Abstract
Although roots in dry soil layers are commonly rehydrated by internal hydraulic redistribution during the nocturnal period, patterns of tissue rehydration are poorly understood. Rates of nocturnal rehydration were examined in roots of different orders in Vaccinium corymbosum L. ‘Bluecrop’ (Northern highbush blueberry) grown in a split-pot system with one set of roots in relatively moist soil and the other set of roots in dry soil. Vaccinium is noted for a highly branched and extremely fine root system. It is hypothesized that nocturnal root tissue rehydration would be slow, especially in the distal root orders because of their greater hydraulic constraints (smaller vessel diameters and fewer number of vessels). Vaccinium root hydraulic properties delayed internal water movement. Even when water was readily available to roots in the wet soil and transpiration was minimal, it took a whole night-time period of 12 h for the distal finest roots (1st to 4th order) under dry soil conditions to reach the same water potentials as fine roots in moist soil (1st to 4th order). Even though roots under dry soil equilibrated with roots in moist soil, the equilibrium point reached before sunrise was about –1.2 MPa, indicating that tissues were not fully rehydrated. Using a single-branch root model, it was estimated that individual roots exhibiting the lowest water potentials in dry soil were 1st order roots (distal finest roots of the root system). However, considered at the branch level, root orders with the highest hydraulic resistances corresponded to the lowest orders of the permanent root system (3rd-, 4th-, and 5th-order roots), thus indicating possible locations of hydraulic safety control in the root system of this species.
Keywords: Blueberry, hydraulic redistribution, root water potential, split-pot
Introduction
The importance of day length for plant photosynthetic carbon gain has been widely recognized. Duration of the nocturnal period may also be important. During the night-time, periods of minimal transpiration allow water potential gradients among plant parts to dissipate by internally redistributing water to tissues of lower water potential, including the movement of water to roots in dry soil layers (Bauerle et al., 2008). Nocturnal internal hydraulic redistribution may be very important to the maintenance of roots in surface soil layers where root densities are highest, as surface soil can be dry for extended periods in many regions (Caldwell et al., 1998). However, the rate at which water redistributes at night depends on a combination of environmental and plant factors, including the magnitude of differences in water potential among tissues and the hydraulic conductivity of the vascular system (Brooks et al., 2002; Burgess and Bleby, 2006).
Many factors influence the rates of tissue rehydration of roots at night. Nocturnal transpiration due to incomplete closure of stomata (Donovan et al., 1999, 2003; Caird et al., 2007) or high vapour pressure deficits (Hinckley and Ritchie, 1973) can create and maintain high water potential gradients between the soil and leaves and thus limit water movement to other parts of the root system. In addition, rehydration of roots in dry soil layers may be slower where there are significant constraints to water movement, including embolisms, small-diameter and curved xylem vessels with extensive branching (Howard, 1932; Tsuda and Tyree, 1997; Brooks et al., 2002), high frequency of pits and end-plate membranes (Orians et al., 2005), and the presence of heartwood, latewood, and rays (Burgess and Bleby, 2006). Lateral water movement may also be limited by the high resistances that form at the stem base–root junction (Brooks et al., 2002; Burgess and Bleby, 2006).
Vaccinium corymbosum has been reported to be a species that does not effectively distribute water laterally (Abbott and Gough, 1986). Using a split-root water application, Abbott and Gough (1986) found that dyes did not move laterally from one stem to another and observed root mortality in the unwatered root container. The main objective of this study was to quantify patterns of nocturnal internal hydraulic redistribution and conductances among the first seven orders of V. corymbosum roots under severe drought conditions and its implications for root tissue rehydration. Vaccinium corymbosum was selected due to its unique root anatomy and morphology and its reported limited ability to redistribute water. It was hypothesized that root tissue rehydration at night would be delayed, especially in the distal root orders because of their greater hydraulic constraints (smaller vessel diameters and fewer number of vessels).
Materials and methods
The experiment was conducted in November 2005 in a greenhouse at The Pennsylvania State University, University Park, PA, USA. Plant material consisted of 9-year-old V. corymbosum L. ‘Nelson’ (Northern highbush blueberry) plants. At the end of the summer (early September) of 2004, individual plants were transplanted to a split-pot system filled with coarse sand (840 μm particle size). The 40 l pots (52 cm length, 36 cm width, 32 cm height) (Sterilite, Townsend, MA) were separated into two sections using a vertical plastic partition (constructed with the lid of the 40 l pot), with the edges sealed with silicone and polyurethane foam. Roots were divided equally and half was carefully placed in each side of the pot. There were five replicate plants. Plants were always watered on the same side of the pot (wet-side), with the other side not irrigated (dry-side) for the duration of the experiment. Drought conditions were induced gradually over a 5-month period previous to the actual measurements, by exposing plants to decreasing amounts of irrigation that began at 500 ml twice a day, then decreased to 250 ml twice a day, 250 ml once a day, and, finally, to 150 ml once a day. Midday stem water potentials were measured with a pressure chamber on bagged and covered (aluminium foil) leaves between 12.00 h and 14.00 h (Soil Moisture Equipment Co. Santa Barbara, CA) and ranged between –2.0 MPa and –2.5 MPa at the lowest level of irrigation. Soil water content was monitored at 09.00 h each day over the drought imposition period and at 20.00 h before the nocturnal patterns of root rehydration measurements (see below) by time domain reflectometry (TDR 100; Campbell Scientific, Inc., Logan, UT). Moisture probes consisted of three parallel stainless steel rods (20 cm length and 3 mm diameter, 2.5 cm apart) that were inserted in each side of the pots perpendicular to the soil surface. Typical night-time leaf transpiration for a well-watered blueberry plants, was about 25-fold lower (0.214 mmol H2O m−2 s−1) than that observed during daytime (5.3 mmol H2O m−2 s−1) (Li-Cor 6400, Li-Cor Inc, Lincoln, Nebraska). Greenhouse temperatures ranged between 22 °C and 30 °C during the daytime and between 13 °C and 18 °C during the night-time. Supplemental light was provided with three, 400 W halide lamps from 07.00 h to 19.00 h each day.
Nocturnal patterns of root rehydration
Once shoots exhibited water potentials below –2.0 MPa, patterns of internal hydraulic redistribution in the root system were examined. Measurements were completed on one plant per day. At 18.00 h, one hour after sunset (17.00 h, month of November), supplemental lights were turned off and the wet-side was irrigated to field capacity. Nocturnal patterns of root rehydration were determined by sampling the roots at 2, 6, and 11 h after irrigation (20.00 h, 24.00 h, and 05.00 h). Roots were collected by cutting a root section using small scissors, at a distance of 10–15 cm from the centre of the plant, and at a soil depth of 10–15 cm. Once roots were detached from the plant, they were immediately transferred to a humidified chamber to prevent tissue dehydration. After cleaning the roots of sand particles (by tapping them), representative root samples were selected based on three root-order categories. The fine root category consisted of a single root branch that included root orders one to four, where order one consisted of the distal and finest unbranched roots in the root system (morphometric classification system, sensu Fitter, 1982); the medium root classification sample consisted of root orders five to seven; and the coarse root classification sample consisted of the distal section (<0.5 cm length) of an 8th root order. Because blueberry has an extremely fine (finest distal roots can be as fine as 20 μm diameter), densely branched root system (Valenzuela-Estrada et al., 2008), it would have been too time-consuming to collect accurate water potential estimates and still precisely separate roots by order in the finest category. As soon as each root sample was collected, it was loaded into a stainless steel chamber with a thermocouple psychrometer (series 74; JRD Merrill Specialty Equipment, Logan, UT). Sampling all root samples for each specific time interval took no more than 30 min. Transferring one root sample to the humidified chamber took less than 5 s, and less than 2 min for cleaning the roots and sealing them into the chambers. As soon as all the psychrometers were loaded, they were transferred to an insulated water bath at 25 °C, connected to a CR-7 data logger (Campbell Scientific, Inc.) and measured every 30 min for the next 4 h until they reached temperature and vapour equilibrium. Estimations of root water potentials were based on the average of three measured points (Bauerle et al., 2008).
Single branch root model
By previously studying the anatomy of V. corymbosum roots for at least seven root orders (data from Valenzuela-Estrada et al., 2008), and determining the internal root water potentials of the three different groups of roots, the internal water potentials were estimated at the junction of each root order for each sampling time. Estimations were based on a model of a single root branch composed of eight root orders (Fig. 1). For this single branch root model, several conditions were assumed. First, it was assumed that all root orders were connected in series; therefore the flux through out the entire branch was the same, based on the law of conservation of matter (Kirchoff's law). Second, it was assumed that all vessels were fully functional and were able to transport water. Third, hydraulic weighted mean vessel diameters were estimated for each root order as 2(Σr5/Σr4), where r is the vessel radius (Sperry et al., 1994). Fourth, the measured root water potential values used for the root-order estimations were assumed to be mainly contributed by the highest root order in that category as that root order would contain a greater volume of water. Thus, for the coarse roots, it was assumed that the final section of a 8th-order root at the junction with a 7th-order root (Ψ8) was their representative value, and the value for the group of fine roots was assumed to be the final end of a 3rd-order root at the junction with a 4th-order root (Ψ4) (Fig. 1). Once the position of Ψ8 and Ψ4 were established, the corresponding water potential values were estimated for an individual root of each root order (at the junctions between root orders, see Fig. 1) by first estimating the root hydraulic conductance by the Hagen–Poiseuille equation:
where Lx (m3 s−1 MPa−1) is root hydraulic conductance, nv is the mean number of vessels per root, Ra (m) is the vessel radius, η (1×10−9 MPa s at 20 °C) is the viscosity of water, and l (cm) is the root length.
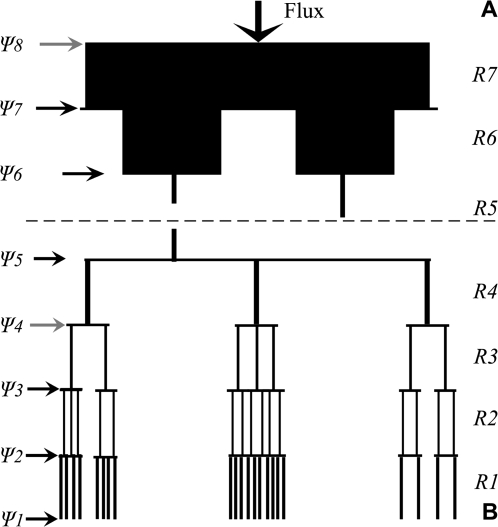
Fig. 1.
Graphic representation of the single root-branch model used to estimate root water potentials for each root order for (A) root orders 5–8 and (B) root orders 1–5. Different root orders (R1–R7) were assumed to be arranged in series and individuals belonging to a specific root order were assumed to be arranged in parallel. In (B) (below the dotted line), the number of roots for each branch order represents the proportion of root length of that root order to the total root length of a branch composed of five orders. For (A) (above the dotted line), the number for each branch order roots is not proportional to that in the higher order. Thickness of vertical lines, representative of roots of a particular order is proportional to the estimated conductance (Lx) (see Table 1). Because conductance is related to root length, 1st-order roots show a thicker line than 2nd-order roots even though the conductivity of 1st-order roots is smaller. Grey horizontal arrows indicate two selected positions, Ψ8 (link between 7th- and 8th-order roots) and Ψ4 (link between 3rd- and 4th-order roots) in a single root-branch, corresponding to the observed water potentials in the Vaccinium experiment. Black horizontal arrows indicate the points (root junctions between root orders) at which root water potentials values for each root order were estimated by the single-root branch model. The values, Ψ8 and Ψ4, and the series of resistances (R4+R5+R6+R7) were used to estimate volumetric water flux, Qt (vertical arrow), where: Qt=(Ψ8–Ψ4)/(R4+R5+R6+R7). Values of Qt were assumed to be the same throughout the entire root branch (Kirchoff's law).
Then, hydraulic resistance for an individual root, Rx, was calculated as the inverse of hydraulic conductance:
where Rx (MPa s m−3) is root hydraulic resistance and Lx (m3 s−1 MPa−1) is root hydraulic conductance. Total resistance per order (all roots belonging to the same order added together as a group) was estimated by:
where Rt (MPa s m−3) is total resistance per root order (for all individual roots that composed one order), Rx (MPa s m−3) is individual root hydraulic resistance and nr is the number of individual roots that correspond to each root order.
Total flux through the entire single branch was estimated by:
where Qt (m3 s−1) is total flux, ΔΨ (MPa) is the difference in internal root water potential between root water potential at the junction of the 3rd- and 4th-order roots (Ψ4) and the 7th- and 8th-order roots (Ψ8) and Rx4, Rx5, Rx6, and Rx7 (MPa s m−3) are the resistances of 4th-, 5th-, 6th-, and 7th-order roots.
Root water potentials for each root order (as group) were estimated by using an analogy of Ohm's law:
where Ψ8 (MPa) is the difference in internal root water potential at the junction of 8th-order and 7th-order roots and, Ψi (MPa) root water potential for the consecutive lower root orders, Rx7 (MPa s m−3) is the resistance for 7th-order roots plus Rxi (MPa s m−3), the resistance for consecutive lower root orders and Qt (m3 s−1) is total flux.
An extrapolation of 2 h before the first root water potential measurement (20.00 h) and 1.5 h after the last measurement (05.00 h) was completed by fitting a polynomial curve over the three different root water potentials estimated by the single branch model for each time point and root order.
Statistical analysis
Root water potential and soil water content over time were analysed using the MIXED procedure and repeated measures analysis of variance (PROC MIXED; SAS Institute Inc., Cary, NC).
Results
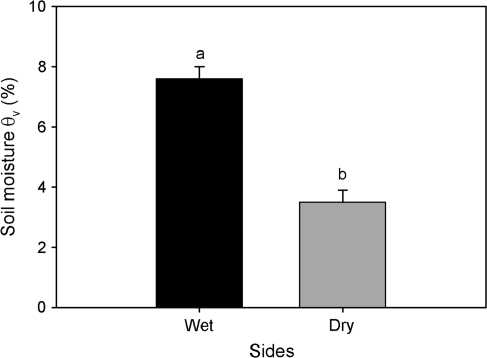
Soil water content decreased over time on both the wet and dry sides of the root system; however, the dry side experienced lower values compared to the wet side (data not shown). At 20.00 h, immediately before the root water potential measurements were taken, soil water contents on the wet sides were almost double that of the dry sides (P <0.006) (Fig. 2). For the 4 weeks prior to the estimation of root water potential, midday stem water potentials were maintained between –2.0 and –2.5 MPa. Also, roots in dry soil experienced more physiological stress than those in the wet soil, as indicated by greater electrolyte leakage (data not shown; Valenzuela-Estrada, 2008).
Fig. 2.
Volumetric soil moisture content (%) on the wet and dry sides of the root system measured at 20.00 h, 2 h after irrigation and right before root water potential measurements. Error bars represent one standard error of the mean (n=5), and different letters indicate a significant difference between sides of the split-pot (P < 0.05).
Nocturnal patterns of root rehydration
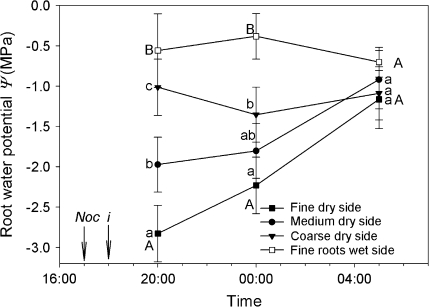
Changes in root water potential over night-time revealed how roots in dry soil may rehydrate under drought conditions. Roots that experienced the lowest water potentials were the finest, most distal, lowest-order roots in the root system. These roots exhibited very low water potentials (about –2.8 MPa) at 20.00 h (2 h after irrigation, and 3 h after ‘sunset’) compared to medium and coarse roots (about –2.0 and –1.0 MPa respectively) (Fig. 3). Four hours later, at 24.00 h, fine and medium roots experienced a significant increase in water potential (reaching values close to –2.3 and –1.8 MPa, respectively). By contrast, coarse roots exhibited a decrease reaching –1.3 MPa. At 05.00 h (near predawn), and 11 h after irrigation (12 h after sunset), all root categories (dry-side) experienced a significant increase in water potential, reaching values between –1.2 to –1.3 MPa. These values were very close to that of fine roots under wet soil conditions, suggesting the plant had evenly redistributed water at this point. The soil in the irrigated compartment was still near field capacity (data not shown).
Fig. 3.
Root water potentials of V. corymbosum plants grown in a split-pot system with one wet side (open symbols) and one dry side (filled symbols). Water potentials were determined over the night-time for fine roots (root orders 1–4) under dry (filled squares) and wet (open squares) conditions, medium roots (orders 5–7) under dry conditions (filled circles), and coarse roots (orders 8 and higher) under dry conditions (filled inverted triangles). Initiation of the nocturnal period is indicated by (↓Noc) and the irrigation event by (↓i). Error bars represent one standard error of the mean (n=5). Statistical differences were found among root orders under dry conditions (P=0.010), over time (P=0.008) and the interaction of wet versus dry of fine roots and time (P=0.003). Lower case letters that differ between root orders (filled symbols) indicate a significant difference (P <0.05) and capital letters that differ between fine roots under dry (filled squares) and wet (open squares) conditions indicate a significant difference (P <0.05).
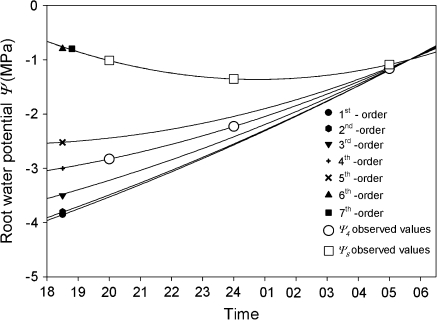
Root water potentials for each root order were estimated by the single-branch root model (Table 1; Fig. 4). Total flux decreased over time (20.00 h, 3.2 E-10 m3 s−1; 24.00 h, 1.5 E-10 m3 s−1; 05.00 h, 1.3 E-11 m3 s−1). Simulations using the single-branch root model indicated that estimated root water potential in dry soil layers decreased as root order decreased and increased with length of night-time (Table 1). It was calculated that root water potentials at 18.00 h for 1st- and 2nd -order roots could have experienced water potential values close to –3.8 MPa. By contrast, estimated water potentials of 6th- and 7th-order roots were close to –0.5 MPa (Fig. 4). By 06.30 h all root orders were estimated to reach the internal root water potential values close to –0.5 MPa (Fig. 4).
Table 1.
Vaccinium corymbosum root anatomical data and hydraulic parameters for seven root orders
| Parameters | Units | Root order | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Measured parameters | ||||||||
| Root diametera | μm | 40 | 48 | 75 | 120 | 177 | 222 | – |
| Vessel diameterb | μm | 3.9 | 4.2 | 4.8 | 5.8 | 6.2 | 15.6 | 17.8 |
| Vessel number (nv)a | 13 | 12 | 22 | 28 | 39 | 501 | 802 | |
| Total root lengtha | m | 1.42 | 1.09 | 1.1 | 0.54 | 0.19 | 0.19 | 0.1 |
| Individual root lengtha | cm | 0. 15 | 0. 38 | 0. 81 | 0. 94 | 1.43 | 0. 98 | 1.15 |
| Number of roots per order (nr)a | 157 | 48 | 23 | 9 | 2 | 3 | 1 | |
| Calculated parameters | ||||||||
| Individual root conductance (Lx) | m3 s−1 MPa−1 | 5.3E-11 | 2.5E-11 | 3.7E-11 | 8.8E-11 | 1.0E-10 | 7.5E-08 | 1.7E-07 |
| Individual root resistance (Rx) | MPa s m−3 | 1. 9E+10 | 4.0E+10 | 2.7E+10 | 1.1E+10 | 9.7E+09 | 1.3E+07 | 5.7E+06 |
| Root resistance (Rt) | MPa s m−3 | 1.2E+08 | 8.3E+08 | 1.2E+09 | 1.2E+09 | 4.4E+09 | 4.1E+06 | 4.0E+06 |
| Estimated internal root Ψ at 20.00 h | MPa | –3.53 | –3.49 | –3.22 | –2.83 | –2.44 | –1.02 | –1.02 |
| Estimated internal root Ψ at 00.00 h | MPa | –2.57 | –2.55 | –2.42 | –2.23 | –2.04 | –1.36 | –1.35 |
| Estimated internal root Ψ at 05.00 h | MPa | –1.19 | –1.19 | –1.18 | –1.16 | –1.15 | –1.09 | –1.09 |
Root anatomical measurements were obtained from Valenzuela-Estrada et al. (2008). Root hydraulic parameters were estimated by the single-branch model. Individual root estimations for each root order are shown for conductance and resistance parameters (Lx and Rx), while other parameters are values of each root order taken as a group.
Average value.
Vessel diameter is expressed as the hydraulic weighted mean (see Materials and methods).
Fig. 4.
Simulated root water potentials over the night of different root orders in the dry soil portion of a split-pot using the single-branch model of V. corymbosum. Night-time was assumed to begin at 17.00 h and soil on the wet side was assumed to be irrigated at 18.00 h. Observed values for the link between 3rd- and 4th-order roots (Ψ4) (indicated by unfilled circles) and the link between 7th- and 8th-order roots (Ψ8) (indicated by unfilled squares) are also shown.
Discussion
The V. corymbosum root system is characterized as being very efficient in terms of biomass allocation for the production of a large amount of root surface area. Its highly branched root system is composed of very fine roots (the finest can be just 20 μm in diameter; Valenzuela-Estrada et al., 2008) that usually proliferate in the top 20–30 cm of the soil (Bryla and Strik, 2007). Water and nutrient uptake presumably occurs mostly in the first three root orders, which correspond to the mycorrhizal and non-woody, ephemeral section of the root system (Valenzuela-Estrada et al., 2008). These non-woody roots have very few and small-diameter vessels. Such root anatomical characteristics may lead to a high cavitation vulnerability of the distal roots during severe drought, especially when water is available to only a fraction of the root system such as might occur with drip irrigation or when there are only a few deeper roots. Plants may cope with drought under conditions of heterogeneous soil moisture conditions by internally redistributing water from roots in moist soil to those in dry soil during the night, such as in grape (Vitis vinifera) (Bauerle et al., 2008), but to a much more limited extent in blueberry. In the present work, patterns of root water potentials measured throughout the night revealed how water redistributes internally within the root system of Vaccinium. Vaccinium rehydrated the distal first five root orders in dry soil from 60% to 70% in a period of 11 h when well irrigated in another portion of the root system. No other work has previously quantified the rate or magnitude of change of tissue rehydration over the night as water moved through different branch orders in the root system.
Root rehydration at night was influenced by several factors, including root hydraulic constraints, duration of the nocturnal period, water availability in the wet-side, and water potential gradients among roots. The overall final rehydration achieved by the distal root orders in dry soil served as an indicator of how efficiently or inefficiently water was hydraulically redistributed through the root system. As suspected, the distal, finest root orders experienced the lowest water potentials at the onset of the nocturnal period, followed by medium and coarse roots. The simulations also demonstrated the importance of the duration of the nocturnal period. Although water was readily available to roots in the wet soil and transpiration was minimal, it took the whole night-time period of 12 h for the distal finest roots under dry soil conditions to reach the same water potentials as fine roots in wet soil. Even though roots in dry soil equilibrated with roots in wet soil, the equilibrium point reached before sunrise was still approximately –1.2 MPa, indicating that the tissues were not fully rehydrated, i.e. not fully in equilibrium with the soil water potential in the wettest portion of the rooted soil. Even with an additional hour and a half of no transpiration, water potentials for all root orders were predicted to range between –0.6 MPa to –0.7 MPa (Fig. 4). Therefore, even with the additional time, roots in dry soil would not be predicted to reach values greater than –0.5 MPa, suggesting that the duration of the nocturnal period was not sufficient for roots in dry soil to be fully rehydrated.
The main factors influencing water transport were the hydraulic properties of the conductive system. In very fine roots, internal water movement was probably delayed by either very high hydraulic resistances due to small diameter vessels in these roots or by additional resistances caused by the occurrence of xylem embolisms associated with severe water stress conditions (Hacke et al., 2000; Kolb and Sperry, 1999; Sperry et al., 2002).
With the single-branch model, it was possible to estimate the water potential of each of the seven root orders over the night and to identify those orders with the highest hydraulic resistance. As expected, the magnitude of hydraulic resistance per individual root was highest in 1st-order roots due to these roots having the fewest number of vessels and the smallest vessel diameters. However, it was found that in a root branch composed of seven root orders, 3rd-, 4th-, and 5th-orders (summing up all resistances belonging to that root order), exhibited the highest overall hydraulic resistances within the root branch (Table 1). Thus, when many resistances are arranged in parallel (as it was assumed to be for each root order level), the total resistance added to the system was not highest in the 1st- and 2nd-order roots but in the medium root orders, which had fewer individuals within the root branch (Table 1). In the case of root orders greater than five, although the number of these roots was small, numerous and wider vessels helped to compensate for the limited length and number of roots (Table 1). Therefore, the high hydraulic resistances exhibited by 3rd-, 4th-, and 5th-order roots may contribute considerably to delayed rehydration of the finest root orders. Interestingly, these roots represented the transition from the more permanent roots with secondary development to the more ephemeral roots without secondary development (Valenzuela-Estrada et al., 2008).
The possibility of intermediate-order roots serving as hydraulic controllers has important implications for the function of the whole root system. The observed pattern of hydraulic resistances in Vaccinium roots is consistent with the segmentation concept proposed by Zimmermann (1983) for above-ground hydraulic architecture. Similar to the occurrence of embolism within stem junctions, which may cause the sacrifice of minor branches and leaves during severe water stress conditions, hydraulic failure in 5th- or 4th-order roots may lead to the sacrifice of the lower root orders under drought conditions, but the maintenance of higher order roots.
In summary, it has been found that under severe water stress conditions the root system of V. corymbosum did not fully redistribute water from roots in wet soil to roots in dry soil. This was mainly attributed to anatomical constraints on water movement and because of the severe degree of water stress of roots in dry soil. Root orders with the highest hydraulic resistances corresponded to the lowest orders of the permanent root system (i.e. 5th-order roots), indicating the possible location of a hydraulic safety control in the root system of this species.
Acknowledgments
We thank Alonso Valenzuela-Estrada, Israel Alarcón, Marc Göbel, and Darryl Cruz for their assistance in data collection and the experimental set-up. Darryl Cruz was supported by the BRIE/CEKA/LOR and undergraduate summer scholars programme at Penn State University. We also want to thank Taryn Bauerle for her helpful ideas and laboratory assistance. We thank David Bryla and Frederick Meinzer for helpful reviews. LRV-E acknowledges the financial support of the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México. Partial support was also provided by NSF IOB 06-13832 to DME.
References
- Abbott JD, Gough RE. Split-root water application to highbush blueberry plants. HortScience. 1986;21:997–998. [Google Scholar]
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell and Environment. 2008;31:177–186. doi: 10.1111/j.1365-3040.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- Brooks JR, Meinzer FC, Coulombe R, Gregg J. Hydraulic redistribution of soil water during summer drought in two contrasting Pacific Northwest coniferous forests. Tree Physiology. 2002;22:1107–1117. doi: 10.1093/treephys/22.15-16.1107. [DOI] [PubMed] [Google Scholar]
- Bryla DR, Strik BC. Effects of cultivar and plant spacing on the seasonal water requirements of highbush blueberry. Journal of the American Society for Horticultural Science. 2007;132:270–277. [Google Scholar]
- Burgess SSO, Bleby TM. Redistribution of soil water by lateral roots mediated by stem tissues. Journal of Experimental Botany. 2006;57:3283–3291. doi: 10.1093/jxb/erl085. [DOI] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA. Night-time stomatal conductance and transpiration in C3 and C4 plants. Plant Physiology. 2007;143:4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MM, Dawson TE, Richards JH. Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia. 1998;113:151–161. doi: 10.1007/s004420050363. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Grise DJ, West JB, Pappert RA, Alder NN, Richards JH. Predawn disequilibrium between plant and soil water potentials in two cold-desert shrubs. Oecologia. 1999;120:209–217. doi: 10.1007/s004420050850. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Richards JH, Linton MJ. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology. 2003;84:463–470. [Google Scholar]
- Fitter AH. Morphometric analysis of root systems: application of the technique and influence of soil fertility on root system development in two herbaceous species. Plant, Cell and Environment. 1982;5:313–322. [Google Scholar]
- Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schafer KVR, Oren R. Influence of soil porosity on water use in Pinus taeda. Oecologia. 2000;124:495–505. doi: 10.1007/PL00008875. [DOI] [PubMed] [Google Scholar]
- Hinckley TM, Ritchie GA. A theoretical model for calculation of xylem sap pressure from climatological data. American Midland Naturalist. 1973;90:56–69. [Google Scholar]
- Howard NF. Twisted trees. Science. 1932;75:132–133. doi: 10.1126/science.75.1935.132-a. [DOI] [PubMed] [Google Scholar]
- Kolb KJ, Sperry JS. Transport constraints on water use by the Great Basin shrub, Artemisia tridentata. Plant, Cell and Environment. 1999;22:925–935. [Google Scholar]
- Orians CM, Smith SDP, Sack L. How are leaves plumbed inside a branch? Differences in leaf-to-leaf hydraulic sectoriality among six temperate tree species. Journal of Experimental Botany. 2005;56:2267–2273. doi: 10.1093/jxb/eri233. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of Northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Sperry JS, Stiller V, Hacke UG. Soil water uptake and water transport through root systems. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York, USA: Marcel Dekker, Inc.; 2002. pp. 663–681. [Google Scholar]
- Tsuda M, Tyree MT. Whole-plant hydraulic resistance and vulnerability segmentation in Acer saccharinum. Tree Physiology. 1997;17:351–357. doi: 10.1093/treephys/17.6.351. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Estrada LR. Above- and below-ground physiology in Vaccinium corymbosum L. (Northern highbush blueberry) in response to water stress and reproductive effort. PhD thesis. University Park: Pennsylvania State University; 2008. [Google Scholar]
- Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM. Root anatomy, morphology and longevity among root orders in Vaccinium corymbosum (Ericaceae) American Journal of Botany. 2008;95:1506–1514. doi: 10.3732/ajb.0800092. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap in plants. New York, USA: Springler-Verlag; 1983. [Google Scholar]