Abstract
During post-harvest storage, potato tubers age as they undergo an evolution of their physiological state influencing their sprouting pattern. In the present study, physiological and biochemical approaches were combined to provide new insights on potato (Solanum tuberosum L. cv. Désirée) tuber ageing. An increase in the physiological age index (PAI) value from 0.14 to 0.83 occurred during storage at 4 °C over 270 d. Using this reference frame, a proteomic approach was followed based on two-dimensional electrophoresis. In the experimental conditions of this study, a marked proteolysis of patatin occurred after the PAI reached a value of 0.6. In parallel, several glycolytic enzymes were up-regulated and cellular components influencing protein conformation and the response to stress were altered. The equilibrium between the 20S and 26S forms of the proteasome was modified, the 20S form that recycles oxidized proteins being up-regulated. Two proteins belonging to the cytoskeleton were also differentially expressed during ageing. As most of these changes are also observed in an oxidative stress context, an approach focused on antioxidant compounds and enzymes as well as oxidative damage on polyunsaturated fatty acids and proteins was conducted. All the changes observed during ageing seemed to allow the potato tubers to maintain their radical scavenging activity until the end of the storage period as no accumulation of oxidative damage was observed. These data are interpreted considering the impact of reactive oxygen species on the development and the behaviour of other plant systems undergoing ageing or senescence processes.
Keywords: Ascorbate, carbonyl, glutathione, oxylipin, phenolic compounds, physiological age index (PAI), radical scavenging activity, reactive oxygen species, sprouting pattern, two-dimensional electrophoresis
Introduction
Potato (Solanum tuberosum L.) seed tubers are used as multiplication organs and have to be stored for up to 1 year at low temperature before planting. During this post-harvest storage, the tubers undergo an evolution of their physiological state that influences their sprouting capacity, hence conditioning the yield of the crop (Coleman, 2000; Delaplace et al., 2008a, b). Apart from their agronomical importance, potato tubers are also considered as a model to study the ageing of non-photosynthetic—and hydrated—storage organs.
In this context, several approaches have been developed to characterize potato tuber ageing based on biophysical, physiological, or biochemical measurements (for a review, see Coleman, 2000). Recently, a physiological age index (PAI) originally developed by Caldiz and co-workers in 2001 was validated (Delaplace et al., 2008a). This PAI ranges from 0 for young dormant tubers to 1 for exhausted tubers unable to regenerate a caulino-foliar axis after long-term storage. It is based on sprouting-related measurements and constitutes a reference frame more representative of the physiological state of the tubers than the storage duration. Moreover, it is correlated with the changes in the sprouting phenotype associated with ageing.
Independently of this physiological approach, many biochemical studies were previously performed during post-harvest storage at low temperature focusing on the changes observed in protein, polyamine, lipid, polysaccharide, or antioxidant contents (Coleman, 2000; Delaplace et al., 2008b). The results obtained in such studies were, however, difficult to compare because of the use of different experimental parameters (storage temperature, duration, and cultivar). Concerning the changes in antioxidant compounds and enzymes observed during tuber ageing, an increase in catalase (CAT), superoxide dismutase (SOD), glutathione reductase, and ascorbate peroxidase (APX) activities as well as in α-tocopherol and glutathione contents was measured (Spychalla and Desborough, 1990; Kumar and Knowles, 1996; Zabrouskov et al., 2002). In contrast, the ascorbate pool seemed to decrease during ageing (Burton, 1989; Dipierro and De Leonardis, 1997; Mizuno et al., 1998). In addition, the potential accumulation of oxidative damage on susceptible molecules such as polyunsaturated fatty acids (PUFAs) and proteins (Sohal, 2002; Spiteller, 2003) was also investigated during tuber ageing. The results of the measurement of the oxidation products of PUFAs—the oxylipins—were variable and depended on the method used to assess lipid oxidation (Kumar and Knowles, 1993; Dipierro and De Leonardis, 1997; Fauconnier et al., 2002; Zabrouskov et al., 2002). The protein oxidation status was also measured based on their carbonyl content. An increase in carbonyl content was observed during long-term storage of up to 30 months, but these measurements were not tentatively correlated with physiological data (Kumar et al., 1999).
The scientific background thus presents heterogeneous data difficult to integrate within a single ageing model. In this study, the PAI was used to establish a reliable reference frame for biochemical studies (Delaplace et al., 2008a). A proteomic approach was performed based on fluorescence difference gel electrophoresis (DIGE) coupled with tandem mass spectrometry to identify the differentially expressed proteins. Concerning potato tubers, the two-dimensional electrophoresis technique has already been applied to various physiological contexts including sink-to-source transition (Borgmann et al., 1994), dormancy break (Desire et al., 1995), low temperature storage (Espen et al., 1999), and tuberization (Lehesranta et al., 2006; Agrawal et al., 2008). However, only the two last studies dealing mainly with tuber development before harvest led to the identification of differentially expressed proteins. Based on the results of the present proteomic study, a targeted approach focused on the quantification of antioxidant compounds and oxidative damage on PUFAs and proteins was used. It included some less frequently studied antioxidant compounds such as the phenolic compounds, and an in-depth profiling of free and esterified oxylipins.
These physiological and biochemical results were combined to provide a better understanding of the ageing process of potato tubers. These data are discussed with regard to the impact of reactive oxygen species (ROS) on potato tuber development and the behaviour of other biological systems undergoing ageing or senescence processes.
Materials and methods
Plant material
Potato tubers (S. tuberosum L. cv. Désirée, 35–40 mm grade) were harvested in autumn 2004 and sampled after 0, 30, 90, 150, 210, or 270 d of storage at 4 °C (95% relative humidity, darkness). Each sample comprised 40 tubers for the assessment of physiological parameters and 15 tubers for biochemical studies.
Tubers sampled for physiological studies were placed in a sprouting chamber (20 °C, 85% relative humidity, darkness), half-buried longitudinally in moisted vermiculite [55% (w/w) water]. Three times a week, the tubers were assessed for sprouting-related parameters and sprayed with 0.01 M CaSO4 in order to reduce the occurrence of terminal necrosis (Dyson and Digby, 1975).
For biochemical studies, each 15 tuber sample was homogenized as follows. Each tuber was cut in half longitudinally. A core (diameter: 17 mm) was taken in each half-tuber using a punch. The length of the resulting cylinders was reduced to reach a weight of 10 g fresh weight (FW) per tuber. After slicing, the tuber tissues were frozen in liquid nitrogen and ground in a mill (IKA A10 type, Staufen, Germany). Finally, the tissue powders obtained from 15 tubers were pooled and stored at –80 °C before analysis.
Physiological parameters
For each tuber, dormancy length was calculated as the length of time between sampling and sprouting (production of at least one sprout >5 mm, Caldiz et al., 2001). The incubation period was defined as the time elapsed between sprouting and new tuber formation on the sprouts (Caldiz et al., 2001), i.e. when sessile tuberous swellings reached 3 mm in diameter (Claver, 1973) or when a tuberous swelling reached twice the diameter of the substanding stolon (Reust, 1986). The PAI used in this study was calculated according to Caldiz et al. (2001):
where T is the sampling date, T0 is the haulm killing date, and T1 is the date corresponding to the end of the incubation period. This index ranges from 0 for very young seed tubers assessed immediately after haulm killing, to 1 for old seed tubers assessed at the ‘no top’ stage (Delaplace et al., 2008a).
Water content measurement at the beginning and at the end of the storage period was based on 10 tubers dried at 105 °C until constant mass was reached.
2D-DIGE experiment
Tuber proteins were extracted according to Delaplace et al. (2006) using a hot SDS lysis buffer. The protein concentration was determined using the RC/DC protein assay from Bio-Rad (Hercules, CA, USA), and an additional clean-up step was performed on ice using the 2-D Clean-Up Kit from GE Healthcare (Little Chalfont, UK). DIGE analytical gels were loaded with cyanine-labelled extracts according to the manufacturer's instructions (GE Healthcare). A common reference was composed using equal amounts of all extracts. After mixing, this was labelled and loaded on each gel for normalization purposes.
The isoelectric focusing was performed using 24 cm pH 4–7 immobilized pH gradient (IPG) strips (GE Healthcare) rehydrated overnight with either 150 μg (DIGE analytical gels, two samples and one common reference) or 450 μg of total proteins (preparative gels) diluted in the rehydration buffer (see above) complemented with 2.3 μl of IPG buffer pH 4–7 and 1.2 μl of IPG buffer pH 3–10 (GE Healthcare) to reach a final volume of 450 μl. After rehydration, the focusing was performed on the IPGphor (GE Healthcare) using the following conditions: 30 V during 1 h, 300 V during 3 h, gradient to 1000 V in 6 h, gradient to 8000 V in 3 h, 8000 V until 100 000 Vh. Prior to the second dimension, the strips were equilibrated for 15 min in 10 ml of equilibration buffer (6 M urea, 30% glycerol, 2% SDS, 50 mm TRIS, pH 8.8) containing 1% (w/v) dithiothreitol (DTT) and subsequently for 15 min in 10 ml of equilibration buffer containing 2.5% (w/v) iodoacetamide. For analytical gels, the second dimension was performed in the Hoefer DALT (GE Healthcare) tank using lab-cast 1 mm SDS–polyacrylamide gels (11%). After a 90 min step at 30 V, gels were run at 100 V overnight. For preparative gels, the separation in the second dimension was realized on the Ettan DALTsix System (GE Healthcare) with lab-cast 1 mm SDS–polyacrylamide gels (12.5%): 1 h at 2 W per gel, 3 h 30 min at 100 W total.
The analytical gels were directly scanned on the Typhoon Variable Mode Imager Trio (GE Healthcare) using the appropriate excitation wavelength and emission filters. Gel images were analysed using the DeCyder v5.0 software (GE Healthcare). For each time point of the ageing kinetics, at least two gel images using independent extracts labelled with different cyanines (dye swap between Cy3 and Cy5) were used for one-way analysis of variance (ANOVA). A protein was considered as differentially expressed when the following criteria were met for the corresponding spot on the gels: ANOVA P-value ≤0.05, amplitude of abundance variation ≥|1.5|, and spot present on at least seven out of eight analysed gels. The preparative gels were individually stained with 200 ml of SYPRO Ruby fluorescent stain (Bio-Rad) according to the manufacturer's instructions. Gel images were acquired with the Typhoon 9200 (GE Healthcare) using an excitation wavelength of 532 nm and an emission wavelength filter of 610 nm. The gel images were matched before manual spot picking and tryptic digestion according to Bohler et al. (2007).
The selected spots were picked using a 2 mm wide punch on a UV-transilluminator. Gel plugs were stored at –20 °C in a multiwell plate containing 50 μl of MilliQ water per well.
Excised spots were digested using the Ettan Spot Handling Workstation (GE Healthcare) as previously described (Bohler et al., 2007). After digestion, the resulting peptides were resolubilized in 2 μl of 50% (v/v) acetonitrile (ACN) containing 0.1% (v/v) trifluoroacetic acid (TFA). For protein identification, 0.7 μl or 1 μl of this solution was spotted on the target of the mass spectrometer and mixed with 0.7 μl of matrix [α-cyano-4-hydroxycinnamic acid 7 mg ml−1 in 50% (v/v) ACN/0.1% (v/v) TFA].
Peptide mass fingerprint and fragmentation spectra were acquired using a 4800 Proteomic Analyzer (Applied Biosystems, Foster City, CA, USA), and the resulting spectra were subjected to a database search through the MASCOT interface (MASCOT 2.2, Matrix Science, London, UK) integrated in the GPS Explorer software suite (Applied Biosystems). Searches against the NCBI nr database and a potato expressed sequence tag (EST) database downloaded from the NCBI server on 27 January 2007 were performed using the following parameters: (i) error tolerance on peptide mass fingerprints of 100 ppm or 0.3 Da [tandem mass spectrometry (MS/MS)]; (ii) fixed modifications: carbamidomethylation (C) and oxidation (M); and (iii) potential modifications: kynurenin (W) and double oxidation (W). Search results were evaluated based on the peptide scores, and identifications were manually validated.
Enzymatic antioxidant activities
Protein extraction was done by homogenizing 500 mg of tuber powder on ice with 1 ml of extraction buffer [50 mM phosphate buffer containing 1 mM EDTA and 1% (w/v) polyvinyl polypyrrolidone (PVPP)]. After centrifugation at 16 000 g for 30 min at 4 °C, the supernatant was stored at –80 °C before analysis. The protein concentrations were assayed using the ‘Protein assay kit II’ (Bio-Rad) according to Bradford (1976). Three independent extracts were used for each point of the kinetics. The same extraction protocol was used for the measurement of the following activities.
SOD (EC 1.15.1.1) activity was measured based on the inhibition of nitro blue tetrazolium photoreduction in the presence of riboflavin (Dhindsa et al., 1980). The reaction medium (final volume: 3 ml) contained 50 mM phosphate buffer pH 7.8 with 0.1 mM EDTA, 78 μM nitro blue tetrazolium, 13 mM methionine, 2 μM riboflavin, and 0, 8, or 16 μl of enzyme extract. These three dilutions were incubated during 20 min at room temperature at 20 cm from a fluorescent light (Sylvania Luxline Plus F36W/827, Raunheim, Germany) before reading the absorbance at 560 nm against a blank stored in the dark. The final absorbance decreased according to the increasing enzyme activity. One SOD unit was defined as the enzyme quantity that inhibited by 50% the initial rate of photoreduction measured in the absence of the enzyme. The curve expressing the absorbance according to the enzyme volume was linearized after log transformation. The equation of the resulting line was used to obtain the enzyme volume corresponding to one SOD unit.
APX (EC 1.11.1.11) activity was measured based on the decrease of the absorbance at 290 nm of ascorbate (reduced form, AsA) in the presence of H2O2 and APX according to Nakano and Asada (1981). Two complementary measurements were performed without H2O2 or enzyme extract, respectively, in order to consider the enzymatic oxidation of AsA by ascorbate oxidase and the non-enzymatic oxidation of AsA by H2O2. One APX unit corresponded to 1 μmol of AsA consumed per minute.
CAT (EC 1.11.1.6) activity measurement was performed according to Claiborne (1985) by monitoring the decrease in absorbance at 240 nm of H2O2 in the presence of CAT. One CAT unit corresponded to 1 μmol H2O2 consumed per minute.
Non-enzymatic antioxidant measurements
Both AsA and dehydroascorbate (DHA) were assayed according to the method of de Pinto et al. (1999). Briefly, 500 mg FW of tuber powder was homogenized on ice with 2 vols of ice-cold 5% (w/v) metaphosphoric acid. After centrifugation at 4 °C for 15 min at 18 000 g, the supernatant was used for ascorbate and glutathione analysis. ‘Total ascorbate’ (TAsA=AsA+DHA) was measured after reduction of DHA in AsA with DTT. The DHA concentration was obtained by substracting the AsA content from the TAsA content. A calibration curve was established using an AsA range comprised between 0 μM and 1000 μM. Four independent replicates were used for each point of the kinetics.
The glutathione assay method was based on the recycling of reduced glutathione (GSH) by glutathione reductase in the presence of 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB) according to the following equations, where GSSG corresponds to the oxidized form of glutathione:
The TNB– formation rate was measured at 412 nm and was proportional to the GSH and GSSG content of the sample (Punchard, 1996). Glutathione measurement was performed according to Zhang and Kirkham (1996), using 400 μl of the aforementioned supernatant neutralized with 600 μl of 0.5 M phosphate buffer at pH 7.5. The GSH concentration was calculated by subtracting the GSSG content (expressed as GSH equivalents) from the total glutathione (GSH+GSSG) content. The linear range for this assay was 0–200 nM. Four independent replicates were used for each point of the kinetics.
The carotenoid content was measured spectrophotometrically according to the method of Morris et al. (2004). The total carotenoid concentration was assayed based on the absorbance at 450 nm, by using a mean extinction coefficient A1%=250 cm−1 l−1 g−1. Three independent extractions were performed for each point of the kinetics.
The extraction of phenolic compounds was carried out according to André et al. (2007) using ortho-anisic acid as internal standard. The extracted phenolic compounds were injected in a Summit high-performance liquid chromatography (HPLC) system (Dionex, Sunnyvale, CA, USA) provided with a P580 gradient pump, a GINA50 autosampler, a diode array detector UVD 340S, and a Bio-Rad thermostated oven set at 40 °C. For each sample, 20 μl were injected on a HPLC Nucleodur C18 Pyramid column (250×4.6 mm internal diameter, particle size: 5 μm, Macherey-Nagel, Düren, Germany). The mobile phase was a mix of (A) an aqueous solution containing 0.1% (v/v) formic acid and (B) ACN containing 0.1% (v/v) formic acid. The flow rate was set at 1 ml min−1. The 95 min gradient comprised the following steps: 0–10 min, 0–9% B; 10–40 min, 9–13% B; 40–80 min, 13–35% B; 80–82 min, 35–100% B; 82–87 min, 100% B; 87–90 min, 100–0% B; 90–95 min, 0% B (re-equilibration). The absorbance changes were monitored simultaneously at 280 nm (tyrosine and tryptophan), 254 nm (phenylalanine), 308 nm (caftaric acid and the internal standard, ortho-anisic acid), and 320 nm (chlorogenic, neochlorogenic, cryptochlorogenic, dichlorogenic, and caffeic acids). Six independent replicates were used for each point of the kinetics.
Radical scavenging activity (RSA)
The method described by Miller et al. (2000) was used, with minor modifications, to measure RSA. A 0.5 mM 2,2-diphenyl-1-pierylhydrazyl (DPPH, Sigma-Aldrich, Saint-Louis, MO, USA) solution was prepared in the dark at room temperature in a 50/50 (v/v) (methanol/water) solution. After filtration (Whatman 595 ½, 150 mm Ø), 50 ml of this solution was mixed with 50 mg FW of frozen potato tuber powder. The frozen samples were directly defrosted in the DPPH solution in order to ensure a rapid interaction between the DPPH and the antioxidant molecules. The reaction flask was placed in a rotating incubator in the dark at 38 °C. After 4 h, the mixture was filtered (Whatman 595 ½, 150 mm Ø), and the absorption recorded at 515 nm using a Shimadzu UV-1601 spectrophotometer. A filtered DPPH solution incubated without the samples was used as the control. A standard curve was calculated using different 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) concentrations ranging from 0 mM to 2.5 mM. The data were then converted using the standard curve in terms of mmol Trolox equivalents g−1 FW. Three independent replicates were performed for each point of the kinetics.
Lipoxygenase (LOX) activity measurement
This assay is based on the measurement of the absorbance of the conjugated dienes [fatty acid hydroperoxides (HPOs)] produced by LOX (EC 1.13.11.12) activity at 234 nm according to the method developed by Surrey (1964). The tuber powder (500 mg) was homogenized for 1 h in 10 ml of ice-cold 0.1 M phosphate buffer at pH 7.5. After centrifugation at 21 000 g for 30 min at 4 °C, the protein concentration was measured according to Waddell (1956). The reaction medium contained 50 μl of a 10 mM linoleic acid emulsion and an enzyme extract volume ranging from 50 μl to 80 μl dissolved in an oxygenated 0.1 M phosphate buffer at pH 7.5 to reach a final volume of 3 ml. One LOX unit corresponds to 1 μmol of linoleic acid HPOs produced per minute, considering a molar extinction coefficient of 25 000 cm−1 M−1. Three independent extracts were used for each point of the kinetics.
Oxylipin profiling
For free oxylipin analysis, (6Z,9Z,11E,13S)-13-hydroxy-6,9,11-octadecatrienoic acid (Cayman Chemical, East Ellsworth, MI, USA) was used as the internal standard and 5 g FW of frozen material were added to 20 ml of extraction medium [isohexane/2-propanol, 3/2 (v/v) with 0.0025% (w/v) butylated hydroxytoluene]. After homogenization, the extract was centrifuged at 1300 g at 4 °C for 10 min. The clear upper phase was collected and a 6.7% (w/v) solution of potassium sulphate was added to reach a volume of 32.5 ml. After vigorous shaking, the extract was centrifuged at 1300 g at 4 °C for 10 min. The upper hexane-rich layer containing the oxylipin fatty acid derivatives was collected and used for further HPLC analysis. For esterified oxylipin analysis, the same protocol was used, with glycerol triricinoleate (Sigma, Saint-Louis, MO, USA) as the internal standard. Subsequently, the esterified oxylipins were transmethylated with sodium methoxide following the method used by Göbel et al. (2002) and Fauconnier et al. (2008).
Similar chromatographic conditions were used for free and esterified oxylipin samples. The protocol was divided into two steps. The first step, performed on the reverse phase column, allowed group separation. Each separated fraction was collected and then injected on a straight-phase column, allowing for the individual separation of oxylipines (Göbel et al., 2002; Fauconnier et al., 2008). The results were expressed in terms of nmol g−1 FW. Two independent extracts were used for each point of the ageing kinetics.
Carbonyl assay
Tuber proteins were extracted according to Delaplace et al. (2006) without acetone precipitation. Briefly, 250 μl of protein extract were derivatized at room temperature with 1 ml of 10 mM 2,4-DNPH in 2.5 M HCl during 1 h in the dark. A derivatization blank was performed by using 2.5 M HCl instead of the 2,4-DNPH solution. Afterwards, 250 μl of 100% (w/v) trichloroacetic acid were added and proteins were allowed to precipitate on ice for 15 min after 5 min of incubation at –20 °C. After centrifugation at 16 000 g during 5 min at 4 °C, the supernatant was discarded and the protein pellets were washed three times with 1 ml of ethanol/ethyl acetate (1:1 v/v). The pellets were dried under a nitrogen stream and solubilized at room temperature for 30 min in 1 ml of rehydration buffer as described in the ‘2D-DIGE experiment’ section. The carbonyl concentration calculation was based on the absorbance at 370 nm by using a molar extinction coefficient of 22 000 M−1 cm−1. These data were expressed in nmol of carbonyls mg−1 protein after assessment of the protein concentration using the RC/DC Protein Assay kit (Bio-Rad). Three independent replicates were performed for each point of the kinetics.
Statistical analysis of data
After checking the application conditions, each data set was subjected to one-way ANOVA using the PAI as fixed factor. When the ageing influence on the studied variables was significant (P <0.05), means were classified using the Newman and Keuls test. Graphically, mean values which are not distinct significantly (α=5%) share the same letter.
Results
Physiological evolution of the tubers during ageing
In a previous work, a PAI was validated based on several ageing kinetics (Delaplace et al., 2008a). It is used in this study to build an ageing scale for biochemical studies (Table 1). In the storage conditions of this work, this index increases markedly from 0.14±0.01 at the beginning of the storage period to 0.83±0.02 after 270 d at 4 °C. This physiological evolution is accompanied by a dormancy break that occurs non-synchronously at the apical and proximal bud level and leads to modifications of the sprouting pattern. The noteworthy points of the present ageing kinetics are the following. The samples with a PAI value <0.46 present a dormancy duration significantly longer than the following samples. Apical dominance corresponds to PAI values <0.5. This apical dominance is broken for PAI values close to 0.6, and multiple vigorous sprouts are observed for PAI values >0.7. A decrease in sprouting vigour is finally observed for PAI values ≥0.8. Complementarily, the incubation period (the time elapsed between sprouting and new tuber formation on the sprouts) decreased from 188±27 d (no storage) to 66±11 d (after 270 d of storage). This latter ageing marker is inversely correlated to the physiological age of the tubers (Delaplace et al., 2008a, b).
Table 1.
Physiological parameters of the tubers harvested in 2004 and stored at 4 °C during 270 d
| Observations | Storage duration at 4 °C (d) |
|||||
| 0 | 30 | 90 | 150 | 210 | 270 | |
| PAI (absolute unit) | 0.14±0.01 | 0.27±0.02 | 0.46±0.03 | 0.62±0.03 | 0.75±0.02 | 0.83±0.02 |
| IP duration (d) | 188±27 | 165±24 | 150±18 | 114±17 | 80±8 | 66±11 |
| Dormancy length (d) | 54±22 | 30±11 | 7±3 | 4±2 | 3±3 | 0±0 |
The physiological age index (PAI) based on the sprouting parameters was calculated according to Caldiz et al. (2001). The incubation period (IP) was defined as the time elapsed between sprouting and new tuber formation on the sprouts. The dormancy length was calculated as the length between sampling and sprouting (production of at least one sprout >5 mm). All data are means of measurements on 40 tubers ±SD.
Dry weight measurements at the beginning and at the end of the storage period also revealed that water loss was low (<1.4%) and that no significant dehydration occurred during storage.
Proteome changes observed during ageing
The purpose of this study was first to correlate proteomic marker expression with the noteworthy points of the physiological scale. Proteome changes were monitored during potato tuber ageing using the 2D-DIGE technique. Based on the criteria presented in the Materials and methods section, 52 and 41 spots were up- or down-regulated, respectively, and four spots exhibited a transient abundance maximum. Among those differentially expressed spots, 43 spots were selected for manual excision on SYPRO-stained preparative gels based on their ANOVA P-value and their spot intensity. Most of the selected spots included in this reduced data set presented a P-value <0.01. After tryptic digestion, the excised spots were submitted to MS/MS sequence analysis, and 31 proteins (72%) were successfully identified (Fig. 1, Supplementary Tables S1, S2 available at JXB online). The relative abundances of the identified proteins are presented in Tables 2, 3, and Supplementary Table S3.
Fig. 1.
Preparative 2D gel showing the manually excised spots for MS/MS analysis. The pH 4–7 IPG strip was loaded with 400 μg of proteins resolved in the second dimension using a 12.5% SDS–polyacrylamide gel. The gel was then stained with Sypro Ruby. A manual matching was performed with DIGE analytical gels in order to locate the differentially expressed proteins. Protein identifications are presented in Supplementary Tables S1 and S2 at JXB online. The evolution of standard abundances of proteins are presented in Tables 2, 3, and Supplementary Table S3 at JXB online.
Table 2.
Abundance kinetics of up-regulated proteins during ageing
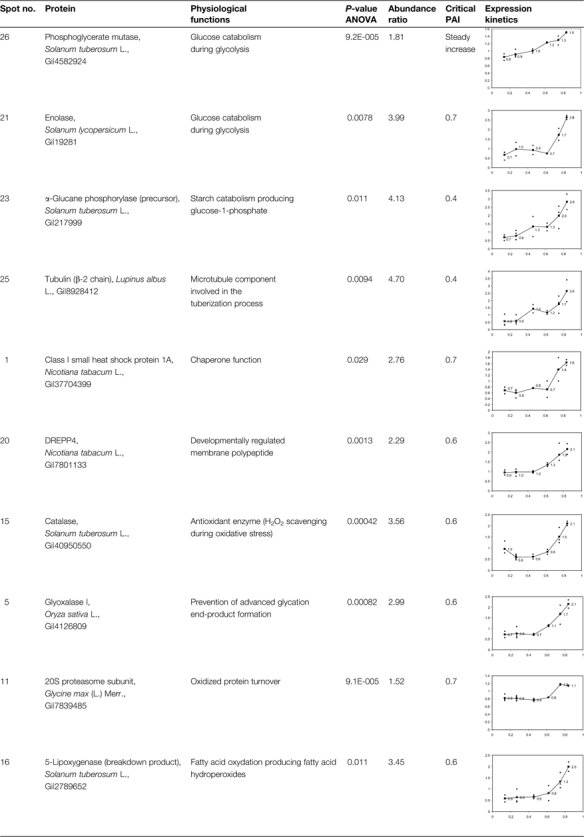 |
The P-values corresponding to ANOVA using PAI as the fixed factor are presented as well as the ratios between extreme abundance values. Critical PAI values corresponding to the major changes are also presented in the table. Standard abundances (y-axis) as a function of PAI (y-axis) are displayed in the graphics.
Table 3.
Abundance kinetics of down-regulated proteins during ageing
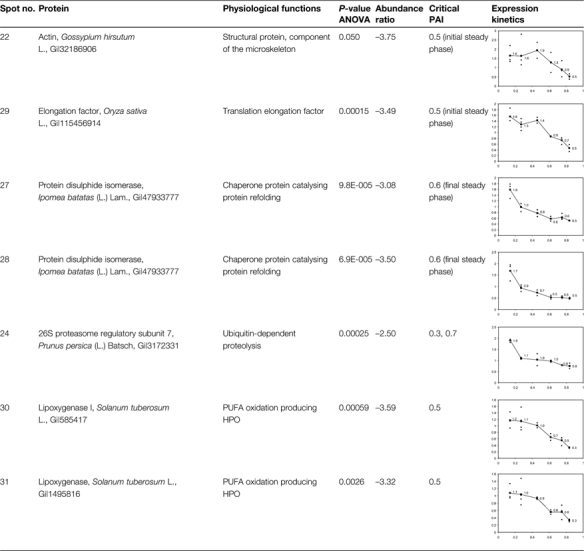 |
The P-values corresponding to ANOVA using PAI as the fixed factor are presented as well as the ratios between extreme abundance values. Critical PAI values corresponding to the major changes are also presented in the table. Standard abundances (y-axis) as a function of PAI (y-axis) are displayed in the graphics.
Among the identified up-regulated proteins, 14 proteins share amino acid sequences with patatin polypeptides but they exhibit a lower molecular weight than that of the intact protein. As the patatin is a 42 kDa storage glycoprotein that is degraded during ageing of potato tubers (Brierley et al., 1997), these 14 proteins should be considered as patatin breakdown products. The abundance of some of these peptides increases generally when a PAI value >0.6 is reached (spots 2, 3, 6, 8, 9, 10, 12, 13, and 14). The abundance kinetics of other breakdown products either increase faster when PAI values >0.4 are reached (spots 4, 7, 17, and 18) or exhibit a terminal steady phase (spots 8, 18, and 19, Supplementary Table S3 at JXB online). Spots 7 and 14 present the highest abundance variations, 4.94 and 4.88, respectively, compared with the mean variation of 3.36. These data confirm thus that a proteolysis of patatin isoforms occurs mainly in the second half of the ageing process, when PAI values >0.4 or 0.6, respectively, are reached.
Three enzymes related to starch catabolism are up-regulated during ageing (Table 2). The α-glucane phosphorylase (spot 23) catalyses the production of glucose-1-phosphate from starch through phosphorolysis. It is up-regulated early in the ageing process when PAI values >0.4 are reached. This point corresponds to the dormancy break of the tubers. Phosphoglycerate mutase (spot 26) and enolase (spot 21) are implicated in the last steps of the glycolysis preceding the formation of pyruvate (Givan, 1999). They are respectively up-regulated from the beginning of the ageing process or after PAI values >0.7 are reached.
Other proteins influencing protein conformation are differentially expressed during ageing. A small heat shock protein (sHSP, spot 1) is up-regulated when the PAI is >0.7. The expression of this sHSP is organ and development dependent (Dafny-Yelin et al., 2008). It has been shown to be up-regulated in tobacco pollen during development and after induction of pollen embryogenesis by heat shock or starvation (Zarsky et al., 1995; Volkov et al., 2005). It protects and stores mRNAs and possesses a chaperone function (Lubaretz and Zur Nieden, 2002; Park and Hong, 2002; Volkov et al., 2005). On the other hand, two isoforms of protein disulphide isomerases (spots 27 and 28) are down-regulated during ageing. These proteins belong to an oxidoreductase protein family containing at least two thioredoxin domains implicated in the formation of disulphide bridges. They act as dithiol oxidases for protein refolding in the endoplasmic reticulum (Buchanan and Balmer, 2005) and are potentially involved in ascorbate recycling as they exhibit a monodehydroascorbate and dehydroascorbate reductase activity (Huang et al., 2005). They also play a role as isomerase and chaperone proteins (Gruber et al., 2006; Wadahama et al., 2007).
Ageing influences the equilibrium of the 20S and 26S proteasome complexes: a 20S subunit (spot 11) is up-regulated when PAI values >0.7 are reached and a 26S regulatory subunit (spot 24) is down-regulated early in the ageing process. These data seem to indicate that the increase in abundance of the 20S proteasome that recycles oxidized proteins is concomitant with the decrease in abundance of the 26S complex (or at least of its 19S regulatory subunit) that degrades denaturated or misfolded proteins after polyubiquitination (Fu et al., 1998; Carrard et al., 2002; Smalle and Vierstra, 2004).
Proteins implicated in the defence against (a)biotic stresses are differentially expressed during ageing. Glyoxalase I (spot 5), an enzyme preventing the formation of advanced glycation end-products by scavenging 2-oxoaldehydes (Martins et al., 2001), is up-regulated when PAI values >0.6 are reached. After an initial decrease in abundance, a CAT (spot 15) is up-regulated for PAI values >0.6. This enzyme scavenges H2O2 produced by SOD. In parallel, two LOX isoforms (spots 30 and 31) are down-regulated from the beginning of the ageing process. These data are consistent with the accumulation of a LOX breakdown product (spot 16) possessing a lower molecular weight than the intact enzyme (comprised between 97 kDa and 103 kDa; Royo et al., 1996).
Finally, two components of the cytoskeleton are differentially expressed during ageing. An actin (spot 22) is down-regulated when PAI values are >0.5. It is involved in microfilament formation and influences organelle positioning within cells (Staiger and Blanchoin, 2006). A tubulin β-2 chain is up-regulated when the PAI is >0.4. This protein is a key component of the microtubules of the cytoskeleton and is implicated in growth and cell cycle progression. It is associated with the tuberization process in the potato tuber (Taylor et al., 1994).
Considering the whole data set, among the changes in protein abundance, the strong up-regulation of a CAT isoform is suggestive of an increased production of ROS (Smirnoff, 1995; Feierabend, 2005). Other variations (e.g. glyoxalase I, class I sHSP, 20S proteasome, and tubulin β chain) are also observed in an oxidative stress context, as will be discussed later. Based on those results, a targeted approach focused on antioxidant compounds and enzymes was developed to complement the understanding of potato tuber metabolism during ageing.
Assay of the antioxidant compounds
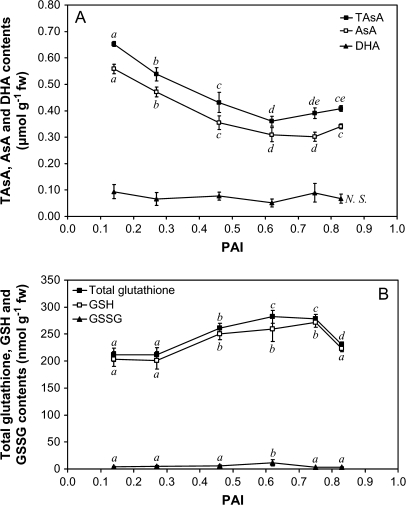
Ascorbate is quantitatively the most important antioxidant compound. It can inactivate most of the ROS. This oxidation generates DHA that can be converted into 2-(threo-1,2,3-trihydroxypropyl)tartronic acid without being recycled into ascorbate in the Halliwell–Asada cycle (Smirnoff, 1995). Total ascorbate and reduced ascorbate contents decrease very highly significantly (P <0.001) during ageing until a steady phase is reached for PAI values >0.6 (Fig. 2A). In parallel, no accumulation of DHA is observed, the measured data being close to the detection limit of the method (0.044 μmol g−1 FW, Kampfenkel et al., 1994). At the end of the storage period, the reduced form still represents 83% of the total pool.
Fig. 2.
Changes in reduced (AsA, GSH), oxidized (DHA, GSSG), and total (TAsA, Total glutathione) forms of ascorbate (A) and glutathione (B) during ageing. After metaphosphoric acid extraction, ascorbate and glutathione were assayed according to de Pinto et al. (1999) and Zhang and Kirkham (1996), respectively. The presented data are the means of four independent replicates ±SD. Means sharing the same letter were not statistically distinct using the Newman and Keuls test.
Glutathione is the main soluble thiol compound in the plant cells. Similarly to ascorbate, it can reduce, enzymatically or not, most of the ROS and is also involved in ascorbate recycling from DHA (Smirnoff, 2005). Its oxidation product is GSSG. The total glutathione content increases according to the PAI before reaching a steady phase, and decreases when PAI values >0.8 are reached. The GSH content follows a similar trend, while no accumulation of GSSG is observed (Fig. 2B). The GSH/GSSG ratio thus increases from 52.3 before storage to 67.7 at the end of the storage period.
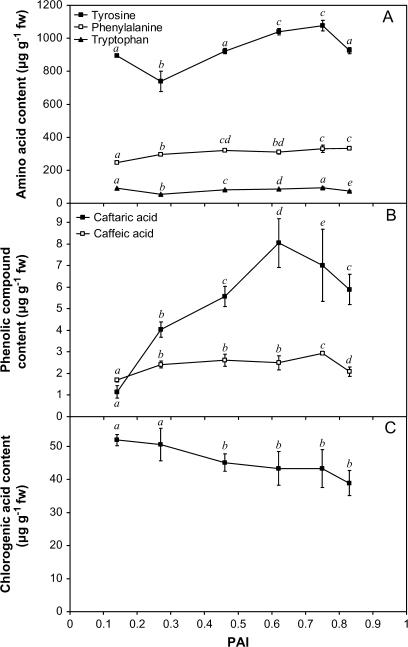
Phenolic compounds are free or cell wall-bound secondary metabolites that are able to detoxify ROS. They are implicated in hydrogen peroxide scavenging pathways together with ascorbate and monodehydroascorbate reductase (Takahama and Oniki, 1997). The analytical method used to quantify phenolic compounds also allowed measurement of the changes in their precursor amino acids (phenylalanine and tyrosine). A significant influence (P <0.001) of ageing is observed for both amino acids (Fig. 3A). After an initial decrease, the tyrosine content increases to reach 1076.4 μg g−1 FW at PAI=0.75. It then decreases to its initial value at PAI=0.83. The phenylalanine content increases steadily during ageing to reach 333.7 μg g−1 FW at the end of the storage period. Caffeic acid content increases until reaching a steady phase that ends when a PAI value of 0.83 is attained (Fig. 3B). The changes observed in caftaric acid content are characterized by a transient maximum (8.05 μg g−1 FW) reached at PAI=0.62 (Fig. 3B). A steady decrease in chlorogenic acid content is finally observed during ageing (Fig. 3C). This phenolic compound is by far the most abundant in the extracts. The evolution of its minor isomers such as the cryptochlorogenic and neochlorogenic acids is less clear (data not shown).
Fig. 3.
Changes in the main phenolic compounds (B, C) and in their precursor amino acids (A) during ageing. After extraction with a methanol/water/acetic acid (70:29.5:0.5; v/v/v) solution, phenolic compounds were measured according to André et al. (2007) using an HPLC-based method combined with a diode array detector. Presented values are means of six independent replicates ±SD. Means sharing the same letter were not statistically distinct using the Newman and Keuls test.
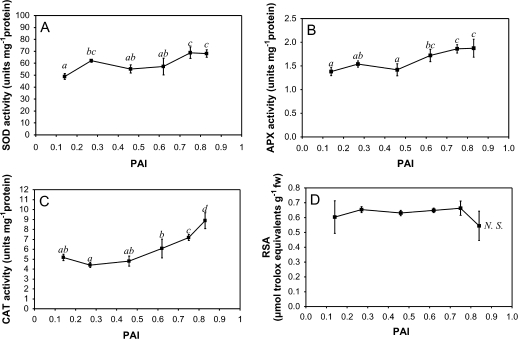
Changes in antioxidant enzymatic activities
In addition to the modifications of the antioxidant compound content, SOD activity increases during ageing (P=0.001, Fig. 4A). The major changes are observed when PAI values of 0.27 and 0.75 are reached. In parallel, the APX activity remains relatively steady until PAI values >0.6 are attained. It then increases to reach a final activity of 1.87±0.19 U mg−1 protein (Fig. 4B). Complementarily, after an initial decrease, the CAT activity significantly increases when PAI values >0.6 are reached (Fig. 4C). Using meristem sampling, the CAT activity has been reported to be—at least transiently—repressed during dormancy break (Bajji et al., 2007). Considering the whole tuber, the changes in CAT activity (Fig. 4C) are, however, comparable with those observed for protein abundance (Table 2).
Fig. 4.
Changes in the activities of SOD (A), APX (B), and CAT (C) during ageing. SOD, APX, and CAT activities were measured spectrophotometrically according to Dhindsa et al. (1980), Nakano and Asada (1981), and Claiborne (1985), respectively. The RSA (D) was measured according to Miller et al. (2000) using a stable free radical (DPPH) which reacts with antioxidants present in the tubers. Three independent measurements were performed for each point of the kinetics. Presented data are means ±SD. Means sharing the same letter were not statistically distinct using the Newman and Keuls test.
Radical scavenging activity
In order to assay the overall antioxidant capacity, the adopted protocol used a stable free radical (DPPH) which forms a deep purple solution and reacts with the antioxidants contained in potato tubers. During ageing, no statistically significant change is observed in the ROS scavenging activity (Fig. 4D).
The results obtained via the targeted approach focused on antioxidant enzymes are consistent with the proteomic data and constitute indirect evidence of a potential oxidative stress due to an increased ROS production. In order to confirm or deny this hypothesis, and find direct evidence of this oxidative stress, the changes in oxidative damage on sensitive biomolecules (e.g. PUFAs and proteins) were measured through an extensive oxylipin profiling and a global assay of the carbonyl content.
Oxylipin profiling and carbonyl content measurement
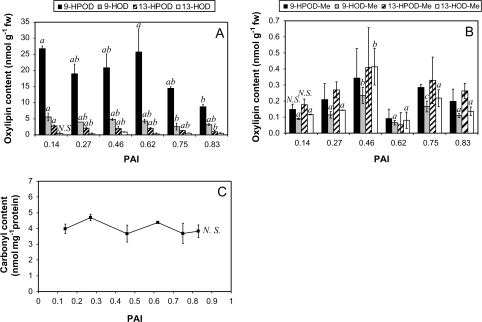
Considering the lipid oxidation context, a significant (P <0.001) increase in carotenoid content is observed during ageing before a steady phase is reached for PAI values >0.6 (Supplementary Fig. S1A at JXB online). The carotenoids constitute an important class of lipophilic antioxidant compounds. They are implicated in membrane protection against ROS and they work synergistically with ascorbate and tocopherols (Smirnoff, 2005). On the other hand, fatty acid oxidation can be due to autoxidative processes or can be promoted by lipoxygenases. These latter enzymes can form either 9- or 13-HPO derived from linoleic (‘D’ derivatives) or linolenic (‘T’ derivatives) acids. The HPOs exist as free or esterified (-Me ending) forms and can be reduced to the corresponding hydroxide (HO; Liavonchanka and Feussner, 2006). In the storage context considered here, this enzymatic activity decreases significantly (P=0.037) during ageing of potato tubers (Supplementary Fig. S1B at JXB online). This decrease does not necessarily imply that no oxylipin accumulation occurs during ageing as these oxidation products can be non-enzymatically formed during oxidative stress.
Therefore, the potential changes in the oxylipin profiles were measured extensively during ageing (Fig. 5A, B). As the concentrations of both esterified and free oxylipins derived from linoleic acid are globally higher than those derived from linolenic acid (data not shown), only the results obtained for the most abundant oxylipins are presented. Moreover, the trends observed for both kinds of oxylipins are similar as no accumulation can be noticed during the ageing process in the storage conditions of this study. For linoleic acid-derived oxylipins, free forms are more abundant than esterified forms. They are mainly represented by 9-HPOD and 9-HOD. The concentrations of esterified oxylipins are close to the detection threshold, and 9-HPOD-Me and 13-HPOD-Me are the most abundant. Chiral-phase analysis also reveals that these main compounds are formed enzymatically during ageing. Indeed, their S enantiomer percentage remains >80% during 270 d of storage at 4 °C (data not shown), which is typical for enzymatically produced oxylipins (Göbel et al., 2003). For these oxylipins, a transient maximum is observed when a PAI value comprised between 0.46 and 0.62 is reached.
Fig. 5.
Changes in the main free (A) and esterified (B) oxylipins during ageing. The fatty acid hydroperoxides (HPOs) and hydroxides (HOs) derived from linoleic acid (‘D’ derivatives) were measured according to Göbel et al. (2002) and Fauconnier et al. (2008) using a two-step HPLC method combining reverse and normal phase analysis. Two independent extracts were used for each point of the kinetics. The change in carbonyl content (C) was measured spectrophotometrically after SDS extraction and DNPH derivatization. The presented data are means of three independent replicates ±SD. Means sharing the same letter were not statistically distinct using the Newman and Keuls test. The non-significant (N.S.) changes are mentioned on the graphs.
Concerning the proteins, it is generally assumed that the measurement of carbonyl content is a good evaluation of the intensity of their oxidation (Dalle-Donne et al., 2003; Shulaev and Oliver, 2006). In the context of this study, although the PAI increases markedly during the 270 d of storage at 4 °C, no accumulation of carbonyl groups is observed on SDS-extracted proteins during ageing (Fig. 5C).
Discussion
In the storage conditions used in this study, the physiological age of the potato tubers increased markedly, with PAI values ranging from 0.14 to 0.83. This physiological evolution was accompanied by a dormancy break that occurred non-synchronously at the apical and proximal bud level and led to modifications of the sprouting pattern (Delaplace et al., 2008a). This progressive dormancy loss corresponded to enhanced respiration, as reflected by the increase in cytochrome c oxidase activity (Burton, 1989; Delaplace et al., 2008c). It should be noted that only extreme PAI values >0.8 induced a loss in sprouting vigour.
The proteomic study indicated that, during ageing, the differentially expressed proteins were involved mainly in starch catabolism, control of protein conformation, protein recycling, and stress response. Moreover, 14 breakdown products of patatin increased during ageing, indicating enhanced patatin proteolysis. Previous proteomic studies have characterized tuber formation (tuberization) before harvest, and it is worth comparing these results with those obtained in the present study on post-harvest development. Indeed, Agrawal et al. (2008) have shown that, apart from the accumulation of various patatin isoforms, the tuberization process is also associated with the overexpression of ROS-catabolizing enzymes (SOD, APX, and CAT). A proteasome subunit (Gi|79325892) was up-regulated, indicating that this proteolytic complex is also involved in tuberization. In contrast to the results obtained here, several HSPs were down-regulated during tuberization, but the developing tubers did not experience extensive cold stress. An earlier study by Lehesrantha et al. (2006) on the potato tuber life cycle, encompassing the tuberization process and the dormancy break, presented similar results in terms of patatin accumulation and up-regulation of antioxidant enzymes. However, the changes they observed in proteasome (down-regulated) and HSP (up-regulated) abundance did not agree with those observed by Agrawal et al. (2008). Complementarily, a transcriptomic study of the dormancy break by Campbell et al. (2008) revealed that two LOX mRNA were down-regulated during this physiological process. This observation is consistent with (i) the decrease in LOX abundance observed in the present study; and (ii) the accumulation of a LOX breakdown product exhibiting a lower molecular weight. However, the changes observed here in protein abundance of a CAT isoform did not correspond to the decrease observed at the transcriptomic level by Campbell et al. (2008). This could be due to a different sampling technique (excised meristems versus whole tubers) or different behaviour of CAT isoforms.
The biochemical results obtained with the targeted approach are consistent with those of the present proteomic study. The ascorbate content decreased initially until apical dominance was lost. In parallel, glutathione and caffeic acid increased markedly before a decrease occurred for PAI values >0.8. When a relatively steady value was reached for ascorbate, an increase in activity was observed for the major enzymatic antioxidants (SOD, APX, and CAT). In contrast, the content of chlorogenic acid decreased steadily during storage, as observed during fruit ripening by Macheix et al. (2005).
Most of the observed changes, at both the proteomic and metabolomic levels, are typical of a biological system facing an increased ROS production. The glyoxalase I, class I sHSP, CAT, 20S proteasome, and tubulin β chain have been shown to be up-regulated during various stresses, including oxidative stress (Davies, 2001; Sun et al., 2002; Feierabend, 2005; Yadav et al., 2005; Schwarzerova et al., 2006; Swindell et al., 2007). The trends observed for SOD, APX, and CAT activities, as well as for the ascorbate and GSH content, are also comparable with those observed during the oxidative stress response (Smirnoff, 1995; Feierabend, 2005; Foyer et al., 2005; Mittler and Poulos, 2005). However, the radical scavenging activity is maintained throughout the ageing process, as no accumulation of oxidative damage was measured in the storage conditions used in this study. The present observations therefore constitute indirect clues of an enhanced oxidative challenge.
The build-up of this oxidative challenge could actually be influenced by several abiotic factors (e.g. cold stress) and intrinsic factors (e.g. dormancy breaks during ageing).
In a similar storage context (180 d of storage at 4 °C), Reverberi et al. (2001) indeed proposed that the observed modifications in the antioxidant content of potato tissues adjacent to the meristems were due to cold stress. Nevertheless, compared with the results reported herein and obtained at 4 °C, similar changes in antioxidant content were observed during 350 d of storage at 20 °C (Delaplace et al., 2008c). Therefore, although a quantitative influence of cold stress cannot be excluded, low temperatures do not seem to affect the main trends related to the ageing process.
However, the asynchronous dormancy break occurring during ageing is concomitant with the enhanced metabolic activity (e.g. respiration) that potentially produces ROS as by-products. The tubers respond efficiently to this oxidative challenge at least until PAI values of 0.8 are reached. The build-up of an oxidative challenge could, therefore, be a consequence of the post-harvest development of the tubers. Furthermore, as signal molecules, ROS can also modify gene expression through oxidation of transcription factors or modification of antioxidant content (Halliwell, 2006). The progression of the cell cycle could indeed be influenced by the changes observed in the concentration of reduced and oxidized forms of ascorbate and glutathione (Potters et al., 2004; Zentgraf, 2007). Sprouting could also be influenced by the decrease in chlorogenic acid content as this compound is known to inhibit seed germination (Macheix et al., 2005). Complementarily, the up-regulation of three enzymes involved in starch catabolism could also increase sugar availability to the developing sprouts acting as physiological sinks, thus influencing the sprouting pattern.
Several hypotheses could explain the decrease in sprout growth for PAI values >0.8. At these PAI values, the decrease in GSH and caffeic acid content, as well as the observed drop in RSA, could suggest a progressive weakening of antioxidant defences. An effective oxidative stress could then follow this developmental stage, as observed by Kumar et al. (1999) based on the carbonyl content measurements during long-term storage (30 months at 4 °C). However, deleterious effects on the sprouting phenotype were noticed without measuring significant damage on lipids and proteins. Other hypotheses (e.g. modifications of the hormonal balance at the meristem level) should therefore be investigated to gain a better understanding of the onset of deleterious ageing.
Altogether, the results obtained in this study suggest that the oxidative metabolism of ageing tubers is distinct from that observed in other ageing or senescence contexts (e.g. true seed ageing or leaf senescence). In these latter contexts, oxidative damage accompanies the degenerative and/or recycling processes leading to death (Procházková and Wilhelmová, 2007). This oxidative stress induces an increase in oxylipin content in both contexts, due mainly to the non-enzymatic oxidations of PUFAs. In the storage conditions used in this study, the potato tubers did not exhibit such enhanced oxidative damage.
Under realistic post-harvest conditions, potato tubers do not actually age in the deleterious (gerontological) sense, as described by Harman (1956) in his oxidative theory of ageing. This theory postulates that the accumulation of non-enzymatic modifications on cellular biomolecules, caused by ROS attacks, is one of the main factors leading to the functional degradation observed during ageing. During potato tuber storage, the physiological evolution of the tubers is concomitant with an increase in both sprout growth rate and number (Delaplace et al., 2008a). Only extreme PAI values >0.8 are associated with deleterious effects on the sprouting pattern and on the concentration of some antioxidants. The biochemical data indicate that the many biochemical changes observed during most of the ageing process allow the tubers to respond efficiently to a putative increase in ROS production. Cellular breakdown and post-harvest ageing should therefore be dissociated, in contrast to other plant—and animal—ageing models.
Conclusion
This study focuses on the post-harvest ageing of potato tubers stored under realistic agronomical conditions (270 d at 4 °C). Under these conditions, the ageing index increases markedly, but the sprouting phenotype does not exhibit deleterious changes until PAI values >0.8 are reached. Taking PAI as a reference point, the physiological and biochemical changes observed during ageing confirm that this process is essentially not deleterious within the given time frame. The post-harvest development of potato tubers actually seems to induce an oxidative challenge that is efficiently taken up by the proteomic and metabolic responses of the tubers, as no significant accumulation of oxidative damage on PUFAs or proteins was noticed, even when a decrease in sprouting vigour was observed. Other metabolic pathways (e.g. phytohormone pathways) should therefore be investigated to complement the understanding of the factors influencing potato tuber ageing. Some of the results of this study are, however, well correlated with the sprouting phenotypes and could also be assessed as potential ageing biomarkers.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Changes in the lipid oxidation context during ageing. The carotenoid content (A) was measured spectrophotometrically according to Morris et al. (2004). The LOX activity assay was based on the method originally developed by Surrey (1964).
Table S1. MS/MS identifications of up-regulated proteins during potato ageing.
Table S2. MS/MS identifications of down-regulated proteins during potato ageing.
Table S3. Standard abundance kinetics of putative breakdown products of patatin during ageing.
Supplementary Material
Acknowledgments
The authors thank Sylvain Lestrade and Sébastien Planchon for their efficient help in 2D gel spot picking and tryptic digestion of proteins, respectively. We are also grateful to Virginie Gosset and Adeline Blondiaux for their excellent help in oxylipin profiling, assessments of sprouting parameters, and measurements of enzymatic activities. This work was financially supported by the Belgian Fonds de la Recherche Scientifique-FNRS (FRFC project 2.4569.00. and short-term grants) and The Netherlands Proteomics Centre Hotel facility (Wageningen, The Netherlands).
Glossary
Abbreviations
- ACN
acetonitrile
- ANOVA
analysis of variance
- APX
ascorbate peroxidase
- AsA
reduced ascorbate
- CAT
catalase
- DHA
dehydroascorbate
- DIGE
fluorescence difference gel electrophoresis
- DPPH
2,2-diphenyl-1-pierylhydrazyl
- DTNB
5,5′-dithiobis(2-nitrobenzoic) acid
- DTT
dithiothreitol
- FW
fresh weight
- HO
fatty acid hydroxide
- HPLC
high-performance liquid chromatography
- HPO
fatty acid hydroperoxide
- IPG
immobilized pH gradient
- LOX
lipoxygenase
- MS/MS
tandem mass spectrometry
- PAI
physiological age index
- PUFAs
polyunsaturated fatty acids
- ROS
reactive oxygen species
- RSA
radical scavenging activity
- sHSP
small heat shock protein
- SOD
superoxide dismutase
- TAsA
total ascorbate (AsA+DHA)
- TFA
trifluoroacetic acid
- Trolox
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
References
- Agrawal L, Chakraborty S, Jaiswal DK, Gupta S, Datta A, Chakraborty N. Comparative proteomics of tuber induction, development and maturation reveal the complexity of tuberization process in potato (Solanum tuberosum L.) Journal of Proteome Research. 2008;7:3803–3817. doi: 10.1021/pr8000755. [DOI] [PubMed] [Google Scholar]
- André C, Oufir M, Guignard C, Hoffmann L, Hausman JF, Evers D, Larondelle Y. Antioxidant profiling of native Andean potato cultivars (Solanum tuberosum L.) reveals tubers with high levels of β-carotene, α-tocopherol, chlorogenic acid and patanin. Journal of Agricultural and Food Chemistry. 2007;55:10839–10849. doi: 10.1021/jf0726583. [DOI] [PubMed] [Google Scholar]
- Bajji M, M'Hamdi M, Gastiny F, Rojas-Beltran JA, du Jardin P. Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) tubers. Biotechnologie, Agronomie. Société et Environnement. 2007;11:121–131. [Google Scholar]
- Bohler S, Bagard M, Oufir M, Planchon S, Hoffmann L, Jolivet Y, Hausman JF, Dizengremel P, Renaut J. A DiGE analysis of developing poplar leaves subjected to ozone reveals major changes in carbon metabolism. Proteomics. 2007;7:1584–1599. doi: 10.1002/pmic.200600822. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Sinha P, Frommer WB. Changes in the two-dimensional protein pattern and in gene expression during the sink-to-source transition of potato tubers. Plant Science. 1994;99:97–108. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brierley ER, Bonner PLR, Cobb AH. Aspects of amino acid metabolism in stored potato tubers (cv. Pentland Dell) Plant Science. 1997;127:17–24. [Google Scholar]
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annual Reviews of Plant Biology. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- Burton WG. Post-harvest physiology. In: Burton WG, editor. The potato. Harlow: Longman Scientific and Technical; 1989. pp. 423–522. [Google Scholar]
- Caldiz DO, Fernandez LV, Struik PC. Physiological age index: a new, simple and reliable index to assess the physiological age of seed potato tubers based on the haulm killing date and length of the incubation period. Field Crops Research. 2001;69:69–79. [Google Scholar]
- Campbell M, Segear E, Beers L, Knauber D, Suttle J. Dormancy in potato tuber meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Functional and Integrative Genomics. 2008;18:317–328. doi: 10.1007/s10142-008-0079-6. [DOI] [PubMed] [Google Scholar]
- Carrard G, Bulteau AL, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. International Journal of Biochemistry and Cell Biology. 2002;34:1461–1474. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods in oxygen radical research. Boca Raton, FL: CRC Press; 1985. pp. 283–284. [Google Scholar]
- Claver FK. Influence of temperature during the formation of tubers in relation with their incubation state (physiological age) and seed value. Experientia. 1973;30:97–98. [Google Scholar]
- Coleman WK. Physiological ageing of potato tubers: a review. Annals of Applied Biology. 2000;137:189–199. [Google Scholar]
- Dafny-Yelin M, Tzfira T, Vainstein A, Adam Z. Non-redundant functions of sHSP-CIs in acquired thermotolerance and their role in early seed development in Arabidopsis. Plant Molecular Biology. 2008;67:363–373. doi: 10.1007/s11103-008-9326-4. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- De Pinto MC, Francis D, De Gara L. The redox state of the ascorbate–dehydroascorbate pair as a specific sensor of cell division in tobacco TBY2 cells. Protoplasma. 1999;209:90–97. doi: 10.1007/BF01415704. [DOI] [PubMed] [Google Scholar]
- Delaplace P, Brostaux Y, Fauconnier ML, du Jardin P. Potato (Solanum tuberosum L.) tuber physiological age index is a valid reference frame in postharvest ageing studies. Postharvest Biology and Technology. 2008a;50:103–106. [Google Scholar]
- Delaplace P, Fauconnier ML, du Jardin P. Méthodes de mesure de l’âge physiologique des tubercules semences de pomme de terre (Solanum tuberosum L. Biotechnologie, Agronomie, Société et Environnement. 2008b;12:171–184. [Google Scholar]
- Delaplace P, Rojas-Beltran J, Frettinger P, du Jardin P, Fauconnier M-L. Oxylipin profile and antioxidant status of potato tubers during extended storage at room temperature. Plant Physiology and Biochemistry. 2008c;46:1077–1084. doi: 10.1016/j.plaphy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Delaplace P, van der Wal F, Dierick JF, Cordewener JHG, Fauconnier ML, du Jardin P, America AHP. Potato tuber proteomics: comparison of two complementary extraction methods designed for 2-DE of acidic proteins. Proteomics. 2006;6:6494–6497. doi: 10.1002/pmic.200600493. [DOI] [PubMed] [Google Scholar]
- Désiré S, Couillerot JP, Hilbert JL, Vasseur J. Protein changes in Solanum tuberosum during storage and dormancy breaking of in vitro microtubers. Plant Physiology and Biochemistry. 1995;33:479–487. [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany. 1981;32:93–101. [Google Scholar]
- Dipierro S, De Leonardis S. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. Journal of Experimental Botany. 1997;48:779–783. [Google Scholar]
- Dyson PW, Digby J. Effects of calcium on sprout growth of ten potato cultivars. Potato Research. 1985;18:363–377. [Google Scholar]
- Espen LS, Morgutti S, Cocucci SM. Changes in the potato (Solanum tubersoum L.) tuber at the onset of dormancy and during storage at 23 °C and 3 °C. II. Evaluation of protein patterns. Potato Research. 1999;42:203–215. [Google Scholar]
- Fauconnier ML, Rojas-Beltran J, Delcarte J, Dejaeghere F, Marlier M, du Jardin P. Lipoxygenase pathway and membrane permeability and composition during storage of potato tubers (Solanum tuberosulm L. cv. Bintje and Désirée) in different conditions. Plant Biology. 2002;4:77–85. [Google Scholar]
- Fauconnier ML, Rojas-Beltran J, Dupuis B, Delaplace P, Frettinger P, Gosset V, du Jardin P. Changes in oxylipin synthesis after Phytophthora infestans infection of potato leaves do not correlate with resistance. Plant Physiology and Biochemistry. 2008;46:823–831. doi: 10.1016/j.plaphy.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Feierabend J. Catalases in plants: molecular and functional properties and role in stress defence. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing; 2005. pp. 101–140. [Google Scholar]
- Foyer CH, Gomez LD, Van Heerden PDR. Glutathione. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing; 2005. pp. 1–24. [Google Scholar]
- Fu H, Doelling JH, Arendt CS, Hochstrasser M, Vierstra RD. Molecular organization of the 20S proteasome gene family from Arabidopsis thaliana. Genetics. 1998;149:677–692. doi: 10.1093/genetics/149.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan CV. Evolving concepts in plant glycolysis: two centuries of progress. Biological Reviews. 1999;74:277–309. [Google Scholar]
- Göbel C, Feussner I, Hamberg M, Rosahl S. Oxylipin profiling in pathogen infected potato leaves. Biochimica Biophysica Acta. 2002;1584:55–64. doi: 10.1016/s1388-1981(02)00268-8. [DOI] [PubMed] [Google Scholar]
- Göbel C, Feussner I, Rosahl S. Lipid peroxidation during the hypersensitive response in potato in the absence of 9-lipoxygenases. Journal of Biochemistry and Molecular Biology. 2003;278:52834–52940. doi: 10.1074/jbc.M310833200. [DOI] [PubMed] [Google Scholar]
- Gruber CW, Cemazar M, Heras B, Martin JL, Craik DJ. Protein disulfide isomerase: the structure of oxidative folding. Trends in Biochemical Sciences. 2006;31:455–464. doi: 10.1016/j.tibs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Huang D-J, Chen H-J, Lin Y-H. Isolation and expression of protein disulfide isomerase cDNA from sweet potato (Ipomoea batatas [L.] Lam ‘Tainong 57’) storage roots. Plant Science. 2005;169:776–784. [Google Scholar]
- Kampfenkel K, Van Montagu M, Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry. 1994;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Kumar GNM, Houtz RL, Knowles NR. Age-induced protein modifications and increased proteolysis in potato seed-tubers. Plant Physiology. 1999;119:89–100. doi: 10.1104/pp.119.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GNM, Knowles NR. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiology. 1993;102:115–124. doi: 10.1104/pp.102.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GNM, Knowles NR. Oxidative stress results in increased sinks for metabolic energy during aging and sprouting of potato seed-tubers. Plant Physiology. 1996;112:1301–1313. doi: 10.1104/pp.112.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehesranta SJ, Davies HV, Shepherd LVT, Koistinen KM, Massat N, Nunan N, McNicol JW, Kärenlampi SO. Proteomic analysis of the potato tuber life cycle. Proteomics. 2006;6:6042–6052. doi: 10.1002/pmic.200600383. [DOI] [PubMed] [Google Scholar]
- Liavonchanka A, Feussner I. Lipoxygenases: occurrence, functions and catalysis. Journal of Plant Physiology. 2006;163:348–357. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Lubaretz O, Zur Nieden U. Accumulation of plant small heat-stress proteins in storage organs. Planta. 2002;215:220–228. doi: 10.1007/s00425-002-0745-1. [DOI] [PubMed] [Google Scholar]
- Macheix J-J, Fleuriet A, Jay-Allemand C. Les composés phénoliques des végétaux. Un exemple de métabolites secondaires d'importance économique. Lausanne: Presses polytechniques et universitaires romandes; 2005. [Google Scholar]
- Martins AM, Mendes P, Cordeiro C, Ponces Freire A. In situ kinetic analysis of glyoxalase I and glyoxalase II in Saccharomyces cerevisiae. European Journal of Biochemistry. 2001;268:3930–3936. doi: 10.1046/j.1432-1327.2001.02304.x. [DOI] [PubMed] [Google Scholar]
- Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. Journal of the American College of Nutrition. 2000;19:312S–319S. doi: 10.1080/07315724.2000.10718966. [DOI] [PubMed] [Google Scholar]
- Mittler R, Poulos TL. Ascorbate peroxidase. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing; 2005. pp. 87–100. [Google Scholar]
- Mizuno M, Kamei M, Tsuchida H. Ascorbate peroxidase and catalase cooperate for protection against hydrogen peroxide generated in potato tubers during low-temperature storage. Biochemistry and Molecular Biology International. 1998;44:717–726. doi: 10.1080/15216549800201762. [DOI] [PubMed] [Google Scholar]
- Morris WL, Ducreux L, Griffiths DW, Stewart D, Davies HV, Taylor MA. Carotenogenesis during tuber development and storage in potato. Journal of Experimental Botany. 2004;55:975–982. doi: 10.1093/jxb/erh121. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiology. 1981;22:867–880. [Google Scholar]
- Park SM, Hong CB. Class I heat-shock protein gives thermotolerance in tobacco. Journal of Plant Physiology. 2002;159:25–30. [Google Scholar]
- Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiology. 2004;134:1479–1487. doi: 10.1104/pp.103.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková D, Wilhelmová N. Leaf senescence and activities of the antioxidant enzymes. Biologia Plantarum. 2007;51:401–406. [Google Scholar]
- Punchard NA. Free radicals: a practical approach. Oxford: IRL Press at Oxford University Press; 1996. [Google Scholar]
- Reust W. EAPR working group physiological age of the potato. Potato Research. 1986;29:268–271. [Google Scholar]
- Reverberi M, Picardo M, Ricelli A, Camera E, Fanelli C, Fabbri AA. Oxidative stress, growth factor production and budding in potato tuber during cold storage. Free Radical Research. 2001;35:833–841. doi: 10.1080/10715760100301331. [DOI] [PubMed] [Google Scholar]
- Royo J, Vancanneyt G, Perez AG, Sanz C, Stormann K, Rosahl S, Sanchez-Serrano JJ. Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. Journal of Biological Chemistry. 1996;271:21012–21019. doi: 10.1074/jbc.271.35.21012. [DOI] [PubMed] [Google Scholar]
- Schwarzerova K, Petrasek J, Panigrahi KCS, Zelenkova S, Opatrny Z, Nick P. Intranuclear accumulation of plant tubulin in response to low temperature. Protoplasma. 2006;227:185–196. doi: 10.1007/s00709-005-0139-x. [DOI] [PubMed] [Google Scholar]
- Shulaev V, Oliver DJ. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiology. 2006;141:367–372. doi: 10.1104/pp.106.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annual Reviews of Plant Biology. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Environment and plant metabolism: flexibility and acclimation. Oxford: BIOS Scientific Publishers; 1995. pp. 217–243. [Google Scholar]
- Smirnoff N. Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing; 2005. pp. 53–86. [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radical Biology and Medicine. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Spiteller G. The relationship between changes in the cell wall, lipid peroxidation, proliferation, senescence and cell death. Physiologia Plantarum. 2003;119:5–18. [Google Scholar]
- Spychalla JP, Desborough SL. Superoxide dismutase, catalase, and α-tocopherol content of stored potato tubers. Plant Physiology. 1990;94:1214–1218. doi: 10.1104/pp.94.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger CJ, Blanchoin L. Actin dynamics: old friends with new stories. Current Opinion in Plant Biology. 2006;9:554–562. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Sun W, Van Montagu M, Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochimica et Biophysica Acta. 2002;1577:1–9. doi: 10.1016/s0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- Surrey K. Spectrophometric method for determination of lipoxidase activity. Plant Physiology. 1964;39:65–70. doi: 10.1104/pp.39.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8:1–15. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama U, Oniki T. A peroxidase/phenolics/acorbate system can scavenge hydrogen peroxide in plant cells. Physiologia Plantarum. 1997;101:845–852. [Google Scholar]
- Taylor MA, Wright F, Davies HV. Characterization of the cDNA clones of 2 beta-tubulin genes and their expression in the potato plant (Solanum tuberosum L.) Plant Molecular Biology. 1994;26:1013–1018. doi: 10.1007/BF00028869. [DOI] [PubMed] [Google Scholar]
- Volkov RA, Panchuk II, Schöffl F. Small heat shock proteins are differentially regulated during pollen development and following heat stress in tobacco. Plant Molecular Biology. 2005;57:487–502. doi: 10.1007/s11103-005-0339-y. [DOI] [PubMed] [Google Scholar]
- Wadahama H, Kamauchi S, Ishimoto M, Kawada T, Urade R. Protein disulfide isomerase family proteins involved in soybean protein biogenesis. FEBS Journal. 2007;274:687–703. doi: 10.1111/j.1742-4658.2006.05613.x. [DOI] [PubMed] [Google Scholar]
- Waddell WJ. A simple ultraviolet spectrophotometric method for determination of protein. Journal of Laboratory and Clinical Medicine. 1956;48:311–314. [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochemical and Biophysical Research Communications. 2005;337:61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Zabrouskov V, Kumar GNM, Spychalla JP, Knowles NR. Oxidative metabolism and the physiological age of seed potatoes are affected by increased α-linolenate content. Physiologia Plantarum. 2002;116:172–185. doi: 10.1034/j.1399-3054.2002.1160206.x. [DOI] [PubMed] [Google Scholar]
- Zarsky V, GArrido D, Eller N, Tupy J, Vicente O, Schöffl F, Heberle-Bors E. The expression of a small heat shock gene is activated during induction of tobacco pollen embryogenesis by starvation. Plant, Cell and Environment. 1995;18:139–147. [Google Scholar]
- Zentgraf U. Oxidative stress and leaf senescence. In: Gan S, editor. Senescence processes in plants. Oxford: Blackwell Publishing; 2007. pp. 39–62. [Google Scholar]
- Zhang F, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist. 1996;132:361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.