Abstract
Kernel weight is an important factor determining grain yield and nutritional quality in sorghum, yet the developmental processes underlying the genotypic differences in potential kernel weight remain unclear. The aim of this study was to determine the stage in development at which genetic effects on potential kernel weight were realized, and to investigate the developmental mechanisms by which potential kernel weight is controlled in sorghum. Kernel development was studied in two field experiments with five genotypes known to differ in kernel weight at maturity. Pre-fertilization floret and ovary development was examined and post-fertilization kernel-filling characteristics were analysed. Large kernels had a higher rate of kernel filling and contained more endosperm cells and starch granules than normal-sized kernels. Genotypic differences in kernel development appeared before stamen primordia initiation in the developing florets, with sessile spikelets of large-seeded genotypes having larger floret apical meristems than normal-seeded genotypes. At anthesis, the ovaries for large-sized kernels were larger in volume, with more cells per layer and more vascular bundles in the ovary wall. Across experiments and genotypes, there was a significant positive correlation between kernel dry weight at maturity and ovary volume at anthesis. Genotypic effects on meristem size, ovary volume, and kernel weight were all consistent with additive genetic control, suggesting that they were causally related. The pre-fertilization genetic control of kernel weight probably operated through the developing pericarp, which is derived from the ovary wall and potentially constrains kernel expansion.
Keywords: Grain size, grain filling, kernel size, meristem, ovary, pericarp, sorghum
Introduction
Kernel weight is an important yield component and also an important factor determining nutritional quality in sorghum [Sorghum bicolor (L.) Moench] (Kriegshauser et al., 2006). Environmental factors, especially those influencing water and assimilate availability, are known to affect kernel weight realized at maturity, but potential kernel weight is, to a large extent, under genetic control. The genetic and physiological mechanisms underlying the genotypic variation in potential kernel weight remain unclear. A better understanding of the developmental processes determining kernel weight could enhance the efficiency of breeding programmes aimed at improving grain yield and quality.
Kernel growth and development are dynamic processes. In cereals, the pre-anthesis structures that will develop into a kernel are the ovule and the ovary wall. The ovule contains two female gametes: the egg cell and the central cell. After pollination, fertilization of the egg cell and central cell produces the embryo and endosperm, respectively (Olsen, 2004). The maternally derived ovary wall, which is formed by the lower part of the carpels, undergoes morphological changes and develops into the mature pericarp, completely enclosing the seed (Earp et al., 2004). From fertilization to physiological maturity, kernel development can be divided into three consecutive phases (Egli, 2006). During the initial lag phase, rapid endosperm cell division and starch granule initiation occurs, but dry matter accumulation is slow. The second phase is often referred to as the linear phase of kernel filling, as kernel dry weight increases rapidly during this period and the rate of dry matter accumulation is near constant. Finally, reserve accumulation slows until the maximum kernel dry weight is attained at physiological maturity, when vascular bundles supplying the kernel cease to function and degrade to form the commonly observed ‘black layer’ on the mature kernels (Eastin et al., 1973).
Kernel-filling processes have been studied in numerous cereal crops. Genotypic and environmental effects on kernel weight at maturity are generally related to changes in kernel-filling rate, duration, or both (Gebeyehou et al., 1982; Bruckner and Frohberg, 1987; Darroch and Baker, 1990; Heiniger et al., 1993; Wang et al., 1999). Some studies indicated that kernel-filling potential was determined at the early stages of kernel filling. It has been shown that genotypic differences in kernel weight were associated with differences in maximum kernel water content achieved early in the kernel-filling period (Schnyder and Baum, 1992; Borras et al., 2004; Gambin and Borras, 2005). Studies on maize and wheat suggested that potential kernel weight was determined soon after anthesis during the initial lag phase of kernel filling (Brocklehurst, 1977; Jones et al., 1996). The number of endosperm cells and starch granules is established during this period, which could limit the capacity of the kernel to convert and store assimilates during the later stages of kernel growth. However, early ovary development prior to fertilization may also play a critical role in the control of potential kernel growth. In wheat and barley, kernel weight at maturity was found to be positively associated with carpel weight prior to fertilization (Scott et al., 1983; Calderini and Reynolds, 2000).
Hence, uncertainty remains as to the developmental mechanisms that determine genotypic differences in kernel weight, and the developmental stage at which potential kernel weight is set. Although cell division, water uptake, and dry matter accumulation are the prerequisites for kernel growth, and genotypic variation in kernel size was found to be related to differences in these filling characteristics, the rate and extent of cell division, water uptake, and dry matter accumulation could be determined by processes that occur earlier in kernel development, even before fertilization. In this study, pre-anthesis floret development and post-anthesis kernel filling were examined in five genotypes of sorghum known to differ in kernel weight at maturity. The aim was to determine the developmental stage at which genetic effects on potential kernel weight were realized, as such knowledge allows a better understanding of the physiological mechanisms by which potential kernel size is controlled.
Materials and methods
Plant material and experimental details
Two field experiments were conducted at Gatton (27º33′ S, 152º20′ E), Queensland, Australia. The soil was a Lawes brown black clay loam, which is a moderately fertile deep alluvial, weakly cracking vertisol (Typic Chromustert). Experiment I included three sorghum inbred lines (KS115, BTx642, and RQL36) and their hybrids (ATx642/KS115 and ATx642/RQL36) and was sown on 17 January 2006. ATx642 is the male-sterile version of BTx642 and is used to produce hybrids. The genotypes represented a wide range in kernel weight. KS115 is a large-seeded inbred line developed at Kansas State University (Tuinstra et al., 2001) and has been used as a source for improving kernel size and quality in sorghum breeding programmes (Kriegshauser et al., 2006). Its kernel weight is approximately twice that of genotypes with normal kernel weight (BTx642, RQL36, and their hybrid). Experiment II included three of the genotypes used in Experiment I (KS115, ATx642/KS115, and ATx642/RQL36) and was sown on 14 November 2006.
Both experiments were laid out as a randomized block design with three replicates. Each plot consisted of four rows, 1 m apart and 15 m long. Plots were over-sown and thinned to a density of five plants m−2 (50 000 plants ha−1) 2 weeks after sowing. Experiment I received pre-sowing fertilizer applications of 291 kg ha−1 single superphosphate (SSP), 112 kg ha−1 muriate of potash (MP), and 281 kg ha−1 urea. For Experiment II, the amounts applied were 322 kg ha−1 SSP, 78 kg ha−1 MP, and 236 kg ha−1 urea. Additional nitrogen (N) was applied twice with irrigation water, to ensure the crop developed without nutrient stress. In Experiment I, 62 kg ha−1 N was applied as Ca(NO3)2 on 24 February 2006 and 60 kg ha−1 N was applied as urea on 16 March 2006. In Experiment II, 42 kg ha−1 N was applied as Ca(NO3)2 on 21 December 2006 and 21 kg ha−1 N was applied as urea on 5 January 2007. Irrigation was applied twice monthly and no significant drought stress occurred. Sorghum midge [Contarinia sorghicola (Coquillett)] was controlled chemically around anthesis and no major outbreak was recorded. However, Experiment II was infected by sugarcane mosaic virus (Potyvirus), which caused some premature leaf senescence, particularly during grain filling.
Post-anthesis kernel observations
Approximately 30 main shoot panicles in each plot of both experiments were tagged and their dates of apical and basal anthesis were recorded. Labelled panicles (1–2 per plot) were harvested twice a week throughout the kernel-filling period. Harvested panicles were sealed in plastic bags and transported to the laboratory. In a humidified box, 15–50 kernels were randomly sampled from each of the apical and basal portions of the harvested panicles to determine their volume, fresh weight, and dry weight. Kernel volume was measured by displacement of water in a pipette and kernel dry weight by drying to a constant weight in an oven at 65 oC for at least 2 d. Maximum kernel water content and volume were determined from the average of the five highest values measured for each panicle section of a genotype. In addition, kernel weight at maturity was determined from a harvest of two rows of one meter length (10 plants) for each plot in both experiments. Main stem panicles were threshed and kernel dry weight was determined from a sample of 100 seeds.
Spikelet removal treatment
To determine whether genotypic variation in kernel-filling capacity was due to difference in resource availability per kernel during the kernel-filling period, alternate primary branches were removed from the main shoot panicle of five adjacent plants in each plot of Experiment I, after anthesis of the basal spikelets. This removed approximately 50% of the spikelets in all the genotypes and resulted in genotypes with normal-sized kernels having the same or fewer spikelets per main shoot panicle compared to the large-seeded KS115 and ATx642/KS115 panicles with no spikelets removed. All tiller heads of the treated plants were removed at the time of spikelet removal on the main shoots. Final kernel weight of control and spikelet-thinned panicles was determined at maturity.
Light microscopy
Between panicle initiation and panicle emergence, developing panicles were harvested and dissected regularly. Excised developing panicle branches were transferred to a microscope slide, and cleared in Hoyer's solution (9 g gum arabic, 60 g chloral hydrate, and 6 ml glycerol in 15 ml H2O). Floral meristems were exposed for viewing. At anthesis, spikelets were dissected when they started to extrude anthers, and dissected ovaries were cleared overnight in lactic acid saturated with chloral hydrate and then mounted on slides in Hoyer's solution. Cleared meristems and ovaries were examined and photographed using an Olympus BX-61 compound microscope equipped with Nomarski differential interference contrast (DIC) optics. Sorghum spikelets are usually borne in pairs, one sessile and fertile, the other pedicellate and sterile. The diameter of the floret meristem of sessile spikelets was measured across the base of the meristem at a stage immediately before the initiation of stamen primordia (approximately 15 d after panicle initiation). For each genotype, 10–18 meristems from three plants were measured using the ImageJ image processing program. For ovary volume at anthesis, 16–22 ovaries from four plants were measured for each genotype.
After pollination, developing kernels were collected at different times and immediately fixed in cold FAA fixative (3.7% formaldehyde, 5% acetic acid, 50% ethanol) for 24 h, followed by dehydration in a series of ethanol and tertiary butyl alcohol mixtures and embedding in paraffin. Paraffin-embedded kernels were sectioned to 12–20 μm thickness (depending on the development stage) with a microtome. Tissue sections were stained in an aqueous solution of 0.05% toluidine blue for 20 min, rinsed in water for 1 min, and allowed to air dry. Paraffin was removed with two changes of xylene and the cover slip was mounted with DPX Mountant for Histology (Fluka). Endosperm cell number was calculated from observations of cell size in kernel cross-sections and estimates of endosperm volume via water displacement. Four kernels were sectioned and measured for each genotype.
Starch granule number
The number of starch granules was estimated using the method of Kiniry (1988). Briefly, kernels sampled around maturity were fixed in 3:1 95% ethanol/glacial acetic acid overnight at 4 oC, and then stored in 70% ethanol. The endosperm was separated from the fixed kernel, put into fresh deionized water, and incubated at 60 oC for 15 min in a shaking water bath. Endosperms were then put into 2 ml of 1 M HCl in an ice bath for 60 min, followed by incubation at 60 oC for 10 min. They were subsequently rinsed three times with deionized water, put into 1 ml of cellulase solution (30 g l−1 of cellulase in 0.1 M sodium acetate buffer, pH 4.7) and incubated at 37 oC overnight. Endosperms were then mashed with a glass rod, incubated for an additional 2 h at 37 oC, and finally forced through a syringe and diluted to 50 ml with deionized water. A subsample was counted using a hemacytometer under ×100 magnification. Three kernels were processed and measured for each genotype. For each kernel, four subsamples were counted.
Data analysis
The time series of kernel dry weight data were fitted against accumulated thermal time after anthesis using a broken linear model in the R2LINES procedure of the GenStat Release 9.1 statistical package. The model includes an initial positive linear increase followed by a phase of constant dry weight. Physiological maturity was defined as the time at which the two line segments intersected (i.e. when maximum kernel dry weight was reached). As linear kernel filling starts a few days after anthesis, only observations for which kernel weight had reached at least 10% of its final value (approximately 130 oCd after anthesis) were included in the analysis. The model was fitted separately to apical and basal kernel data for each genotype. Daily thermal time accumulation was calculated from hourly temperature records, using a base temperature of 5.7 oC and an optimal temperature of 23.5 oC (Hammer and Muchow, 1994). Analysis of variance was performed using GenStat Release 9.1. Main effects of genotypes, treatments, and their interactions were tested using Fisher's LSD method.
Results
Kernel weight, number, and filling rate
Final kernel dry weight showed significant genotypic differences. In Experiment I, the final dry weight of apical kernels of the main shoot panicle was 61.0 mg for KS115, compared to 24.6–28.4 mg for the normal-seeded genotypes (Table 1). The kernel weight of ATx642/KS115 (45.4 mg) was close to the average of its two parents (44.7 mg), significantly lower than KS115, but significantly higher than its maternal parent BTx642 and other normal-seeded genotypes (Table 1). In Experiment II, final kernel dry weight was consistently less than in Experiment I, but genotypic effects were similar across experiments (Table 1).
Table 1.
Final kernel dry weight, kernel number, kernel-filling rate, maximum water content, and maximum kernel volume for apical kernels of main shoot panicles
| Experiment | Genotype | Final kernel dry weighta (mg kernel−1) | Kernel number of main shoot paniclea | Kernel-filling ratea (mg oCd−1 kernel−1) | Maximum water contenta (mg kernel−1) | Maximum kernel volumea (μl kernel−1) |
| Experiment I | KS115 | 61.0 a | 1053 d | 0.141 a | 48.8 a | 79.9 a |
| ATx642/KS115 | 45.4 b | 1524 c | 0.114 b | 28.8 b | 56.8 b | |
| BTx642 | 28.4 c | 2454 b | 0.070 c | 17.8 d | 35.9 c | |
| ATx642/RQL36 | 24.9 cd | 3654 a | 0.069 c | 19.9 c | 33.9 c | |
| RQL36 | 24.6 d | 2504 b | 0.056 d | 19.6 c | 30.8 d | |
| Experiment II | KS115 | 49.8 a | 795 c | 0.123 a | 39.7 a | 64.5 a |
| ATx642/KS115 | 42.2 b | 1338 b | 0.087 b | 23.9 b | 48.9 b | |
| ATx642/RQL36 | 18.7 c | 3094 a | 0.053 c | 13.6 c | 23.6 c |
Final kernel weight and kernel-filling rate were determined by fitting the broken linear model (Fig. 1). Kernel number was determined at final harvest at maturity. Maximum kernel water content and volume were determined from the average of the five highest values measured for each panicle section of a genotype.
For each experiment, means in columns that are followed by the same letter do not differ significantly (P <0.05).
There was a trade-off between kernel size and number, as the higher kernel weight of KS115 and its hybrid was offset by a significantly lower kernel number per panicle compared with the normal-seeded genotypes (Table 1). This decreased kernel number was associated with reduced panicle branching (data not shown). Genotypic and experimental effects on final kernel weight were predominantly associated with differences in filling rate during the linear phase of kernel filling (Table 1; Fig. 1). In both experiments, kernel-filling rate of ATx642/KS115 was intermediate (close to the average of KS115 and normal-seeded hybrids), significantly lower than KS115, but significantly higher than the normal-seeded genotypes.
Fig. 1.
Kernel dry weight as a function of thermal time after anthesis for apical kernels of five genotypes studied in Experiment I. Solid lines show the broken linear model fitted for each genotype. Slopes are presented in Table 1 as kernel-filling rates.
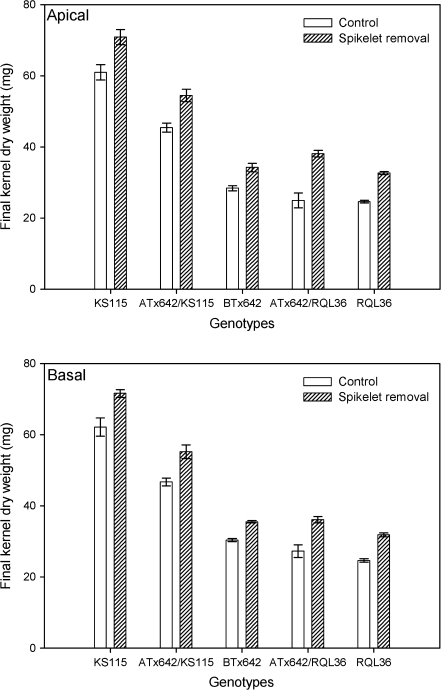
Decreasing the number of kernels by spikelet removal at anthesis significantly increased the final kernel weight of the remaining kernels in all genotypes (Fig. 2). There was no significant effect of panicle position on the responses to sink reduction through spikelet removal. The increase in final kernel weight by spikelet removal treatment was mainly due to a change in filling rates. The significant genotypic difference in final kernel weight remained after spikelet-removal, even though the relative response was greater for the normal-seeded genotypes (Fig. 2). Importantly, the kernel dry weight of normal-seeded genotypes after spikelet removal was still significantly lower than that of KS115 and ATx642/KS115 plants without spikelet removal (Fig. 2), despite similar kernel number per panicle.
Fig. 2.
Response of final kernel dry weight for apical and basal kernels of main stem panicles to the removal of approximately 50% of the spikelets at anthesis for each genotype in Experiment I. The vertical bars indicated the standard errors of the means.
Size of floret apical meristems and ovaries
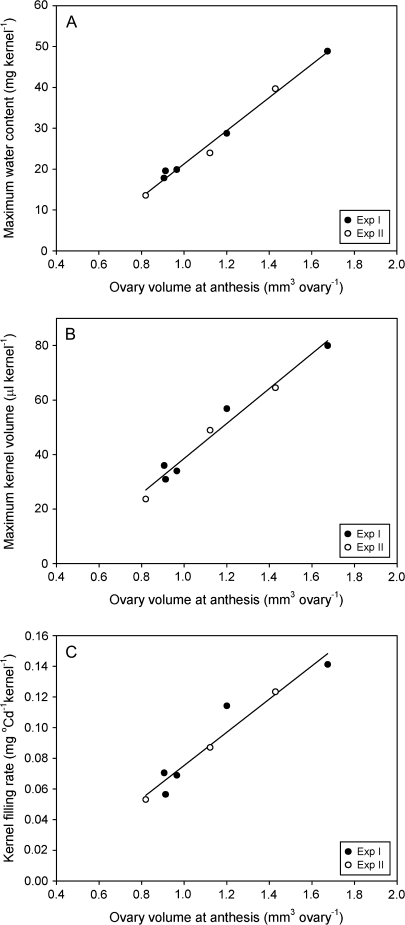
Genotypic differences in size of reproductive organs were apparent early in floret development. Immediately before initiation of stamen primordia, sessile spikelets of KS115, ATx642/KS115, and ATx642/RQL36 differed significantly in the diameter of floret apical meristems (Table 2; Fig. 3), with the meristem diameter of ATx642/KS115 (146.7 μm) close to the mean of the other two genotypes (146.9 μm). Assuming the meristem is a hemisphere, these diameters translated into a volume of 10.8, 8.3, and 6.3×105 μm3 for KS115, ATx642/KS115, and ATx642/RQL36, respectively. Ovary volume at anthesis differed similarly among genotypes. KS115 produced the largest ovaries in both experiments (Table 2; Fig. 4), whereas the ovary volume of ATx642/KS115 (1.20 mm3 and 1.12 mm3 in Experiments I and II, respectively) was close to the mean of KS115 and the normal seeded genotypes (1.30 mm3 and 1.13 mm3 in Experiments I and II, respectively). The lower final kernel weight in Experiment II compared with Experiment I was associated with a reduction in ovary volume at anthesis. Across experiments and genotypes, ovary volume at anthesis was positively associated with the maximum kernel water content attained during early kernel filling (r2=0.99, P <0.001), the maximum kernel volume attained during mid-kernel-filling (r2=0.97, P <0.001), the filling rate during the linear filling period (r2=0.94, P <0.001) (Fig. 5) and, ultimately, the kernel dry weight at maturity (r2=0.93, P <0.001).
Table 2.
Diameter of floret meristem, ovary volume at anthesis, number of vascular bundles in the ovary wall, number of starch granules per kernel, number of endosperm cells per kernel, and the cross-sectional area of endosperm cells in the central region of the endosperm for apical kernels of main shoot panicles
| Genotype | Diameter of floret meristem (μm) | Ovary volume at anthesisb (mm3) | Number of vascular bundles in the ovary wallb | Number of endosperm cellsb (×104 kernel−1) | Cross–sectional area of endosperm cells (×103 m2) | Number of starch granules (×106 kernel−1) | |
| Experiment 1 | KS115 | – | 1.67 a | 5.3 a | – | – | 14.9 a |
| ATx642/KS115 | – | 1.20 b | 4.0 b | – | – | 10.6 b | |
| BTx642 | – | 0.91 c | 2.5 c | – | – | 6.3 c | |
| ATx642/RQL36 | – | 0.97 c | 2.3 c | – | – | 7.2 c | |
| RQL36 | – | 0.91 c | 2.3 c | – | – | 7.1 c | |
| Experiment 2 | KS115 | 160.2 a | 1.43 a | 5.3 a | 8.21 a | 12.9 a | 14.4 a |
| ATx642/KS115 | 146.7 b | 1.12 b | 4.1 b | 6.60 b | 12.5 a | 8.9 b | |
| ATx642/RQL36 | 133.6 c | 0.82 c | 2.7 c | 4.57 c | 10.6 b | 6.3 c |
Measured immediately before the initiation of stamen primordia in developing florets.
For each experiment, means in columns that are followed by the same letter do not differ significantly (P < 0.05).
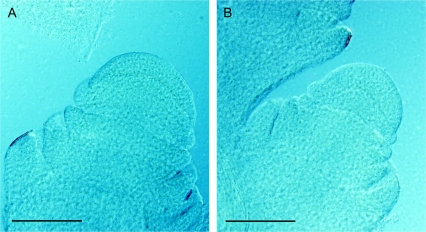
Fig. 3.
Whole-mount cleared floret meristems at a stage immediately before the initiation of stamen primordia in developing florets. (A) KS115; (B) ATx642/RQL36. Scale bars: 100 μm.
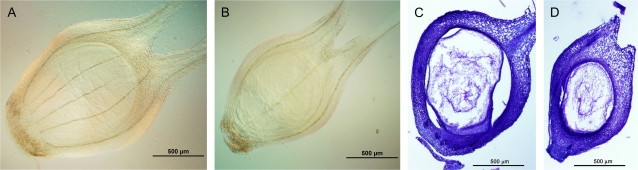
Fig. 4.
Ovaries at anthesis for KS115 (A, C) and RQL36 (B, D). (A, B) Whole-mount cleared ovaries; (C, D) cross-sections of ovaries. Scale bars: 500 μm.
Fig. 5.
Association of maximum kernel water content (A; r2=0.99, P <0.001), maximum kernel volume (B; r2=0.97, P <0.001) and kernel-filling rate (C; r2=0.94, P <0.001) with ovary volume at anthesis for apical kernels of sorghum genotypes grown in two field experiments.
The large ovary volume of KS115 was mainly due to the presence of more cells per layer in the ovary wall (Fig. 4C, D). In addition, the vascular supply to each ovary differed significantly among genotypes (Table 2; Fig. 4A, B). Ovaries of KS115 had 5–6 vascular bundles in their wall, including a right and a left stylar bundle, whereas ovaries of normal-seeded genotypes had only 2–3 vascular bundles in the ovary wall, including the stylar bundles. In both experiments, the number of vascular bundles for ATx642/KS115 (4.0–4.1) was close to the mean of KS115 and the normal-seeded genotypes (3.9–4.0). Within experiments, the number of vascular bundles was significantly related to the kernel-filling rate (r2=0.98, P <0.001 for Experiment I), but unlike ovary volume and kernel-filling rate, the number of vascular bundles was similar across the two experiments (Table 2). The size of other floral organs of the spikelets (i.e. glumes, lemma, and palea) also differed among genotypes. Those that produced large kernels had large floral organs (Fig. 6) and genotypic differences in the size of these organs became evident shortly after their initiation (data not shown).
Fig. 6.
Branches of panicles collected at anthesis showing genotypic difference in spikelet size. From left to right: KS115, ATx642/KS115, and ATx642/RQL36.
Endosperm cells and starch granules
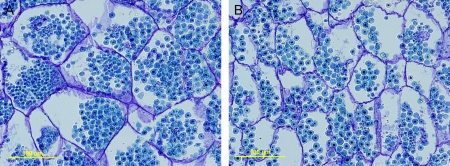
Within individual kernels, endosperm cells differed in size and shape, with small cells in the outer layers and large cells in the central parts surrounding the embryo. Large kernels of KS115 and its hybrid contained more endosperm cells and also had larger cells in the central region of the endosperms compared with the normal size kernels of ATx642/RQL36 (Table 2; Fig. 7). This suggests that the increase in total endosperm volume in KS115 and its hybrid was due to an increase in both cell size and cell number. Pericarps of large kernels appeared to contain more cells per layer than those of normal-sized kernels, because there was no significant difference in pericarp cell size (data not shown). The size of starch granules at maturity varied considerably within genotypes, but the mean diameter was similar across genotypes, ranging from 16.9 μm (KS115) to 18.1 μm (ATx642/KS115). The increase in total starch volume in the endosperm of large kernels was associated with an increase in the number of starch granules (Table 2), rather than their size.
Fig. 7.
Cells in the central part of the endosperm 3 weeks after anthesis for KS115 (A) and RQL36 (B). Scale bars: 100 μm.
Discussion
Kernel weight in sorghum is under strong genetic control
Kernel dry weight is an important factor determining final grain yield and nutritional quality in sorghum (Kriegshauser et al., 2006). Genotypes used in this study showed a more than 2-fold range in kernel dry weight at maturity (Table 1). Hybrid ATx642/KS115 was intermediate in final kernel dry weight compared with its parents, suggesting that gene action for kernel weight was additive. This is in agreement with the results for previous studies on the inheritance of kernel-filling characteristics of other cereals (Bhatt, 1972; Ketata et al., 1976; Katsantonis et al., 1986; Wang et al., 1999). Kernel-filling rate during the linear phase of kernel filling showed a similar additive gene action as kernel weight for the genotypes studied (Table 1), which is consistent with the observations for other grain crops that greater kernel weight is predominantly associated with a higher filling rate (Bruckner and Frohberg, 1987; Santiveri et al., 2002; Egli, 2006).
The significant reduction in kernel number of large-seeded genotypes compared to normal-sized genotypes resulted in a higher level of resource availability per kernel. However, removing 50% of the spikelets at anthesis did not increase kernel weight of normal-seeded genotypes to the levels found for KS115 or ATx642/KS115 without spikelet removal (Fig. 2), even though it resulted in a comparable kernel number. Therefore, it is unlikely that the genotypic differences in kernel-filling rate and final kernel weight were solely a consequence of differences in resource availability per kernel during the kernel-filling period. Genetic control of potential kernel weight had most likely occurred through processes that were established before the start of kernel filling.
Genetic control of potential kernel weight occurs before fertilization and is related to ovary development
In the genotypes examined in this study, a significant difference in ovary volume prior to anthesis was observed. Across experiments and genotypes, the volume of the ovary at anthesis was closely associated with maximum kernel water content, maximum kernel volume, kernel-filling rate, and final kernel dry weight (Fig. 5). Due to the large initial volume of ovaries, large kernels showed a faster rate of kernel expansion and water uptake after fertilization, and attained a higher level of maximum water content early in kernel filling. The maximum water content in the kernel is closely related to the kernel-filling rate in sorghum (Gambin and Borras, 2005) and other cereal crops (Bradford, 1994; Funaba et al., 2006; Gambin et al., 2007b). Hence, the genotypic differences in kernel-filling rate in this study were probably a consequence of differences in ovary volume at the start of kernel filling.
The effect of ovary volume on kernel weight of sorghum could operate through the ovary wall. In sorghum, the lower part of the carpel forms the ovary wall, which encloses the ovule. After fertilization, the ovary wall develops into the pericarp which is fused to the seed coat (testa) at maturity. Studies on sorghum kernel development found that growth of the pericarp was confined to the enlargement of cells present at pollination, along with some thickening of their walls, as no cell division was observed after pollination (Artschwager and McGuire, 1949; Sanders, 1955; Paulson, 1969). In the genotypes studied here, the ovary wall at anthesis and the pericarp at maturity of large kernels contained more cells in each layer than that of normal size kernels. It is probable therefore that the pre-fertilization ovary structure, in particular, the cell number in the ovary wall, determined the ability of the kernel to increase in volume and consequently imposed a limitation on the potential for kernel growth. Consistent with this, the reduction in kernel weight of wheat by Rht dwarfing genes was associated with a reduction in kernel volume and the total number of cells in the pericarp (Miralles et al., 1998). A positive relationship between carpel weight at anthesis and kernel dry weight at maturity has been reported in wheat and spring barley (Scott et al., 1983; Calderini and Reynolds, 2000). The differences observed in the extent of endosperm cell division and expansion, starch granule formation, kernel water uptake, and dry matter accumulation could be a consequence of volume restriction imposed by the developing pericarp that was derived from the ovary wall. In wheat and maize, physically restricting the capacity of a kernel to expand significantly reduced kernel size at maturity (Millet and Pinthus, 1984; Yu and Egli, 1990; Gambin et al., 2007a). Physical limitation therefore can play an important role in the control of kernel development and final size.
Kernel development depends on a constant supply of assimilates, water, and other resources from the mother plant. The capacity of the transport system to supply these resources into a kernel could therefore limit its growth. Significant genotypic differences were observed in the number of vascular bundles in the ovary wall, and within an experiment, the vascular bundle number was positively associated with the kernel-filling rate and final kernel weight. However, it was not possible to discern whether more serving vascular links was a cause or consequence of greater ovary and kernel size.
In our experiments, the F1 seeds produced by pollinating ATx642 (the male-sterile version of BTx642) plants with KS115 pollen were similar in size to those produced on the self-pollinated BTx642 line (data not shown). Other reciprocal cross studies have also shown that the potential for seed growth is under strong maternal control, such that the size of F1 seeds of reciprocal hybrids at maturity is similar to that obtained from their maternal, rather than their paternal (pollen) parent (Jones et al., 1996; Lemontey et al., 2000; Chaudhury and Berger, 2001). The mechanism involved in the maternal control of potential seed size has not been elucidated. The results of this study suggest that the maternally derived reproductive components, and in particular the pericarp developed from the ovary wall, could serve as a site of maternal control of seed growth in cereals. Control of seed development by maternally derived components has also been observed in Arabidopsis. Several studies have shown that mutations causing abnormal integument development in Arabidopsis affected the size and shape of mature seeds (Leon-Kloosterziel et al., 1994; Ohto et al., 2005; Schruff et al., 2006). The determination of potential kernel size through processes operating before fertilization is consistent with the concept of maternal control of seed growth.
Ovary size might be determined at the meristem stage
The physiological mechanisms that determine ovary size are still unclear. Because the large-seeded genotypes produce fewer spikelets than the normal-seeded genotypes, the increase in the size of floral organs could be a consequence of a reduced competition for resources among fewer spikelets. However, in a preliminary greenhouse experiment on commercial hybrid MR-Buster, in which a large percentage (50–75%) of the panicle was removed before any major increase in the size of the panicle structure (about 17 d after panicle initiation), significant changes in the size of ovaries and other floral organs at anthesis were not detected (Z Yang, unpublished data). Genotypic difference in ovary size observed in the current study seems to be a consequence of processes occurring early in floret development. Immediately before the appearance of stamen primordia in developing florets, KS115 and its hybrid ATx642/KS115 had larger floret apical meristems than the normal-seeded genotype ATx642/RQL36 (Fig. 3; Table 2). After stamen formation, the carpel initiated from the floret meristem forms the ovary wall and encloses the ovule. It is possible that the size of the floret meristems exerts a direct effect on the size of carpels and ovaries formed from them. The difference in size of other organs of the spikelets (i.e. glumes, lemma, and palea) could also be related to the difference in the size of meristems from which they were formed. The observation that the relative genotypic differences in the size of floret meristem closely matched the differences in ovary volume supports the hypothesis of a direct and causal effect of meristem size on ovary size.
The negative correlation between kernel weight and number (Table 1) is consistent with observations for other grain crops (Fischer and HilleRisLambers, 1978; Sadras, 2007). If ovary size, and hence potential kernel size, is determined at the meristem stage, then an intrinsic developmental link could exist, thus setting potential kernel size and number simultaneously during the panicle branching and floret formation period. In sunflower, QTLs associated with seed mass were found to be tightly linked to an apical branching gene (Tang et al., 2006), suggesting a potential genetic link between potential seed size and seed number. Floret and kernel development at the meristem stage might therefore be crucial to a better understanding of the developmental processes that regulate both kernel size and number.
Conclusions
This study showed that genotypic effects on potential kernel weight of sorghum were established before fertilization through differences in ovary volume, which in turn were associated with the size of the floret apical meristem during floret development. The genotypic effects on meristem size and subsequently on ovary volume, and kernel weight were all consistent with additive genetic control, supporting the hypothesis that the relationship between meristem size, ovary volume, and final kernel weight is causal. The effects of ovary volume on kernel growth could operate via limitations imposed on kernel expansion by the developing pericarp which is derived from the ovary wall. The determination of potential kernel weight prior to fertilization coincides with the timing of kernel number determination and could explain the trade-off between potential kernel number and kernel weight.
Acknowledgments
We thank Kurt Deifel and Ian Broad for excellent technical assistance. This study was supported financially by the Australian Research Council, through project LP0560484.
References
- Artschwager E, McGuire RC. Cytology of reproduction in Sorghum vulgare. Journal of Agricultural Research. 1949;78:659–673. [Google Scholar]
- Bhatt GM. Inheritance of heading date, plant height, and kernel weight in two spring wheat crosses. Crop Science. 1972;12:95–98. [Google Scholar]
- Borras L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research. 2004;86:131–146. [Google Scholar]
- Bradford KJ. Water stress and water relations of seed development: a critical review. Crop Science. 1994;34:1–11. [Google Scholar]
- Brocklehurst PA. Factors controlling grain weight in wheat. Nature. 1977;266:348–349. [Google Scholar]
- Bruckner PL, Frohberg RC. Rate and duration of grain fill in spring wheat. Crop Science. 1987;27:451–455. [Google Scholar]
- Calderini DF, Reynolds MP. Changes in grain weight as a consequence of de-graining treatments at pre- and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum×T. tauschii) Australian Journal of Plant Physiology. 2000;27:183–191. [Google Scholar]
- Chaudhury AM, Berger F. Maternal control of seed development. Seminars in Cell and Developmental Biology. 2001;12:381–386. doi: 10.1006/scdb.2001.0267. [DOI] [PubMed] [Google Scholar]
- Darroch BA, Baker RJ. Grain filling in three spring wheat genotypes: statistical analysis. Crop Science. 1990;30:525–529. [Google Scholar]
- Earp CF, McDonough CM, Rooney LW. Microscopy of pericarp development in the caryopsis of Sorghum bicolor (L.) Moench. Journal of Cereal Science. 2004;39:21–27. [Google Scholar]
- Eastin JD, Hultquis JH, Sullivan CY. Physiologic maturity in grain sorghum. Crop Science. 1973;13:175–178. [Google Scholar]
- Egli DB. The role of seed in the determination of yield of grain crops. Australian Journal of Agricultural Research. 2006;57:1237–1247. [Google Scholar]
- Fischer RA, HilleRisLambers D. Effect of environment and cultivar on source limitation to grain weight in wheat. Australian Journal of Agricultural Research. 1978;29:443–458. [Google Scholar]
- Funaba M, Ishibashi Y, Molla AH, Iwanami K, Iwaya-Inoue M. Influence of low/high temperature on water status in developing and maturing rice grains. Plant Production Science. 2006;9:347–354. [Google Scholar]
- Gambin BL, Borras L. Sorghum kernel weight: growth patterns from different positions within the panicle. Crop Science. 2005;45:553–561. [Google Scholar]
- Gambin BL, Borras L, Otegui ME. Is maize kernel size limited by its capacity to expand? Maydica. 2007a;52:431–441. [Google Scholar]
- Gambin BL, Borras L, Otegui ME. Kernel water relations and duration of grain filling in maize temperate hybrids. Field Crops Research. 2007b;101:1–9. [Google Scholar]
- Gebeyehou G, Knott DR, Baker RJ. Rate and duration of grain filling in durum-wheat cultivars. Crop Science. 1982;22:337–340. [Google Scholar]
- Hammer GL, Muchow RC. Assessing climatic risk to sorghum production in water-limited subtropical environments. I. Development and testing of a simulation model. Field Crops Research. 1994;36:221–234. [Google Scholar]
- Heiniger RW, Vanderlip RL, Kofoid KD. Caryopsis weight patterns within the sorghum panicle. Crop Science. 1993;33:543–549. [Google Scholar]
- Jones RJ, Schreiber BMN, Roessler JA. Kernel sink capacity in maize: genotypic and maternal regulation. Crop Science. 1996;36:301–306. [Google Scholar]
- Katsantonis N, Gagianas A, Sfakianakis J, Fotiadis N. Inheritance of duration and rate of grain filling and their relationship to grain yield in maize. Plant Breeding. 1986;96:115–121. [Google Scholar]
- Ketata H, Edwards LH, Smith EL. Inheritance of eight agronomic characters in a winter wheat cross. Crop Science. 1976;16:19–22. [Google Scholar]
- Kiniry JR. Kernel weight increase in response to decreased kernel number in sorghum. Agronomy Journal. 1988;80:221–226. [Google Scholar]
- Kriegshauser TD, Tuinstra MR, Hancock JD. Variation in nutritional value of sorghum hybrids with contrasting seed weight characteristics and comparisons with maize in broiler chicks. Crop Science. 2006;46:695–699. [Google Scholar]
- Lemontey C, Mousset-Declas C, Munier-Jolain N, Boutin JP. Maternal genotype influences pea seed size by controlling both mitotic activity during early embryogenesis and final endoreduplication level/cotyledon cell size in mature seed. Journal of Experimental Botany. 2000;51:167–175. doi: 10.1093/jexbot/51.343.167. [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Keijzer CJ, Koornneef M. A seed shape mutant of Arabidopsis that is affected in integument development. The Plant Cell. 1994;6:385–392. doi: 10.1105/tpc.6.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet E, Pinthus MJ. Effects of removing floral organs, light penetration and physical constraint on the development of wheat grains. Annals of Botany. 1984;53:261–269. [Google Scholar]
- Miralles DJ, Calderini DF, Pomar KP, D'Ambrogio A. Dwarfing genes and cell dimensions in different organs of wheat. Journal of Experimental Botany. 1998;49:1119–1127. [Google Scholar]
- Ohto M, Fischer RL, Goldberg RB, Nakamura K, Harada JJ. Control of seed mass by APETALA2. Proceedings of the National Academy of Sciences, USA. 2005;102:3123–3128. doi: 10.1073/pnas.0409858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA. Nuclear endosperm development in cereals and Arabidopsis thaliana. The Plant Cell. 2004;16:S214–S227. doi: 10.1105/tpc.017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson IW. Embryogeny and caryopsis development of Sorghum bicolor (L) Moench. Crop Science. 1969;9:97–102. [Google Scholar]
- Sadras VO. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Research. 2007;100:125–138. [Google Scholar]
- Sanders EH. Developmental morphology of the kernel in grain sorghum. Cereal Chemistry. 1955;32:12–25. [Google Scholar]
- Santiveri F, Royo C, Romagosa I. Patterns of grain filling of spring and winter hexaploid triticales. European Journal of Agronomy. 2002;16:219–230. [Google Scholar]
- Schnyder H, Baum U. Growth of the grain of wheat (Triticum aestivum L.). The relationship between water content and dry matter accumulation. European Journal of Agronomy. 1992;1:51–57. [Google Scholar]
- Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development. 2006;133:251–261. doi: 10.1242/dev.02194. [DOI] [PubMed] [Google Scholar]
- Scott WR, Appleyard M, Fellowes G, Kirby EJM. Effect of genotype and position in the ear on carpel and grain growth and mature grain weight in spring barley. Journal of Agricultural Science. 1983;100:383–391. [Google Scholar]
- Tang S, Leon A, Bridges WC, Knapp SJ. Quantitative trait loci for genetically correlated seed traits are tightly linked to branching and pericarp pigment loci in sunflower. Crop Science. 2006;46:721–734. [Google Scholar]
- Tuinstra MR, Liang GL, Hicks C, Kofoid KD, Vanderlip RL. Registration of KS 115 sorghum. Crop Science. 2001;41:932–933. [Google Scholar]
- Wang GL, Kang MS, Moreno O. Genetic analyses of grain-filling rate and duration in maize. Field Crops Research. 1999;61:211–222. [Google Scholar]
- Yu Z, Egli DB. Effect of physical restraint on kernel growth in winter wheat. Acta Agronomica Sinica. 1990;16:161–167. [Google Scholar]