest that the oxidation of the arsenic species from AsIII to AsV may occur in the rhizosphere and subsequently be reduced to AsIII after absorption through the phosphate pathway in the plant root. A prior study showed considerable amounts of AsIII found in Solanum lycopersicum (tomato), Zea mays (corn), Pisum sativum (pea), and Cucumis melo (melon) after supplementation with AsV (Nissen and Benson, 1982). As an alternative metabolic pathway, AsIII may be passively absorbed in the root with subsequent partial oxidation to AsV. Previous work has shown that AsIII oxidation and AsV reduction can occur in plant roots (Tu et al., 2004). Although past studies have shown the production of phytochelatins as a means of arsenic detoxification within a plant (Schulz et al., 2008), C. comosum utilizes an alternate detoxification pathway. However, the production of phytochelatins may facilitate arsenic transport to the vacuole for storage in plant cells, as previously shown during a plant's heavy metal detoxification process (Shaw et al., 2006).
The predominant species observed in root extracts of the SeIV and AsIII supplemented plants were SeVI, AsIII and, to a lesser extent, SeIV, SeMet, SeCys2, and AsV. The major metabolites detected in the root extracts from the SeVI and AsIII supplemented C. comosum were SeVI and AsIII with AsV as a minor species. For selenium species, the overall concentration of SeVI was similar in the plants supplemented with SeIV compared with the SeIV and AsIII supplementation at 74.6% of the total selenium concentration. A similar trend was noted for SeVI supplemented plants compared with SeVI and AsIII supplementation. However, the overall concentration of SeIV was reduced by more than half in plants supplemented with SeIV compared with SeIV and AsIII supplementation at 6.6% and 14.3%, respectively, of the total extracted selenium concentration. These results suggest a greater restriction on the passive absorption of SeIV in the roots of C. comosum than the direct absorption of SeVI, which may have been caused by an interaction with arsenic in the rhizosphere. For arsenic species, the overall concentration set as a ratio of AsV/AsIII yielded 21.9% for AsIII supplemented plants, but 7.2% and 8.6% for AsIII and SeIV and AsIII and SeVI supplemented plants, respectively. The observed loss of AsV suggests the metabolic pathway used by C. comosum for arsenic absorption and metabolism. If AsIII was oxidized in the rhizosphere to AsV, then subsequently absorbed directly through the phosphate pathway before being reduced to AsIII, a decrease in the arsenic concentration absorbed from the simultaneous addition of selenium should decrease the amount of AsIII observed. Since the contrary was found, the supplemented form of arsenic, AsIII, is suggested to be absorbed passively as AsIII and partially oxidized to AsV in the plant root.
Leaf extract characterization of selenium and arsenic species
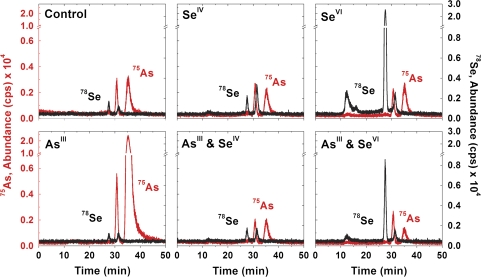
In order to monitor the selenium and arsenic species after translocation and possible further metabolism in the leaf compartment, 100 μl from the TRIS-HCl extraction of C. comosum leaves were injected into the SEC-ICPMS and the resulting chromatograms are depicted in Fig. 4. As noted in the chromatograms from the root extract, the major selenium and arsenic species in the leaf extract eluted after the 1.4 kDa standard, thus depicting small molecules such as peptides or inorganic species. Upon observing the selenium and arsenic chromatographic profile similarities and the decrease in elemental abundance from root to leaf regardless of supplementation type, it is suggested that compounds metabolized in C. comosum roots are not readily translocated nor further metabolized in the leaves, which supports the earlier total elemental analysis results.
Fig. 4.
78Se and 75As SEC-ICPMS chromatograms of the leaf extracts from C. comosum after varying supplementation combinations; the profiles shown are highly similar to several other chromatograms collected from identically treated plant material.
However, an exception was observed for plants supplemented with SeVI. In contrast to observations made from the plant root extracts, high molecular weight species were more prevalent in the leaves of plants supplemented with SeVI than SeIV indicating an alteration in the selenium metabolism. The reason may simply be due to the increased solubility of SeVI versus SeIV, which allows for greater mobility resulting in an increased rate of translocation. In addition, the lack of a void volume peak in the selenium plant profile when supplemented with SeIV indicates sequestering high molecular weight selenium species (greater than 12 kDa) in the roots of C. comosum.
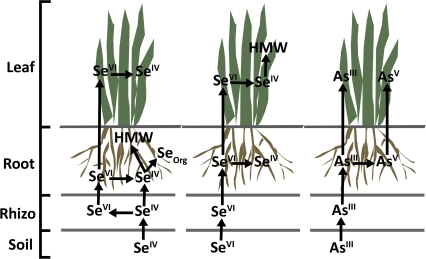
In comparing the selenium and arsenic profiles of the plant leaves supplemented singly versus simultaneously with selenium and arsenic, several similar peaks were observed. While the overall concentration of selenium and arsenic was reduced during simultaneous supplementation, the chromatographic peak profile consistency illustrates that the metabolic pathway remained predominantly unaffected. After further investigation of the selenium and arsenic profiles, a lack of chromatographic peak overlap reveals that a selenium and arsenic containing molecule was not present in the plant leaves regardless of the supplementation administered. Considering the low concentration and general conservation of translocated selenium and arsenic species in C. comosum leaves, IPRP-ICPMS was not performed. A summary of the proposed metabolic pathways after arsenic or selenium supplementation in C. comosum can be found in Fig. 5. Future studies will work towards a universal model by elucidating the metabolism of selenium and arsenic in other non-hyperaccumlating plants.
Fig. 5.
A summary of the metabolism pathway for the water-soluble selenium and arsenic species after varying supplementation types in soil, rhizosphere, roots, and leaves of C. comosum. HMW, high molecular weight compounds; Seorg, organic selenium species.
Acknowledgments
The authors would like to acknowledge Pam Bishop (Rieveschl Green House, University of Cincinnati) for assistance in C. comosum cultivation, support from the NIEHS-SBRP grant ES04908, and Agilent Technologies and the CEM Corporation for their instrumentation and continuing support.
References
- Afton S, Kubachka K, Catron B, Caruso JA. Simultaneous characterization of selenium and arsenic analytes via ion-pairing reversed phase chromatography with inductively coupled plasma and electrospray ionization ion trap mass spectrometry for detection. Journal of Chromatography A. 2008;1208:156–163. doi: 10.1016/j.chroma.2008.08.077. [DOI] [PubMed] [Google Scholar]
- B'Hymer C, Caruso JA. Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. Journal of Chromatograph, A. 2004;1045:1–13. doi: 10.1016/j.chroma.2004.06.016. [DOI] [PubMed] [Google Scholar]
- B'Hymer C, Caruso JA. Selenium speciation analysis using inductively coupled plasma-mass spectrometry. Journal of Chromatograph, A. 2006;1114:1–20. doi: 10.1016/j.chroma.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Welch AH, Stollenwerk KG, McLaughlin MJ, Bundschuh J, Panaullah G. Arsenic in the environment: biology and chemistry. Science of the Total Environment. 2007;379:109–120. doi: 10.1016/j.scitotenv.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Raab A, Meharg AA, Charnock JM, Feldmann J. Can we trust mass spectrometry for determination of arsenic peptides in plants: comparison of LC-ICP-MS and LC-ES-MS/ICP-MS with XANES/EXAFS in analysis of Thunbergia alata. Analytical and Bioanalytical Chemistry. 2008;390:1739–1751. doi: 10.1007/s00216-007-1724-y. [DOI] [PubMed] [Google Scholar]
- Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutrition. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- Bujdos M, Mulova A, Kubova J, Medved J. Selenium fractionation and speciation in rocks, soils, waters and plants in polluted surface mine environment. Environmental Geology. 2005;47:353–360. [Google Scholar]
- Combs GF, Jr, Clark LC, Turnbull BW. An analysis of cancer prevention by selenium. BioFactors. 2001;14:153–159. doi: 10.1002/biof.5520140120. [DOI] [PubMed] [Google Scholar]
- Dubois KP, Moxon AL, Olson OE. Further studies on the effectiveness of arsenic in preventing selenium poisoning. Journal of Nutrition. 1940;19:477–482. [Google Scholar]
- Fayiga AO, Ma LQ, Rathinasabapathi B. Effects of nutrients on arsenic accumulation by arsenic hyperaccumulator Pteris vittata L. Environmental and Experimental Botany. 2008;62:231–237. [Google Scholar]
- Freeman JL, Zhang LH, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EAH. Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiology. 2006;142:124–134. doi: 10.1104/pp.106.081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailer J, George GN, Pickering IJ, Prince RC, Ringwald SC, Pemberton JE, Glass RS, Younis HS, DeYoung DW, Aposhian HV. A metabolic link between arsenite and selenite: the seleno-bis(S-glutathionyl) arsinium ion. Journal of the American Chemical Society. 2000;122:4637–4639. [Google Scholar]
- He PP, Lu XZ, Wang GY. Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environment International. 2004;30:167–172. doi: 10.1016/S0160-4120(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Hedley MJ, Nye PH, White RE. Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings. II. Origin of the pH change. New Phytologist. 1982;91:31–44. [Google Scholar]
- Hoagland DR, Arnon DI. Water-culture method for growing plants without soil. California Agriculaural Experimental Station, Circular. 1938;347:1–39. [Google Scholar]
- Iwaoka M, Ooka R, Nakazato T, Yoshida S, Oishi S. Synthesis of selenocysteine and selenomethionine derivatives from sulphur-containing amino acids. Chemistry and Biodiversity. 2008;5:359–374. doi: 10.1002/cbdv.200890037. [DOI] [PubMed] [Google Scholar]
- Kapolna E, Shah M, Caruso JA, Fodor P. Selenium speciation studies in Se-enriched chives (Allium schoenoprasum) by HPLC-ICP-MS. Food Chemistry. 2006;101:1398–1406. [Google Scholar]
- Kopsell DA, Kopsell DE. Selenium. In: Barker AV, Pilbeam DJ, editors. Handbook of plant nutrition. Boca Raton, USA: CRC Press; 2007. pp. 515–550. [Google Scholar]
- Li B, Bergmann J, Lassen S, Leonhard P, Prange A. Distribution of elements binding to molecules with different molecular weights in aqueous extract of Antarctic krill by size-exclusion chromatography coupled with inductively coupled plasma mass spectrometry. Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences. 2005;814:83–91. doi: 10.1016/j.jchromb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED. A fern that hyperaccumulates arsenic. Nature. 2001;411:438. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Mounicou S, Shah M, Meija J, Caruso JA, Vonderheide AP, Shann J. Localization and speciation of selenium and mercury in Brassica juncea: implications for Se-Hg antagonism. Journal of Analytical Atomic Spectrometry. 2006a;21:404–412. [Google Scholar]
- Mounicou S, Vonderheide AP, Shann JR, Caruso JA. Comparing a selenium accumulator plant (Brassica juncea) to a nonaccumulator plant (Helianthus annuus) to investigate selenium-containing proteins. Analytical and Bioanalytical Chemistry. 2006b;386:1367–1378. doi: 10.1007/s00216-006-0707-8. [DOI] [PubMed] [Google Scholar]
- Navaza AP, Montes-Bayon M, LeDuc DL, Terry N, Sanz-Medel A. Study of phytochelatins and other related thiols as complexing biomolecules of As and Cd in wild type and genetically modified Brassica juncea plants. Journal of Mass Spectrometry. 2006;41:323–331. doi: 10.1002/jms.992. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Casey WH, Sison JD, Mack EE, Ahmad A, Pollack JS. Selenium uptake by sulphur-accumulating bacteria. Geochimica et Cosmochimica Acta. 1996;60:3531–3539. [Google Scholar]
- Nissen P, Benson AA. Arsenic metabolism in freshwater and terrestrial plants. Physiologia Plantarum. 1982;54:446–450. [Google Scholar]
- Nye PH. Changes of pH across the rhizosphere induced by roots. Plant and Soil. 1981;61:7–26. [Google Scholar]
- Pedrero Z, Elvira D, Camara C, Madrid Y. Selenium transformation studies during broccoli (Brassica oleracea) growing process by liquid chromatography-inductively coupled plasma mass spectrometry (LC-ICP-MS) Analytica Chimica Acta. 2007;596:251–256. doi: 10.1016/j.aca.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. Reduction and coordination of arsenic in Indian mustard. Plant Physiology. 2000;122:1171–1177. doi: 10.1104/pp.122.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Haertling S, Tanneberg H. The identification and quantification of arsenic-induced phytochelatins: comparison between plants with varying As sensitivities. Plant and Soil. 2008;303:275–287. [Google Scholar]
- Shaw BP, Prasad MNV, Jha VK, Sahu BB. Detoxification/defense mechanisms in metal-exposed plants. In: Prasad MNV, Sajwan KS, Naidu R, editors. Trace elements in the environment: biogeochemistry, biotechnology and bioremediation. Boca Raton, New York, London: CRC Press; 2006. pp. 291–324. [Google Scholar]
- Shrift A. Aspects of selenium metabolism in higher plants. Annual Review of Plant Physiology. 1969;20:475–494. [Google Scholar]
- Stolz JF, Oremland RS. Bacterial respiration of arsenic and selenium. FEMS Microbiology Reviews. 1999;23:615–627. doi: 10.1111/j.1574-6976.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Terry N, Zayed AM, De Souza MP, Tarun AS. Selenium in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- Tu S, Ma LQ, MacDonald GE, Bondada B. Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction and thiol formation in excised parts of Pteris vittata L. Environmental and Experimental Botany. 2004;51:121–131. [Google Scholar]
- Wang S, Mulligan CN. Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Science of the Total Environment. 2006;366:701–721. doi: 10.1016/j.scitotenv.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wenzel WW, Lombi E, Adriano DC. Biogeochemical processes in the rhizosphere: role in phytoremediation of metal-polluted soils. In: Prasad NMV, Hagemeyer J, editors. Heavy metal stress in plants: from molecules to ecosystems. Heidelberg: Springer Verlag; 1999. pp. 273–303. [Google Scholar]
- Yathavakilla SKV, Caruso JA. A study of Se-Hg antagonism in Glycine max (soybean) roots by size exclusion and reversed phase HPLC-ICPMS. Analytical and Bioanalytical Chemistry. 2007;389:715–723. doi: 10.1007/s00216-007-1458-x. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Oremland RS. Reduction of selenate to selenide by sulphate-respiring bacteria: experiments with cell suspensions and estuarine sediments. Applied and Environmental Microbiology. 1987;53:1365–1369. doi: 10.1128/aem.53.6.1365-1369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]