Abstract
Whereas the interplay of multiple hormones is essential for most plant developmental processes, the key integrating molecular players remain largely undiscovered or uncharacterized. It is shown here that a member of the tomato auxin/indole-3-acetic acid (Aux/IAA) gene family, Sl-IAA3, intersects the auxin and ethylene signal transduction pathways. Aux/IAA genes encode short-lived transcriptional regulators central to the control of auxin responses. Their functions have been defined primarily by dominant, gain-of-function mutant alleles in Arabidopsis. The Sl-IAA3 gene encodes a nuclear-targeted protein that can repress transcription from auxin-responsive promoters. Sl-IAA3 expression is auxin and ethylene dependent, is regulated on a tight tissue-specific basis, and is associated with tissues undergoing differential growth such as in epinastic petioles and apical hook. Antisense down-regulation of Sl-IAA3 results in auxin and ethylene-related phenotypes, including altered apical dominance, lower auxin sensitivity, exaggerated apical hook curvature in the dark and reduced petiole epinasty in the light. The results provide novel insights into the roles of Aux/IAAs and position the Sl-IAA3 protein at the crossroads of auxin and ethylene signalling in tomato.
Keywords: Auxin, differential growth, ethylene, hormone cross-talk, tomato
Introduction
Development in multicellular organisms is a highly complex process that requires the precise coordination of inter- and intracellular signalling and responses. Before the molecular era, the regulation of plant developmental processes was most often described as modifications in the hormonal balance, rather than as changes in the level of a single hormone. Subsequently, genetic screens led to tremendous advances in our understanding of the key components of the individual hormone metabolism and response pathways. As the understanding of these mechanisms grew, it became more apparent that the growth of plant organs is dependent on an intricate orchestration of hormonal and non-hormonal signals (Stepanova et al., 2007; Swarup et al., 2007). Identifying the central players in the interplay between different signalling pathways is critical to unravelling the complex mechanisms underlying the control of plant growth and development. Despite interactions between ethylene and auxin being among the most frequently addressed in hormonal cross-talk studies, little is known about the main actors that take part in this dialogue (Chae et al., 2000; Stepanova et al., 2005, 2007).
The plant hormone auxin, indole-3-acetic acid (IAA), has long been recognized as being a major regulator of plant growth and developmental processes. It exerts its effects by modulating the expression of downstream genes that encode proteins involved in a vast array of physiological processes. Recent genetic and molecular studies in Arabidopsis have revealed that auxin regulates gene expression through an ubiquitin-dependent proteolytic signal transduction system (Dharmasiri and Estelle, 2004). At the centre of the signalling cascade is the ubiquitin–ligase complex; auxin binding to Transport Inhibitor Response1/TIR1 (or its paralogues, the F-box protein AUXIN RECEPTOR F-BOX/AFB1 and AFB3) promotes the ubiquitin-dependent proteolysis of a family of transcriptional regulators known as Aux/IAAs in an auxin-dependent manner (Gray et al., 2001, Dharmasiri et al., 2005a, b; Kepinski and Leyser, 2005). Aux/IAA proteins inhibit the activity of the DNA-binding auxin response factors (ARF) whereas their degradation leads to the activation of ARFs and to subsequent auxin-responsive gene expression (Reed, 2001; Tiwari et al., 2001; Zenser et al., 2001; Hagen and Guilfoyle, 2002; Liscum and Reed, 2002). Aux/IAAs are therefore central to the regulation of auxin-mediated processes. The Arabidopsis genome encodes 29 Aux/IAA proteins (Remington et al., 2004; Overvoorde et al., 2005). Biochemical and genetic studies indicate that they generally function as transcriptional repressors of auxin-regulated genes (Ulmasov et al., 1997; Tiwari et al., 2004; Woodward and Bartel, 2005).
Gain-of-function mutations in several Aux/IAA genes have pleiotropic effects on plant growth, including altered root formation, apical dominance, stem/hypocotyl elongation, leaf expansion, and phototropism/gravitropism. These mutants have been identified in a variety of developmental and auxin-specific genetic screens. Each of these mutants is caused by a single mutation in domain II that results in the stabilization of the Aux/IAA. Strikingly, with the exception of the shy2 mutant that displays subtle modifications (Tian and Reed, 1999), none of the Arabidopsis ‘null mutants’ show obvious visible phenotypes, suggesting considerable functional redundancy among Aux/IAA family members (Overvoorde et al., 2005). The wide diversity of auxin responses and the tissue-specific expression of gene family members suggest, however, that individual Aux/IAAs have precise and distinct functions during normal plant growth and development. In both Arabidopsis and tomato, Aux/IAAs are themselves auxin responsive. Moreover, it has been reported previously that tomato Aux/IAA gene family members can be regulated by ethylene (Jones et al., 2002). Here, it is shown that Sl-IAA3, a tomato Aux/IAA, is critical to both auxin and ethylene signalling and is a key molecular link between ethylene and auxin responses in tomato plants.
Materials and methods
Plant material and growth conditions
Tomato [Solanum lycopersicum cv. MicroTom] plants were grown under standard greenhouse conditions. The culture chamber room was set as follows: 14-h-day/10-h-night cycle, 25/20 °C day/night temperature, 80% relative humidity, 250 μmol m−2 s−1 intense light. Seeds were sterilized, rinsed in sterile water, and sown in recipient Magenta vessels containing 50 ml of 50% Murashige and Skoog (MS) culture medium to which was added R3 vitamin (0.5 mg l−1 thiamine, 0.25 mg l−1 nicotinic acid, and 0.5 mg l−1pyridoxine), 1.5% (w/v) sucrose, and 0.8% (w/v) agar, pH 5.9.
Plant transformation
To generate AS-IAA3 transgenic plants, the forward 5′-AACAAGACTCAGCTCCTGCACC-3’ and reverse 5′-CATCACCAACAAGCATCCAATC-3’ primers were used to amplify a partial Sl-IAA3 clone (antisense construct in Fig. 1). The percentage sequence identity of the amplified fragment relative to the other members of the tomato Aux/IAAs family was checked (see Table S1 in Supplementary data available at JXB online) in order to validate its use in the antisense strategy. This 297 bp fragment was then cloned into the pGA643 binary vector in the antisense orientation under the transcriptional control of the 35S-CaMV promoter and the nopaline synthase (Nos) terminator. Transgenic plants were generated according to Wang et al. (2005) and all experiments were carried out using homozygous lines from F3 or later generations.
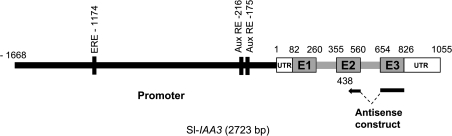
Fig. 1.
Genomic structure of the tomato Sl-IAA3 gene. The black portion represents the promoter region, the grey lines the introns, the grey boxes the exons, and the white boxes the untranslated regions (UTR). The putative auxin and ethylene cis-acting elements are indicated by black bars. The black arrow represents the antisense construct used to generate the silenced lines.
Isolation of the Sl-IAA3 genomic clone
Sl-IAA3 genomic clone was isolated by PCR amplification on genomic DNA template using primers encompassing the coding sequence. The Universal Genome Walker Kit (Clontech Laboratories, Inc., Palo Alto, CA, USA) was used to isolate the Sl-IAA3 gene promoter region. The Sl-IAA3 promoter was then fused to the β-glucuronidase (GUS) reporter gene in the plp100 binary vector (Szabados et al., 1995) and used for stable tomato transformation. DNA sequences were analysed with BLAST network services at the National Center for Biotechnology Information (Altschul et al., 1997) and by PlantCARE (Lescot et al., 2002).
Transient expression using a single cell system
For nuclear localization of the Sl-IAA3 fusion protein, the coding sequence of Sl-IAA3 was cloned as a C-terminal fusion in frame with green fluorescent protein (GFP) into the pGreen vector (Hellens et al., 2000) and expressed under the control of the 35S CaMV, a cauliflower mosaic virus promoter. Protoplasts were obtained from suspension-cultured tobacco (Nicotiana tabacum) BY-2 cells and transfected according to the method described previously (Leclercq et al., 2005). Transfected protoplasts were incubated for 16 h at 25 °C and analysed for GFP fluorescence by confocal microscopy. For co-transfection assays, the coding sequence of Sl-IAA3 was cloned into the pGreen vector and expressed under the control of the 35S CaMV promoter. Aliquots of protoplasts (0.5×106) were transformed either with 10 μg of the reporter vector alone containing the DR5 synthetic auxin-response element fused to the GFP reporter gene (gift from Prof. K Palme, Freiburg, Germany) or in combination with 10 μg of the effector plasmid, allowing the constitutive expression of the Sl-IAA3 protein. Transformation assays were performed in three independent replicates. After 16 h of incubation in the presence or absence of 2,4-D (50 μM), GFP expression was analysed and quantified by flow cytometry (FACS Calibur II instrument, BD Biosciences, San Jose, CA, USA) as indicated in Hagenbeek and Rock (2001). All transient expression assays were repeated at least three times with similar results.
Auxin and ethylene treatment
For auxin dose-response (0, 1, 10, 100 μM NAA) and NPA treatment, experiments were carried out as described by Wang et al. (2005). For quantitative real-time PCR (qRT-PCR) studies, 21-d-old seedlings were treated for 16 h with 1 μl l−1 1-methyl cyclopropene (1-MCP), the ethylene perception inhibitor (Agrofresh, USA) and then incubated in presence or absence of 20 μM IAA. For GUS analysis, 21-d-old tomato seedlings and sections of mature green (MG) fruit (Vibratom, Leica VT 1000 S, Vetzlar, Germany) were incubated for 2 h with or without 20 μM IAA. MG and breaker (Br) fruit were treated for 5 h with 50 μl l−1 ethylene and 1-MCP (1 μl l−1) for 16 h, respectively. Ethylene treatment (10 μl l−1) was performed on 5-d-old etiolated PIAA3::GUS, DR5::GUS transformed seedlings. For the epinastic response, light-grown plants were treated with ethylene (50 μl l−1) for 16 h.
For histochemical GUS analysis, PIAA3::GUS or DR5::GUS transgenic lines were incubated at 37 °C for 5–15 h with GUS-staining solution as indicated by Wang et al. (2005)
qRT-PCR
RNAs extraction and qRT-PCR analyses were performed as described previously (Pirrello et al., 2006). The primer sequences are listed in Table S2 in Supplementary data available at JXB online.
Results
Isolation and structure of the Sl-IAA3 gene
It has previously been shown that Sl-IAA3 (formerly named DR3) is ethylene inducible and differentially expressed during tomato fruit ripening (Jones et al., 2002). Subsequently the full-length Sl-IAA3 cDNA (U 320812, now available from the Solanaceae Genome Network Database, http://www.sgn.cornell.edu) has been isolated and the transcription start site determined by 5’ Race-PCR. The 558 bp cDNA encoded a predicted Sl-IAA3 protein of 185 amino acids comprising the four conserved domains (I–IV) characteristic of Aux/IAA proteins. Sl-IAA3 falls into sub-family I of the four Aux/IAA sub-families (Wang et al., 2005). A genomic fragment of 2723 bp was also isolated comprising 1668 bp of upstream sequence containing promoter and 1055 bp of gene sequence composed of three exons and two introns (Fig. 1) matching that of its closest Arabidopsis homologues, At-IAA3 (AT1G04240) and At-IAA4 (AT5G43700). The Sl-IAA3 nucleotide coding and predicted amino acid sequences displayed 65.8% and 56% identity, respectively, with At-IAA3 and 65.4% and 56.3% identity, respectively, with At-IAA4. Analysis of the 1668 bp promoter fragment with the PlantCare software (Lescot et al., 2002) identified two degenerate auxin-response elements (TGTCNC) at positions –216 and –175, and an ethylene-response element ERE (ATTTCAAA) at position –1174 (Fig. 1).
Sl-IAA3 transcripts are ubiquitous in all plant tissues but show higher accumulation during fruit ripening
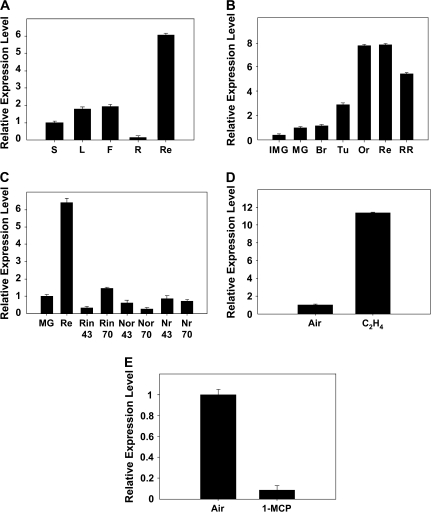
qRT-PCR showed that Sl-IAA3 transcripts were present in all tissues tested (Fig. 2A), with the highest levels in red fruit, where they were 6-fold higher than in the reference (stem) tissue. In wild-type fruit, Sl-IAA3 transcript levels increased commensurate with endogenous ethylene production levels throughout the ripening process (Fig. 2B). In the ripening and ethylene response-impaired monogenic tomato mutants, rin (ripening inhibitor), nor (non-ripening), and Nr (Never-ripe), Sl-IAA3 transcript levels were substantially lower than in the wild-type at the equivalent to ripening stages (Fig. 2C), indicating that Sl-IAA3 is integral to normal ethylene-responsive fruit-ripening processes. To verify that the ripening-associated Sl-IAA3 transcript accumulation was ethylene-dependent, the effect of exogenous ethylene was assessed on MG fruit that are responsive to exogenous ethylene but not yet producing elevated levels of ripening-associated ethylene, and, conversely, the effect of 1-MCP, a potent inhibitor of ethylene perception, on Br fruit producing elevated endogenous ethylene. Five hours of ethylene treatment of MG fruit (50 μl l−1) resulted in an almost 11-fold increase in Sl-IAA3 transcript accumulation (Fig. 2D). Conversely, in Br-stage fruit, an overnight treatment with 1-MCP (1 μl l−1) led to a 10-fold reduction in Sl-IAA3 transcripts (Fig. 2E). Given that SI-IAA3 is a presumptive auxin response regulator, these results reveal that one of the roles for ethylene during climacteric fruit ripening is the modification of auxin responsiveness in ripening fruit.
Fig. 2.
Tissue-specific and ethylene-dependent expression of Sl-IAA3. The expression analyses were carried out by qRT-PCR using RNA samples extracted from various tomato tissues. (A) Analysis of Sl-IAA3 transcript levels in different organs. SI-IAA3 mRNA accumulation was monitored in stem (S), leaf (L), flower (F), root (R), and red fruit (Re). (B) Expression pattern of SI-IAA3 during the late stages of fruit development: immature green fruit, IMG; mature green, MG; breaker, Br; turning, Tu; orange, Or; red, Re; red-ripe, RR. (C) Expression pattern of Sl-IAA3 in wild type (WT) and rin, nor, and Nr ripening mutants. RNA samples were extracted from fruit collected 43 d and 70 d after anthesis, corresponding in the WT to MG and Re stages, respectively. (D) Ethylene responsiveness of the Sl-IAA3 gene. RNA samples were extracted from MG fruit treated for 5 h with air or with 50 μl l−1 ethylene. (E) Br fruit treated with 1 μl l−1 of 1-MCP for 16 h. Relative expression level on the y-axis refers to the fold difference in Sl-IAA3 expression relative to stem in (A), MG stage in (B, C), and untreated control fruit in (D, E). The expression data are means of three replicates ±standard error.
Sl-IAA3 transcript accumulation is positively regulated by auxin and ethylene in tomato seedlings
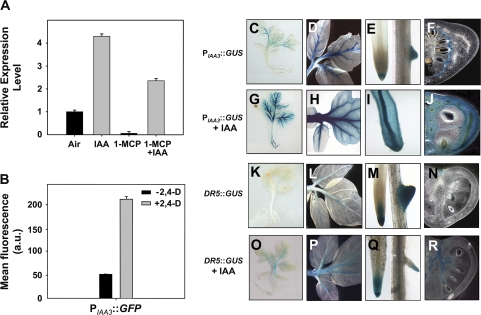
In dark-grown seedlings, qRT-PCR analysis revealed that ethylene induction of Sl-IAA3 transcript accumulation mimicked both the dose-response and the time-course gradient of the well-characterized ethylene-responsive gene, E8 (see Fig. S1 in Supplementary data available at JXB online). Sl-IAA3 transcript levels also increased 4-fold in light-grown tomato seedlings after 2 h of auxin (20 μM IAA) treatment (Fig. 3A). In tobacco BY2 protoplasts transfection assays, Sl-IAA3 promoter (1668 bp)-driven GFP levels increased 4-fold after auxin treatment (50 μM 2,4-D) (Fig. 3B). As auxin is known to stimulate ethylene production (Abel et al., 1995), it was decided to determine whether this auxin-responsiveness resulted from an increase in ethylene production. Light-grown tomato seedlings were treated overnight with 1-MCP (1μl l−1) and then incubated in presence or absence of auxin. Similarly to the observation in fruit, 1-MCP almost completely abolished SI-IAA3 transcripts in untreated tomato seedlings (Fig. 3A). In the presence of both 1-MCP and auxin, however, Sl-IAA3 transcript levels were only partially reduced (Fig. 3A), indicating that in light-grown tomato seedlings SI-IAA3 is both auxin and ethylene-inducible and that the auxin-responsiveness is partially mediated by ethylene.
Fig. 3.
Auxin responsiveness of the Sl-IAA3 gene. (A) qRT-PCR analysis of Sl-IAA3 transcript levels in 3-week-old light-grown control and auxin-treated (20 μM IAA for 2 h) seedlings in presence or absence of 1 μl l−11-MCP applied 16 h prior to auxin treatment. Relative expression level on the y-axis refers to the fold difference in SI-IAA3 transcript levels relative to the non-treated plantlets. (B) Auxin responsiveness of the Sl-IAA3 promoter. Tobacco protoplasts were transformed by PIAA3::GFP and incubated in the presence or absence of 2,4-D (50 μM). Transformation was performed in triplicate and, in each experiment, GFP fluorescence was measured by flow cytometry 16 h after transfection. Values are expressed in arbitrary units (a.u.) ±standard error. (C–F) Tissue-specific expression of Sl-IAA3 assessed in transgenic tomato expressing GUS reporter gene driven by the Sl-IAA3 promoter (PIAA3::GUS). The expression pattern was analysed in 3-week-old seedlings (C), leaves (D), roots (E), and MG fruit (F). (G–J) These images correspond to the same tissues treated for 2 h with 20 μM IAA. (K–N) These images correspond to the same tissues expressing the DR5 auxin-responsive promoter fused to the GUS reporter gene (DR5::GUS) and those in (O–R) to DR5::GUS treated with 20 μM IAA. The data are representative of at least three independent experiments with n > 20 seedlings examined per experiment.
Sl-IAA3 displays tightly regulated tissue-specific expression
To gain further insight into Sl-IAA3 expression, the Sl-IAA3 promoter was fused to the GUS reporter gene (PIAA3::GUS) and this construct stably introduced into tomato plants. In untreated vegetative tissues, the Sl-IAA3 promoter drove GUS expression predominantly in the leaf vasculature, root cap, and developing lateral roots (Fig. 3C–E). A brief auxin treatment (20 μM for 2 h) of light-grown seedlings led to a dramatic increase in GUS expression throughout the roots and shoots (Fig. 3G–I). In MG fruit, GUS staining was restricted to a narrow band in the placental exo-layer at the junction between the placenta and pericarp tissues (Fig. 3F). Auxin treatment, led to GUS staining throughout the pericarp and columella tissues, while it remained excluded from placental tissues (Fig. 3J). As a control for auxin responsiveness, GUS expression driven by the synthetic auxin-responsive promoter, DR5, was also assessed. Interestingly, in the absence of exogenous auxin, DR5 drove GUS expression in the leaf midrib and root tips (Fig. 3K–M), but not in the fruit (Fig. 3N). Exogenous auxin treatment resulted in enhanced staining in vegetative tissues but the fruit expression remained restricted to the vascular tissues (Fig. 3O–R), providing evidence that, although Sl-IAA3 is auxin responsive, its transcriptional control is more complex than that of DR5.
Sl-IAA3 down-regulation results in vegetative growth phenotypes
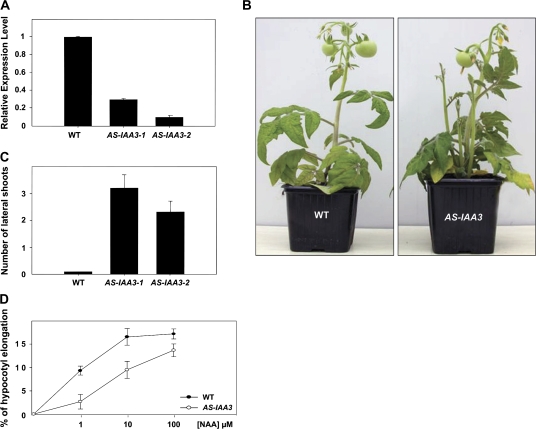
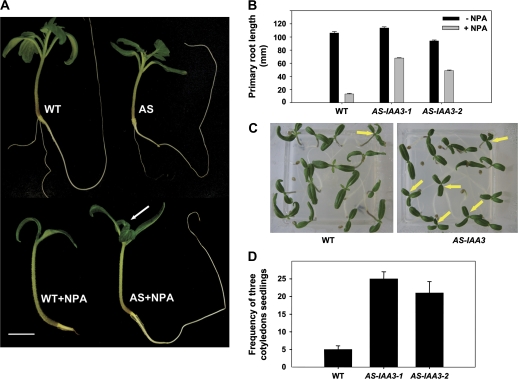
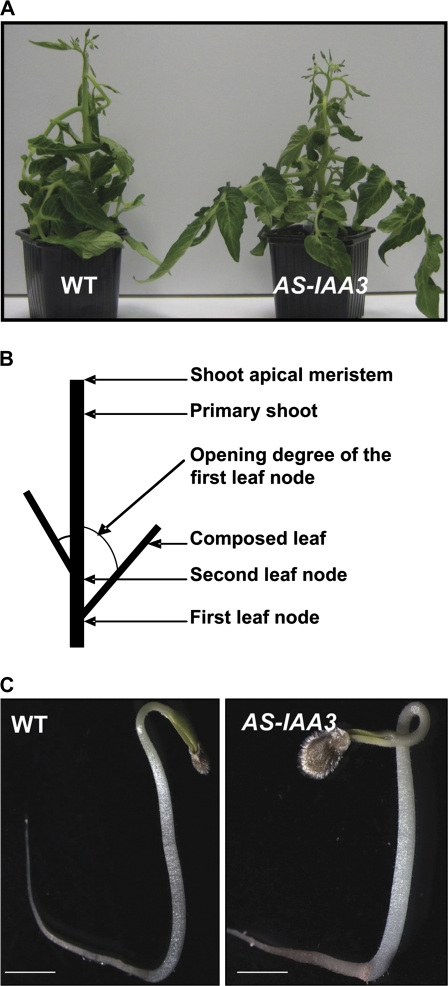
Several independent homozygous Sl-IAA3-suppressed antisense lines (AS-IAA3) were generated and two representative lines (1 and 2) with 3.5-fold and 10-fold reductions, respectively, in Sl-IAA3 transcript levels were selected for further study (Fig. 4A). Down-regulation of Sl-IAA3 resulted in a variety of vegetative growth phenotypes (Figs 4, 5). In determinate wild-type tomato plants, lateral shoots develop only after floral transition, and their growth is initiated in an apical–basal sequence along the primary shoot axis. In the AS-IAA3 plants, by contrast, axillary shoot development began in the lowest leaf node (Fig. 4B) and the number of lateral shoots was greater in the transgenic lines (Fig. 4C). This loss of apical dominance suggests a reduced response to endogenous auxin in the transgenic lines. Similarly, auxin-induced hypocotyl elongation was reduced in AS-IAA3 hypocotyls compared with the wild type (Fig. 4D), further indicating a reduction in auxin responsiveness in the transgenic lines. To investigate this apparent reduction in auxin responsiveness, the effects of the auxin transport inhibitor N-1-napthylphthalamic acid (NPA) on the growth of wild-type and AS-IAA3 seedlings were examined. Wild-type seedlings grown in the presence of 1 μM NPA showed a marked reduction in primary root elongation and a complete suppression of lateral root formation (Fig. 5A, B). By contrast, NPA only weakly affected primary and lateral root growth in the AS-IAA3 plants (Fig. 5A, B). Also, leaf emergence was strongly inhibited in NPA-treated wild-type seedlings, but not in the AS-IAA3 plants (arrow in Fig. 5A). The AS-IAA3 lines also had a higher frequency of ectopic cotyledons than the wild type (Fig. 5C, D). The frequency of polycotyledons was 25% and 20% in AS-AA3-1 and AS-IAA3-2 lines, respectively, compared with only 5% in the wild type (Fig. 5D).
Fig. 4.
Altered vegetative growth phenotypes in antisense Sl-IAA3 plants. (A) Down-regulation of Sl-IAA3 in transgenic tomato plants. The level of Sl-IAA3 transcripts in antisense lines (1 and 2) was assessed by qRT-PCR. Relative expression level refers to the fold difference in Sl-IAA3 transcript levels relative to the wild type (WT). (B) Reduced apical dominance in 7-week-old AS-IAA3 plants compared with WT. (C) The number of lateral shoots branching from the first leaf node in WT and AS-IAA3 plants. The data are the mean ±standard error of 30 plants and are representative of three independent experiments. (D) Auxin dose-response in hypocotyl segments. Hypocotyl fragments (8 mm long) from 3-week-old light-grown seedlings were incubated for 2 h in the presence of the indicated concentration of NAA. Elongation is given as percentage increase in final length over the initial length. The results are representative of data obtained with two independent AS-IAA3 lines and with two replicates for each line. Standard errors are indicated (n ≥25). (This figure is available in colour at JXB online.)
Fig. 5.
Auxin-associated phenotypes of Sl-IAA3 down-regulated lines. (A) Effect of NPA treatment on the development of light-grown wild-type (WT) and AS-IAA3 seedlings. WT and AS-IAA3 tomato seedlings (19-d-old) were grown in the presence or absence of 1 μM NPA. Leaf emergence is inhibited in WT but not in AS-IAA3 lines (white arrow). The scale bar indicates 10 mm. (B) Primary root length upon NPA treatment of light-grown WT and AS-IAA3 lines. Error bars represent mean ±standard error (n ≥60). (C) Triple cotyledon phenotype occurring at higher frequency in AS-IAA3 lines compared with WT. Three cotyledon structures are indicated by arrows in 7-d-old light-grown plantlets. (D) Frequency of triplicate cotyledons occurring in AS-IAA3 and WT seedlings expressed as a percentage of the total population. Error bars represent mean ±standard error of 40 plants. (This figure is available in colour at JXB online.)
Sl-IAA3 suppression results in modified ethylene sensitivity
The ethylene responsiveness of Sl-AA3 prompted the examination of the role of the encoded protein in two classical ethylene response processes, epinastic petiole curvature in light-grown plants and the formation of an apical hook in etiolated seedlings. Tomato leaf petioles typically curve downwards in response to exogenous ethylene (Kazemi and Kefford, 1974). To investigate the impact of the down-regulation of Sl-IAA3 on this epinastic response, light-grown wild-type and AS-IAA3 tomato plantlets were treated with exogenous ethylene (50 μl l−1) for 16 h. The subsequent angles of the petioles to the main stem were measured for leaves 1 and 2 (Fig. 6B). In both AS-IAA3 lines 1 and 2, the leaf angle after ethylene treatment was 87° and 75°, respectively (Fig. 6A, Table 1). In the wild type, the leaf angle was 100° (Fig. 6A, Table 1), indicating a reduced epinastic response in the transgenic lines.
Fig. 6.
Ethylene-associated phenotypes of AS-IAA3 lines. (A) Petiole epinasty in wild-type (WT) and AS-IAA3 plants in response to ethylene. Five-week-old light-grown plants were treated by 50 μl l−1 ethylene for 16 h. (B) Diagram depicting the position of the first and second leaf node in tomato plants. (C) Hook curvature in 5-d-old WT (left panel) and AS-IAA3 (right panel) etiolated seedlings. The scale bar indicates 5 mm. (This figure is available in colour at JXB online.)
Table 1.
Altered petiole epinastic response in AS-IAA3 plants
| Petiole opening degree |
||
| Air | C2H4 | |
| WT | 70.8±2.8 | 100±4.46 |
| AS-IAA3-1 | 70.1±3.5 | 87±4.31 |
| AS-IAA3-2 | 72.2±1.8 | 75±2.87 |
Petiole opening degree of the first and the second leaf node was measured before and after ethylene treatment in wild-type and AS-IAA3 plants. The data are means ±standard error of at least 36 plants and are representative of three independent experiments.
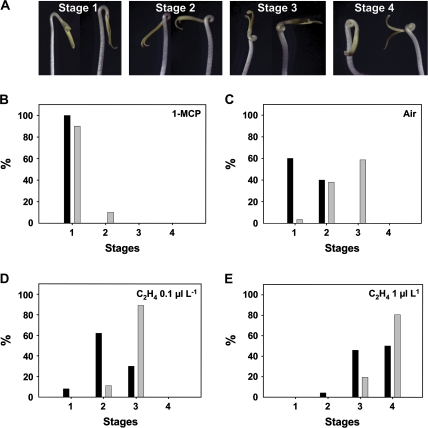
The exaggeration of the apical hook is one of the hallmarks of the classical ethylene triple response, although the process is known to involve changes in both ethylene and auxin signalling (Ecker, 1995). One of the most striking phenotypes in the AS-IAA3 seedlings was the exaggerated apical hook formation in dark-grown seedlings in the absence of exogenous ethylene (Fig. 6C). To characterize this phenotype better, different grades of hook formation (Fig. 7A) were defined ranging from stage 1, corresponding to minimal exaggerated hook with a curvature angle lower than 180°, to stage 4, corresponding to a maximal exaggerated hook with a curvature angle higher than 360°. Sixty percent of air-grown AS-IAA3 seedlings displayed hook curvatures corresponding to stage 3 and 35% corresponded to stage 2. In the same growth conditions, most wild-type seedlings had hook curvatures of either stage 1 (60 % of seedlings) or stage 2 (37% of seedlings) (Fig. 7C). A low level of exogenous ethylene (0.1 μl l−1) shifted hook curvature to stage 2 (63% of seedlings) and stage 3 (25% of seedlings) in the wild-type and to stage 3 (90% of seedlings) in the antisense plants (Fig. 7D). Increasing the exogenous ethylene to 1 μl l−1 shifted hook curvature to stages 4 (50% of seedlings) and 3 (45% of seedlings) in the wild-type and to stages 4 (80% of seedlings) and stage 3 (20% of seedlings) in the transgenic seedlings (Fig. 7E). Treatment with 1-MCP (Fig. 7B) strongly reduced the difference between wild type (98% of seedlings at stage 1) and antisense (90% of seedlings at stage 1), suggesting that the exaggerated apical hook curvature phenotype of the AS-IAA3 plants requires active ethylene signalling.
Fig. 7.
Hook formation in AS-IAA3 lines upon ethylene treatment. (A) Assessment of different grades of hook formation in etiolated tomato seedlings treated with different concentrations of ethylene (0–1 μl l−1). Four stages have been defined corresponding to minimal exaggerated hook with a curvature angle lower than 180° (stage 1) to a maximal exaggerated hook with a curvature angle higher than 360° (stage 4). (B–E) Proportion of wild-type (black columns) and AS-IAA3 (grey columns) plants corresponding to the four stages of hook formation upon treatment with 1 μl l−1 1-MCP for 16 h (B), air (C), or 0.1 (D) and 1 μl l−1 exogenous ethylene (E).
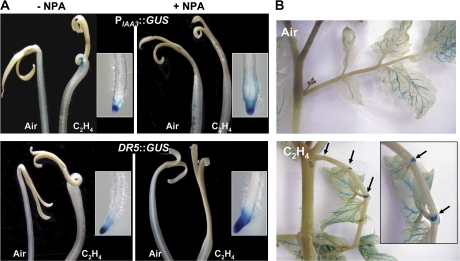
To get more insight on the role of Sl-IAA3 in apical hook formation and epinastic response, the expression pattern of this gene was analysed in tomato lines expressing the PIAA3::GUS construct. In the absence of exogenous ethylene treatment there was minimal GUS staining associated with the apical hook in dark-grown wild-type PIAA3::GUS lines. By contrast, after 48 h ethylene treatment (10 μl l−1), a strong band of GUS staining was observed on the inner surface of the apical hook (Fig. 8A). The same ethylene treatment did not result in detectable DR5-driven GUS staining in the hook. The putative role of auxin in mediating the ethylene-associated expression of Sl-IAA3 was then investigated by performing the ethylene treatment in the presence of NPA, a known inhibitor of auxin transport. NPA completely prevented ethylene-induced apical hook formation and simultaneously suppressed Sl-IAA3 expression, suggesting that auxin is required for apical hook formation and for the expression of IAA3 in the inner side of the hook. Noteworthy, upon ethylene treatment, intense staining was present in the root tips of both transgenic lines, attesting that DR5 and IAA3 promoters exhibit similar capacity to drive GUS activity in tissues accumulating high amounts of auxin. Taken together these data suggest that the higher ethylene-induced expression of Sl-IAA3 in the inner side of the apical hook could not be ascribed only to increased auxin levels (Fig. 8A).
Fig. 8.
Expression of PIAA3::GUS is associated with differential growth during hook formation and leaf epinastic response. (A) Tissue-specific expression of PIAA3::GUS and DR5::GUS in etiolated seedlings. PIAA3::GUS and DR5::GUS seedlings were dark-grown for 5 d and then treated for 48 h with air or 10 μl l−1 of ethylene in absence (left panel) or presence of NPA (right panel). The upper-panel shows the ethylene-dependent GUS staining in the apical hook of PIAA3::GUS tomato plants. The lower-panel shows GUS staining in the DR5::GUS-transformed plants used for detection of active auxin signalling in the hook. Inserts correspond to the expression of PIAA3::GUS and DR5::GUS in the root caps following ethylene treatment. (B) Expression of PIAA3::GUS in epinastic petioles. Six-week-old light-grown plants were placed in airtight chambers for 16 h in the absence (upper-panel) or presence (lower-panel) of 50 μl l−1 of ethylene. The arrows indicate the expression of GUS in the leaf nodes of the petiole. The images are representative of at least three independent experiments with n > 30 seedlings per experiment.
The role of Sl-IAA3 in ethylene-induced differential growth was further investigated by assessing the expression of Sl-IAA3 in light-grown epinastic tissues. Ethylene treatment of epinastic petioles led to PIAA3::GUS expression in restricted zones on the upper side of the leaf nodes (Fig. 8B) whereas no expression was detected in untreated non-epinastic petioles (Fig. 8B). These data indicate that Sl-IAA3 expression is associated with tissues undergoing differential growth, albeit in opposite directions relative to the ethylene-induced expression in the two tissues.
Down-regulation of Sl-IAA3 specifically impacts on the expression of selected auxin and ethylene transcription factors
An Sl-IAA3:GFP fusion protein localized exclusively to the nucleus in transient expression assays in tobacco protoplasts (see Fig. S2 in Supplementary data available at JXB online) consistent with the native Sl-IAA3 being a transcriptional regulator. To address the ability of the Sl-IAA3 protein to regulate the activity of auxin-responsive promoters, a DR5-driven GFP reporter construct was used (Ottenschlager et al., 2003) in a protoplast transient expression assay. In the absence of effector construct, DR5-driven GFP expression was enhanced up to 10-fold by the auxin (2,4-D) treatment (see Fig. S3 in Supplementary data) whereas the presence of 35S-driven Sl-IAA3 in co-transfection assays, strongly reduced this auxin induction. These data indicate that Sl-IAA3 acts in protoplast as a repressor of auxin-dependent transcription and is consistent with Sl-IAA3 being a member of the Aux/IAA family.
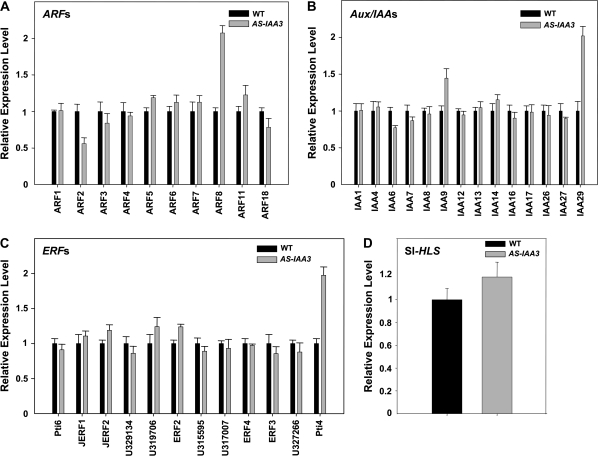
To provide mechanistic insight into how SI-IAA3 functions to bring about the observed phenotypes in the transgenic lines, the expression of transcription factors known to mediate auxin and ethylene responses, including 14 Aux/IAA, 10 ARF, and 12 ERF (Ethylene Response Factor) genes was analysed (Fig. 9). While most of the genes showed similar expression in 5-d-old wild-type and transgenic line seedlings, there was a clear down-regulation of the tomato homologue of Arabidopsis ARF2 (SGN-U314233) and conversely a significant up-regulation of transcript levels for the tomato homologue of ARF8 (SGN-U327976) (Fig. 9A). The expression of IAA29 (SGN-U320261) and Pti4 (SGN-U317071), a tomato ERF gene, were also significantly up-regulated in the transgenic lines (Fig. 9B, C), indicating that down-regulation of Sl-IAA3 alters the expression of specific auxin and ethylene transcriptional mediators. In Arabidopsis, Hookless1 (At-HLS1) is a key regulator of apical hook formation and the hls1 mutant showed no differential growth in the apical region of the hypocotyl even after ethylene treatment (Lehman et al., 1996). Notably, accumulation of transcripts of the tomato Hookless gene (Sl-HLS) was not altered in antisense lines (Fig. 9D).
Fig. 9.
Impact of Sl-IAA3 down-regulation on the expression of auxin and ethylene response genes. The expression of members of the ARF (A), Aux/IAA (B), and ERF (C) gene families of transcription factors as well as the Sl-HLS gene (D) was assessed by qRT-PCR in 5-d-old dark-grown wild-type (WT) and AS-IAA3 etiolated seedlings. Primers used are listed in Table S2 in Supplementary data available at JXB online. Relative expression level on the y-axis refers to the fold difference in expression of each gene relative to that in WT seedlings taken as reference tissues. The data correspond to mean values of three replicates ±standard error.
Discussion
Aux/IAA proteins are critical components of the auxin response. In Arabidopsis, dominant gain-of-function mutations in individual Aux/IAAs have provided telling insights into the roles played by the various family members in eliciting specific auxin responses. It is shown here that Sl-IAA3, a tomato Aux/IAA, is an integral component of both auxin and ethylene response pathways. Indeed, transcripts for the gene accumulate in response to both hormones, and its down-regulation results in auxin- and ethylene-related phenotypes. Phenotypic responses to Sl-IAA3 down-regulation include alterations to the classical auxin-regulated processes of apical dominance and hypocotyl elongation, and to typical ethylene responses such as apical hook formation in etiolated seedlings and leaf epinasty in light-grown plants.
Sl-IAA3 and a number of other partial tomato Aux/IAA clones were initially isolated from fruit tissues. The Sl-IAA3 gene has strong sequence and structural similarities with its putative Arabidopsis orthologues, At-IAA4 and At-IAA3. An Arabidopsis At-IAA4 mutant with an insertion in the first exon shows no obvious growth phenotype (Overvoorde et al., 2005). In fact, although loss-of-function mutations have been identified in Arabidopsis for several Aux/IAA genes, the only phenotypes reported are subtle changes in plants mutated in one of the putative orthologues of tomato Sl-IAA3, SHY2/IAA3 (Tian and Reed, 1999). Double or triple mutants of closely related Aux/IAA genes, such as iaa8-1/iaa9-1 or iaa5-1/iaa6-1/iaa19-1 also exhibit wild-type phenotypes, indicating extensive functional redundancy among Arabidopsis Aux/IAA family members (Overvoorde et al., 2005). It has previously been shown that down-regulation of a tomato Aux/IAA gene, Sl-IAA9, resulted in altered leaf architecture and parthenocarpic fruit, consistent with a pivotal role for auxin in tomato fruit set and leaf morphogenesis (Wang et al., 2005). In the present study, it is shown that the down-regulation of Sl-IAA3 (AS-IAA3) also leads to well-defined phenotypes in transgenic tomato lines. The possibility that the observed changes might result from a lack of specificity of the antisense strategy was ruled out by verifying that the expression of closely related Aux/IAA genes was not altered in the AS-IAA3 transgenic lines. The sequence homology rule predicts that IAA3 antisense would primarily target IAA1, IAA4, and IAA17 among all members of the Aux/IAA gene family. However, none of the best potential Aux/IAA targets displayed detectable change in transcript accumulation in the AS-IAA3 lines (Fig. 9). Moreover, ARF2 which showed down-regulation in the antisense lines displayed an extremely poor sequence match with IAA3. The present data strongly support the hypothesis that different members of the Aux/IAA family are involved in distinct developmental processes. This is also supported by the work of Kloosterman et al. (2006) who showed that suppression of St-IAA2 in potato results in distinctive phenotypes, including increased plant height, petiole hyponasty, and curvature of growing leaf primordia in the shoot apex.
Sl-IAA3 mediates auxin-dependent gene transcription and auxin-associated phenotypes
Aux/IAA genes were originally identified based on their rapid induction by auxin in etiolated soybean (Glycine max) and pea (Pisum sativum) tissues (Walker and Key, 1982; Theologis et al., 1985). Many Arabidopsis auxin-responsive genes contain the canonical auxin response elements (AuxRE), TGTCTC or GAGACA, in their promoters (Guilfoyle and Hagen, 2007). The present in silico search led to the identification of two degenerate AuxRE elements in the Sl-IAA3 promoter that may be responsible for the auxin responsiveness observed in this study (Figs 1, 3).
Sl-IAA3 transcript levels varied dramatically among the different tomato tissues, and analyses of tomato PIAA3::GUS lines revealed that basal levels of expression were spatially restricted within organs. In the root, Sl-IAA3-driven GUS expression was restricted to the root cap and lateral root meristems, in the leaves to the vasculature, and in the fruit to a narrow band defining the junction between placenta and pericarp. This well-defined tissue-specific expression pattern was abolished by exogenous auxin treatment leading to GUS staining throughout the whole fruit pericarp and leaf and root tissues. While the auxin responsiveness is in agreement with previous data (Jones et al., 2002), the expression pattern of Sl-IAA3 in the hook differed from that of the artificial auxin-responsive promoter, DR5, suggesting that a combination of promoter elements contributes to the precise tissue-specific pattern of Sl-IAA3 expression. Because the expression of PIAA3::GUS and DR5::GUS gave similar staining in the root tips but not in the apical hook, the ethylene-induced expression of Sl-IAA3 in the inner side of the apical hook cannot be ascribed to increased levels of auxin only. Nevertheless, auxin is also contributing to both the apical hook formation and the associated Sl-IAA3 expression as suggested by the abolished hook and Sl-IAA3 expression in NPA-treated seedlings (Fig. 8A).
In Arabidopsis, Aux/IAA gain-of-function mutations that stabilize the Aux/IAA proteins (Reed, 2001) are, in most cases, associated with phenotypes reminiscent of reduced auxin responsiveness (Nagpal et al., 2000; Rogg et al., 2001; Tian et al., 2002). Since Arabidopsis Aux/IAAs have been shown to repress DR5-driven transcription (Ulmasov et al., 1997; Tiwari et al., 2001), it was hypothesized that the down-regulation of Sl-IAA3 would lead to enhanced auxin responses. Unexpectedly, the AS-IAA3 lines have many phenotypes consistent with reduced auxin sensitivity. This suggests that, even though Sl-IAA3 has the capacity to repress auxin-responsive gene expression in protoplasts (see Fig. S2 in Supplementary data available at JXB online), in planta the protein seems to act as a positive regulator of auxin responses. One possible explanation for this apparent discrepancy is that in planta Sl-IAA3 may repress the expression of negative regulators of auxin responses. Two ARFs (ARF2 and ARF8) and one Aux/IAA (IAA29) that were differentially regulated in the AS-IAA3 lines, may contribute to the reduced auxin-responsiveness in AS-IAA3.
Ethylene-related expression and phenotypes
It has been shown previously that the accumulation of Sl-IAA3 transcripts is enhanced by ethylene treatment in MG fruit (Jones et al., 2002). In the present work, it was shown that Sl-IAA3 transcript accumulation mimicked both the dose-response and the time-course gradient of the well-characterized ethylene-responsive gene, E8 (Lincoln et al., 1987). Importantly, Sl-IAA3 had an ethylene-dependent, ripening-associated expression pattern that was revealed by a sharp reduction in Sl-IAA3 transcripts when Br fruit were treated with the ethylene inhibitor, 1-MCP. Moreover, accumulation of Sl-IAA3 transcripts was dramatically reduced in the tomato ripening mutants (rin, nor, and Nr) that lack the capacity to respond to autocatalytic ethylene and to undergo normal ethylene-regulated ripening processes (Giovannoni, 2007). Given that SI-IAA3 is a presumptive auxin response regulator, these results strongly suggest that one of the roles for ethylene during climacteric fruit ripening is the modification of auxin responsiveness in the ripening fruit. Whereas these observations suggested that down-regulation of Sl-IAA3 in transgenic lines may have resulted in a fruit ripening phenotype, none of the ripening features examined in the present study differed between antisense and wild-type lines (timing of the onset of ripening, levels of climacteric ethylene production, and pigment accumulation). Though it cannot be excluded that other ripening aspects may have been altered, the present data suggest that either the Sl-IAA3 is functionally redundant in fruit tissues or that residual levels of Sl-IAA3 were sufficient to drive the ripening processes that rely on the IAA3 protein.
Two other phenotypes in the AS-IAA3 lines, the exaggerated apical hook formation and reduced epinasty, indicated that Sl-IAA3 is important for physiological responses involving ethylene. Apical hook formation in etiolated seedlings forms the classical ethylene triple response together with reduced hypocotyl and root elongation (Bleecker et al., 1988; Ecker, 1995). The involvement of both ethylene and auxin in this differential cell elongation has been demonstrated through the analysis of ethylene- and auxin-signalling mutants that are altered in the process of hook formation. In Arabidopsis, mutants that are defective in ethylene perception and signalling, such as etr1-1, ein2, and ein3, do not form an exaggerated hook in response to ethylene treatment. By contrast, the constitutive ethylene response mutant, ctr1, develops an exaggerated hook in the absence of ethylene (Guzman and Ecker, 1990; Kieber et al., 1993). Auxin promotes hypocotyl cell elongation and is unequally distributed in the apical hook (Schwark and Schierle, 1992). The axr1 mutant, which is altered in auxin responses, lacks a normal apical hook and the inhibition of auxin transport disrupts formation of the hook (Lincoln et al., 1990). Clearly, the apical hook is established and maintained by interplay between ethylene and auxin. The exaggerated apical hook phenotype in the AS-IAA3 lines provides direct evidence that Sl-IAA3 is important in physiological processes that rely on both auxin and ethylene. Active ethylene signalling is essential for the appearance of the exaggerated hook phenotype since blocking ethylene perception with 1-MCP prevents hook formation in the AS-IAA3 plants. The other aspects of the triple response, namely exaggerated hypocotyl elongation and the thickening and shortening of roots, were not altered in the AS-IAA3 lines, indicating that Sl-IAA3 is specifically involved in differential growth processes. Ethylene treatment of etiolated seedlings increased the PIAA3::GUS expression in the inner surface of the apical hook (Fig. 8). Likewise, PIAA3::GUS staining was also clearly delimited in epinastic petioles, suggesting that the ethylene-induced gradient of Sl-IAA3 expression is involved in the differential growth associated with both apical hook formation and the petiole epinastic response. However, whereas down-regulation of Sl-IAA3 resulted in an exaggerated ethylene-response of etiolated seedlings, it conferred reduced ethylene sensitivity in light-grown plants. The ability of ethylene to induce opposite growth responses in the dark and in the light have been described previously (Smalle et al., 1997) and could explain the seemingly contradictory phenotypes displayed by AS-IAA3 plants in the seedlings and petioles. In keeping with this complex regulation of Sl-IAA3, the ethylene-induced expression of this gene in light-grown plants was found in the upper side of epinastic petioles, opposite to the pattern observed in the hook of etiolated seedlings.
Arabidopsis plants with a loss-of-function mutation in HLS1 are unable to form an apical hook even in the presence of ethylene (Lehman et al., 1996). A mutation that reverses the hls1 phenotype has been identified and was found to encode the auxin-response factor, ARF2 (Li et al., 2004). Interestingly, the putative tomato orthologue of ARF2 is also down-regulated in the AS-IAA3 lines, suggesting that the process of hook formation may require an interplay between HLS1, IAA3, and ARF2. The previous model proposed by Li et al. (2004) postulates that ARF2 acts downstream of HLS1. It was shown here that the expression of Sl-HLS is not altered in the AS-IAA3 plants, suggesting that Sl-IAA3 and Sl-HLS may act in parallel pathways both of them involving ARF2 as a downstream component. On the other hand, it cannot be ruled out that Sl-HLS may also act upstream of Sl-IAA3.
The altered apical dominance found in the AS-IAA3 lines was also observed in the previously described antisense Sl-IAA9 plants (Wang et al., 2005). Unlike Sl-IAA9, however, Sl-IAA3 has distinct roles in ethylene-related responses. By revealing that a number of transcription factors from the ARF (Sl-ARF2 and Sl-ARF8), Aux/IAA (Sl-IAA29), and ERF (Ethylene Response Factor Pti4) families are under direct or indirect regulation by Sl-IAA3, the present study provides insights into how SI-IAA3 functions to bring about some of the observed phenotypes. While continued effort is required to gain a more complete understanding of the hormonal dialogue mediated by Sl-IAA3, the data described here confirm that Aux/IAA proteins have both distinct and overlapping roles and reveal that these proteins can be integral auxin as well as ethylene response regulators.
Supplementary data
Table S1. Percentage identity of the antisense region relative to the other members of tomato Aux/IAAs family.
Table S2. Auxin- and ethylene-response genes.
Fig. S1. Subcellular localization of Sl-IAA3 protein.
Fig. S2. Sl-IAA3 protein represses the in vivo activity of DR5.
Fig. S3. Ethylene regulation of Sl-IAA3.
Supplementary Material
Acknowledgments
This work forms part of the requirement for the degree of Ph.D. for S Chaabouni. We thank F Regad for technical support in real-time PCR, A Jauneau for microscopy analyses, M Zouine for in silico sequence analyses, S Albert, L Lemonnier, H Mondies, O Berseille, and D Saint-Martin for tomato genetic transformation. This research was funded by the European Integrated Project EU-SOL (FOOD-CT-2006-016214) and by Midi Pyrénées Region Council. S Chaabouni was recipient of a national scholarship from the Tunisian government.
References
- Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indole acetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: structural, characterization, expression in Escherichia coli, and expression characteristics in response to auxin. Journal of Biological Chemistry. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST, a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;26:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Chae HS, Cho YG, Park MY, Lee MC, Eun MY, Kang BG, Kim WT. Hormonal cross-talk between auxin and ethylene differentially regulates the expression of two members of the 1-aminocyclopropane-1-carboxylate oxidase gene family in rice (Oryza sativa L) Plant and Cell Physiology. 2000;41:354–362. doi: 10.1093/pcp/41.3.354. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005a;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Developmental Cell. 2005b;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Estelle M. Auxin signaling and regulated protein degradation. Trends in Plant Science. 2004;9:302–308. doi: 10.1016/j.tplants.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Current Opinion in Plant Biology. 2007;10:283–289. doi: 10.1016/j.pbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G. Auxin response factors. Current Opinion in Plant Biology. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response to identify ethylene-related mutants. The Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. Auxin-responsive gene expression, genes, promoters and regulatory factors. Plant Molecular Biology. 2002;49:373–385. [PubMed] [Google Scholar]
- Hagenbeek D, Rock CD. Quantitative analysis by flow cytometry of abscisic acid-inducible gene expression in transiently transformed rice protoplasts. Cytometry. 2001;45:170–179. doi: 10.1002/1097-0320(20011101)45:3<170::aid-cyto1160>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards AE, Leyland NR, Bean S, Mullineaux P. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Molecular Biology. 2000;42:819–832. doi: 10.1023/a:1006496308160. [DOI] [PubMed] [Google Scholar]
- Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latché A, Pech JC, Bouzayen M. Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. The Plant Journal. 2002;32:603–613. doi: 10.1046/j.1365-313x.2002.01450.x. [DOI] [PubMed] [Google Scholar]
- Kazemi S, Kefford NP. Apical correlative effects in leaf epinasty of tomato. Plant Physiology. 1974;54:512–519. doi: 10.1104/pp.54.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kloosterman B, Visser RGF, Bachem CWB. Isolation and characterization of a novel potato Auxin/indole-3-acetic Acid family member (StIAA2) that is involved in petiole hyponasty and shoot morphogenesis. Plant Physiology and Biochemistry. 2006;44:766–775. doi: 10.1016/j.plaphy.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Leclercq J, Ranty B, Sanchez-Ballesta MT, Li Z, Jones B, Jauneau A, Pech JC, Latché A, Ranjeva R, Bouzayen M. Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. Journal of Experimental Botany. 2005;56:25–35. doi: 10.1093/jxb/eri003. [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Developmental Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH Estelle M. Growth and development of the axr1 mutants of Arabidopsis. The Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Cordes S, Read E, Fischer RL. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proceedings of the National Academy of Sciences, USA. 1987;84:2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Molecular Biology. 2002;49:387–400. [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiology. 2000;123:563–573. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proceedings of the National academy of Sciences, USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. The Plant Cell. 2005;17:3282–3300. doi: 10.1105/tpc.105.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad Q, Regad F, Latché A, Pech JC, Bouzayen M. Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant and Cell Physiology. 2006;47:1195–1205. doi: 10.1093/pcp/pcj084. [DOI] [PubMed] [Google Scholar]
- Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends in Plant Science. 2001;6:420–425. doi: 10.1016/s1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- Remington DL, Vision TJ, Guilfoyle TJ, Reed JW. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiology. 2004;135:1738–1752. doi: 10.1104/pp.104.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in IAA28 suppresses lateral root development. The Plant Cell. 2001;13:465–480. doi: 10.1105/tpc.13.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwark A, Schierle J. Interaction of ethylene and auxin in the regulation of hook growth. I. The role of auxin in different growing regions of the hypocotyl hook of Phaseolus vulgaris. Journal of Plant Physiology. 1992;140:562–570. [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of the National Academy of Sciences, USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. The Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, van der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L, Charrier B, Kondorosi A, de Bruijn FJ, Ratet P. New plant promoter and enhancer testing vectors. Molecular Breeding. 1995;1:419–423. [Google Scholar]
- Theologis A, Huynh TV, Davis RW. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. Journal of Molecular Biology. 1985;183:53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Tian Q, Uhlir NJ, Reed JW. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. The Plant Cell. 2002;14:301–319. doi: 10.1105/tpc.010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. The Plant Cell. 2004;16:533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. The Plant Cell. 2001;13:2809–2822. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Key JL. Isolation of cloned cDNAs to auxin-responsive poly(A)þ RNAs of elongating soybean hypocotyl. Proceedings of the National Academy of Sciences, USA. 1982;79:7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jones B, Li ZG, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. The Plant Cell. 2005;17:2676–2692. doi: 10.1105/tpc.105.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin, regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J. Auxin modulates the degradation rate of Aux/IAA proteins. Proceedings of the National Academy of Sciences, USA. 2001;98:11795–11800. doi: 10.1073/pnas.211312798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.