Abstract
A systematic review and meta-analysis was conducted to evaluate the comparative efficacy and tolerability of second-generation antidepressants in social anxiety disorder. Studies were identified by searching MEDLINE®, Embase, The Cochrane Library, PsychLit, and the International Pharmaceutical Abstracts from January 1980 through October 2006. Comparative evidence was summarized and indirect comparisons were made using network meta-analysis. Only three head-to-head trials were identified; comparative trials found only minimal differences in efficacy between escitalopram and paroxetine, and no statistically significant differences in efficacy between extended-release venlafaxine and paroxetine. Pooled evidence from 15 placebo-controlled trials suggests that escitalopram (relative benefit 1.3; 95% CI 1.2 to 1.5), paroxetine (relative benefit 1.9; 95% CI 1.5 to 2.3), sertraline (relative benefit 1.8; 95% CI 1.5 to 2.2), and venlafaxine (relative benefit 1.7; 95% CI 1.5 to 1.9) all produce significantly more responders than placebo; evidence favored fluvoxamine over placebo but was not significant (relative benefit 1.5; 95% CI 0.9 to 2.4). Network meta-analysis did not reveal differences in efficacy among drugs. Overall, fair evidence supports the efficacy of escitalopram, fluvoxamine, paroxetine, sertraline, and venlafaxine in social anxiety disorder. The drugs do not differ in efficacy, although their adverse event profiles do.
Keywords: social anxiety disorder, social phobia, second-generation antidepressant, network meta-analysis, indirect comparison
INTRODUCTION
Social anxiety disorder (SAD), or social phobia, officially was recognized as a psychiatric illness in 1980. The Diagnostic and Statistical Manual of Mental Disorders – fourth edition (DSM-IV) (American Psychiatric Association, 2000) characterizes SAD as anxiety due to a marked and persistent fear of one or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. SAD may be subdivided into generalized and nongeneralized or specific subtypes. The latter is further classified as anxiety related to a few social or performance situations (most often public speaking). The symptoms must interfere with a person’s normal routine, occupational functioning, relationships, or social activities, or they must cause marked distress. Although these symptoms may be severe enough to present in the form of a panic attack, the occurrence of such symptoms only in social situations distinguishes SAD from panic disorder
A 1994 epidemiological study - the National Comorbidity Survey (NCS) - found SAD to be the third most common of the psychiatric disorders (Kessler et al., 1994), trailing in prevalence only to depression and alcohol dependence. The more recent NCS - replication survey reported 12-month prevalence of SAD to be 6.8% and the lifetime prevalence to be 12.1% (Kessler et al., 2005). Symptoms of SAD typically onset in the middle teen years and appear more common in women than men (Fehm et al., 2005; Westenberg and Liebowitz, 2004).
Estimating the burden of SAD is difficult because it is often accompanied by coexisting psychiatric conditions. However, a lifetime diagnosis of SAD accounts for a 38% high school drop-out rate, which remains significant even after controlling for age, sex, socioeconomic status, and co-existing major depression (Stein and Kean, 2000). In one study, one-third of patients with SAD reported suicidal ideation, and the rate of suicidal attempts was higher among patients with major depression (Schneier et al., 1994). Perhaps the greatest burden of SAD falls on the patient in the form of functional impairment and decreased quality of life; this burden is difficult to quantify.
Current treatment options include pharmacologic and psychotherapeutic interventions, which are often combined (Muller et al., 2005). Pharmacologic interventions have received increased study as a first-line treatment (Van Ameringen and Mancini, 2001), with treatment based on the specific subtype of SAD. “As-needed” doses of beta-blockers are prescribed for performance-related anxiety; generalized SAD requires at least a 12-month course of scheduled medication. Various pharmacological classes of drugs have been used to treat patients with generalized SAD. These include beta-blockers, benzodiazepines, monoamine oxidase inhibitors (MAOIs), and the newer antidepressant therapies sometimes referred to as second-generation antidepressants; the latter include selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and others.
As with performance-related anxiety, beta-blockers are efficacious in generalized SAD for controlling autonomic arousal such as tachycardia, palpitations, and tremor. Although benzodiazepines produce a beneficial anxiolytic effect, typically they are considered second-line therapy because of physical dependence and withdrawal difficulties (Versiani, 2000). Because of drug interactions and possible tyramine-induced hypertensive crisis necessitating diet restrictions, MAOIs often are avoided regardless of promising efficacy (Versiani, 2000). Certain second-generation antidepressants often are considered as first-line therapy because of their beneficial effects and relatively benign safety profile (Davidson, 2006). Currently, sertraline, paroxetine, and venlafaxine XR are approved by the U.S. Food and Drug Administration (FDA) and other regulatory agencies for treating SAD. Although other agents such as bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, and mirtazapine have shown beneficial effects in treating related anxiety disorders, they are not FDA approved for SAD.
Previous reviews have qualitatively and quantitatively summarized response rates for select SSRIs and other older agents (Blanco et al., 2003; Fedoroff and Taylor, 2001; Gould et al., 1997; Hedges et al., 2007; Stein et al., 2004; van der Linden et al., 2000). These reviews have compared SSRIs as a class to placebo and to other classes of drugs such as MAOIs, reversible inhibitors of monoamine oxidase A (RIMAs), and benzodiazapines. Evidence consistently suggests that SSRIs as a class are better than placebo. For example, the review by Van der Linden and colleagues (van der Linden et al., 2000) found that 53% of patients responded to an SSRI; the pooled likelihood of responding to an SSRI was 3.3 times that of responding to placebo (95% CI 2.6 – 4.2).
One review pooled placebo-controlled data for individual SSRIs and qualitatively compared effect sizes on various social and occupational function scales (Hedges et al., 2007). However, this review focused on only SSRIs rather than all second-generation antidepressants and did not evaluate comparative differences among drugs. Little is known about how newer antidepressants compare with each other in the treatment of SAD. We improve upon previously published systematic reviews and meta-analyses in that we focus on second-generation antidepressants and provide the first summary of comparative evidence for these drugs in the treatment of SAD.
METHODS
Key Questions
Key questions designed to address the efficacy and safety of second-generation antidepressants in treating SAD guided our work. A consortium of 14 US state Medicaid programs, the Canadian Coordinating Office for Health Technology Assessment (CCOHTA), and the California HealthCare Foundation helped formulate the questions and provided funding for this research, but did not contribute to the conduct or interpretation of this review.
Literature Search
We searched MEDLINE®, Embase, The Cochrane Library, PsychLit, and the International Pharmaceutical Abstracts. Our searches covered 1980 through October 2006. We manually searched reference lists of relevant review articles and letters to the editor. Pharmaceutical manufacturers were invited to submit dossiers, including citations, as outlined by the Drug Effectiveness Review Project (Fox, 2005). We contacted the FDA and requested unpublished studies, although the FDA did not release any unpublished data.
Study Selection
Two persons independently reviewed titles and abstracts. If both reviewers agreed that the trial did not meet pre-established eligibility criteria (Table 1), we excluded it. We included active- and placebo-controlled trials that randomized patients with a DSM-defined diagnosis of SAD to bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, mirtazapine, nefazodone, paroxetine, sertraline, or venlafaxine. Although observational studies with large sample sizes can detect adverse events not frequent enough to be apparent in smaller trials, we did not include observational evidence because of insufficient evidence specific to patients with SAD.
Table 1.
Systematic review inclusion and exclusion criteria
| Outcome | Inclusion | Exclusion Criteria | ||
|---|---|---|---|---|
| Efficacy / Effectiveness | • | Randomized, controlled trials comparing one second-generation antidepressant to another second-generation antidepressant or placebo | • | Statistically significant differences between treatment groups deemed to affect outcomes (e.g., baseline severity of illness) |
| • | Study duration 12 weeks or longer | • | Fatal flaws in study design or data analysis that contribute to a “poor” quality rating for internal validity | |
| • | Adult and pediatric outpatients | |||
| • | Outcomes include: Liebowitz Social Anxiety Scale, Clinical Global Impression of Improvement scale, or the Sheehan Disability Scale | |||
| Safety / Tolerability | • | Randomized, controlled trials comparing one second-generation antidepressant to another second-generation antidepressant or placebo | • | Statistically significant differences between treatment groups deemed to affect outcomes (e.g., baseline severity of illness) |
| • | Study duration 12 weeks or longer | • | Fatal flaws in study design or data analysis that contribute to a “poor” quality rating for internal validity | |
Data Abstraction and Quality Assessment
Trained reviewers abstracted data from each study; a senior reviewer read each abstracted article and evaluated completeness of data extraction. We recorded intention-to-treat results if available. We assessed the internal validity (quality) of trials based on predefined criteria from the US Preventive Services Task Force (ratings: good-fair-poor)(Harris et al., 2001) and the National Health Service Centre for Reviews and Dissemination (Centre for Reviews and Dissemination, 2001). Elements of internal validity assessment included randomization, allocation concealment, similarity of compared groups at baseline, use of intention-to-treat analysis, and overall and differential loss to follow-up. Loss to followup was defined as the number of persons randomized who did not reach the study endpoint (Egger et al., 2001), independent of the reason and use of intention-to-treat analysis.
Data Synthesis
We abstracted data for several psychiatric rating scales including the Liebowitz Social Anxiety Scale (LSAS) (Liebowitz, 1987), the Sheehan Disability Scale (SDS) (Leon et al., 1997), and the Clinical Global Impression of Improvement scale (CGI-I) (Guy, 1976). The LSAS is a 24-item scale that can be summed to create a total score; higher values of the LSAS total score represent more severe disease. The SDS contains three domains (family, social, and work), with higher scores in each respective domain representing more severe impairment. LSAS total scores and each of the three domains of the SDS were coded as the mean change from baseline to endpoint and standard deviations for the mean change. When standard errors or standard deviations were not reported, we imputed values using estimates from similarly designed trials. Treatment response was coded as the number of patients characterized as 1 “very much” or 2 “much” improved on the CGI-I at endpoint.
We first qualitatively summarized the studies. For rating scales that were consistently used across trials (i.e., LSAS and CGI-I), we did quantitative analyses. For the LSAS we calculated the overall size of effect for a treatment. When we had two or more trials for a single drug, we used the pooled sample standard deviation (Hedges and Olkin, 1985) to calculate a pooled weighted mean difference. For the CGI-I we calculated the likelihood of treatment response using a relative risk (risk ratio) meta-analysis. Because this analysis reflects benefits rather than risks, we report this as a relative benefit (RB). When trials included multiple dosing arms, we included the highest FDA-approved dose. For studies with an active comparator, we analyzed each drug separately but reduced the sample size of the placebo group proportionately (e.g., for studies with two active treatments and placebo, the placebo sample size was halved for each comparison so as not to double count). For each meta-analysis we tested for heterogeneity of treatment effects by using I2 statistics (Higgins et al., 2003). If heterogeneity appeared we explored potential reasons for inconsistency. To estimate possible publication bias caused by the tendency of published studies to be positive, we used funnel plots, the Begg adjusted rank correlation test (Begg and Mazumdar, 1994), and the Egger regression approach (Egger et al., 1997).
Because no head-to-head evidence was available for the majority of drug comparisons, we conducted indirect comparisons using network meta-analysis (Lumley, 2002) of placebo-controlled trials. This method assesses the relative benefits of two treatments when they have not been compared directly with each other, but have each been compared to other treatments (Lumley, 2002). Evidence suggests that indirect comparisons agree with head-to-head trials if component studies are similar and treatment effects are expected to be consistent in patients included in different trials (Glenny et al., 2005). The outcome of this analysis was the CGI-I, with response categorized as improved or very much improved on the CGI-I. Individual drugs were included in the network meta-analysis when at least two similarly designed trials provided CGI-I data.
For adverse events we calculated the pooled mean incidence and 95% confidence intervals (CIs) from included trials. We focused on events previously documented with the drugs of interest in treating major depression (Hansen et al., 2005), such as nausea, vomiting, diarrhea, headache, dizziness, insomnia, sexual side effects, and weight gain.
Statistical analyses used StatsDirect Statistical Software, version 2.3.8 (StatsDirect, Ltd., Cheshire, UK, 2004) and R (Free Software Foundation, Inc., Boston, MA, 2007).
RESULTS
Three head-to-head trials (Allgulander et al., 2004; Lader et al., 2004; Liebowitz et al., 2005) and 15 placebo-controlled trials (Allgulander, 1999; Baldwin et al., 1999; Blomhoff et al., 2001; Davidson et al., 2004; Kasper et al., 2005; Kobak et al., 2002; Lepola et al., 2004; Liebowitz et al., 2003; Rickels et al., 2004; Stein et al., 1999; Stein et al., 1998; Stein et al., 2005; Van Ameringen et al., 2001; Wagner et al., 2004; Westenberg et al., 2004) were included (see Appendix 1). We excluded five trials: one because it assessed relapse prevention (Stein et al., 2003); one because it did not assess the LSAS, CGI-I, or SDS (van Vliet et al., 1994); one because it lasted less than 12 weeks (Katzelnick et al., 1995); and two trials because they did not meet our pre-defined quality criteria (Allgulander and Nilsson, 2001; Martins et al., 1994). Included trials lasted from 12 to 28 weeks. The mean age in most trials was between 35 and 45 years, with a relatively equal distribution of males and females (Table 2). One study included children and adolescents (mean age, 13 years) (Wagner et al., 2004). Although all trials required a diagnosis of SAD consistent with the DSM-IV, trials varied with regard to baseline severity of disease; mean baseline LSAS scores ranged from 74 (Allgulander, 1999) to 96 (Kasper et al., 2005). Some studies included patients with coexisting psychiatric conditions.
Table 2.
Characteristics of trials included for efficacy
| Trial (author, year) | Drug | Mean Dose (mg) |
Sample Size |
Mean Age (years) |
Percent Female |
Duration (weeks) |
Mean Baseline LSAS |
Statistically Significant Differences* |
Quality Rating |
|---|---|---|---|---|---|---|---|---|---|
| Lader et al., 2004 | Escitalopram | 20 | 505** | 37 | 52 | 24 | 94 | LSAS, CGI-I, SDS (W, F, S) |
Fair |
| Kasper et al., 2005 | Escitalopram | 18 | 358 | 38 | 46 | 12 | 96 | LSAS, CGI, SDS (W, S) |
Fair |
| Kobak et al., 2001 | Fluoxetine | 44 | 60 | 39 | 58 | 14 | 82 | None | Fair |
| Stein et al., 1999 | Fluvoxamine | 202 | 92 | 39 | 36 | 12 | 80 | LSAS, CGI, SDS (W, F) |
Fair |
| Davidson et al., 2004 | Fluvoxamine CR | 174 | 279 | 37 | 36 | 12 | 90 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Westenberg et al., 2004 | Fluvoxamine CR | 209 | 300 | 38 | 52 | 12 | 97 | LSAS, CGI, SDS (W) |
Fair |
| Lader et al., 2004 | Paroxetine | 20 | 505** | 37 | 52 | 24 | 94 | LSAS, CGI, SDS (W, S) |
Fair |
| Stein et al., 1998 | Paroxetine | 37 | 187 | 36 | 57 | 12 | 81 | LSAS, CGI, SDS (W, S) |
Fair |
| Allgulander 1999 | Paroxetine | 20–50 | 92 | 39 | 48 | 12 | 74 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Allgulander et al., 2004 | Paroxetine | 44 | 389** | 39 | 53 | 12 | 84 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Liebowitz et al., 2005 | Paroxetine | 46 | 440** | 36 | 46 | 12 | 86 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Wagner et al., 2004 | Paroxetine | 33 | 322 | 13 | 50 | 16 | 78 | LSAS, CGI | Good |
| Baldwin et al., 1999 | Paroxetine | 35 | 323 | 36 | 54 | 12 | 87 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Lepola et al., 2004 | Paroxetine CR | 32 | 375 | 39 | 50 | 12 | 78 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Van Ameringen et al., 2001 | Sertraline | 147 | 204 | 36 | 44 | 20 | NR | CGI, SDS (W, F, S) |
Fair |
| Liebowitz et al., 2003 | Sertraline | 159 | 415 | 35 | 40 | 12 | 92 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Blomhoff et al., 2001 | Sertraline | 126 | 175 | 40 | 60 | 24 | NR | SDS (W, S) | Fair |
| Allgulander et al., 2004 | Venlafaxine ER | 192 | 389** | 39 | 53 | 12 | 84 | LSAS, CGI, SDS (W, F, S) |
Fair |
| Stein et al., 2005 | Venlafaxine ER | 214 | 395 | 37 | 39 | 24 | 89 | LSAS, CGI SDS (W, F, S) |
Fair |
| Rickels et al., 2004 | Venlafaxine ER | 152–178 | 261 | 41 | 42 | 12 | 89 | LSAS, CGI, SDS (W, S) |
Fair |
| Liebowitz et al., 2005 | Venlafaxine ER | 202 | 440** | 36 | 46 | 12 | 86 | LSAS, CGI, SDS (W, F, S) |
Fair |
P < 0.05 for comparison with placebo
Head-to-head trial; placebo sample size was halved for each comparison with active treatment LSAS = Liebowitz Social Anxiety Scale; CGI-I = Clinical Global Impression of Improvement; SDS = Sheehan Disability Scale; SDS(W) = Work subscale; SDS(F) = Family subscale; SDS(S) = Social subscale; NR = not reported
Clinical Response: Anxiety Severity
Two trials that compared venlafaxine with paroxetine (Allgulander et al., 2004; Liebowitz et al., 2005) and one trial that compared escitalopram with paroxetine (Lader et al., 2004) reported LSAS measures. No statistically significant differences in the reduction in LSAS scores were reported between venlafaxine and paroxetine. Mean doses of venlafaxine and paroxetine were generally comparable. Statistically significantly larger differences were noted for patients on higher doses (i.e., 20mg/day) of escitalopram compared with paroxetine 20mg/day in observed cases analysis, although these were not statistically significant in the intention to treat analysis. Differences were not statistically significant for lower doses of escitalopram in either the observed cases or intention to treat analysis.
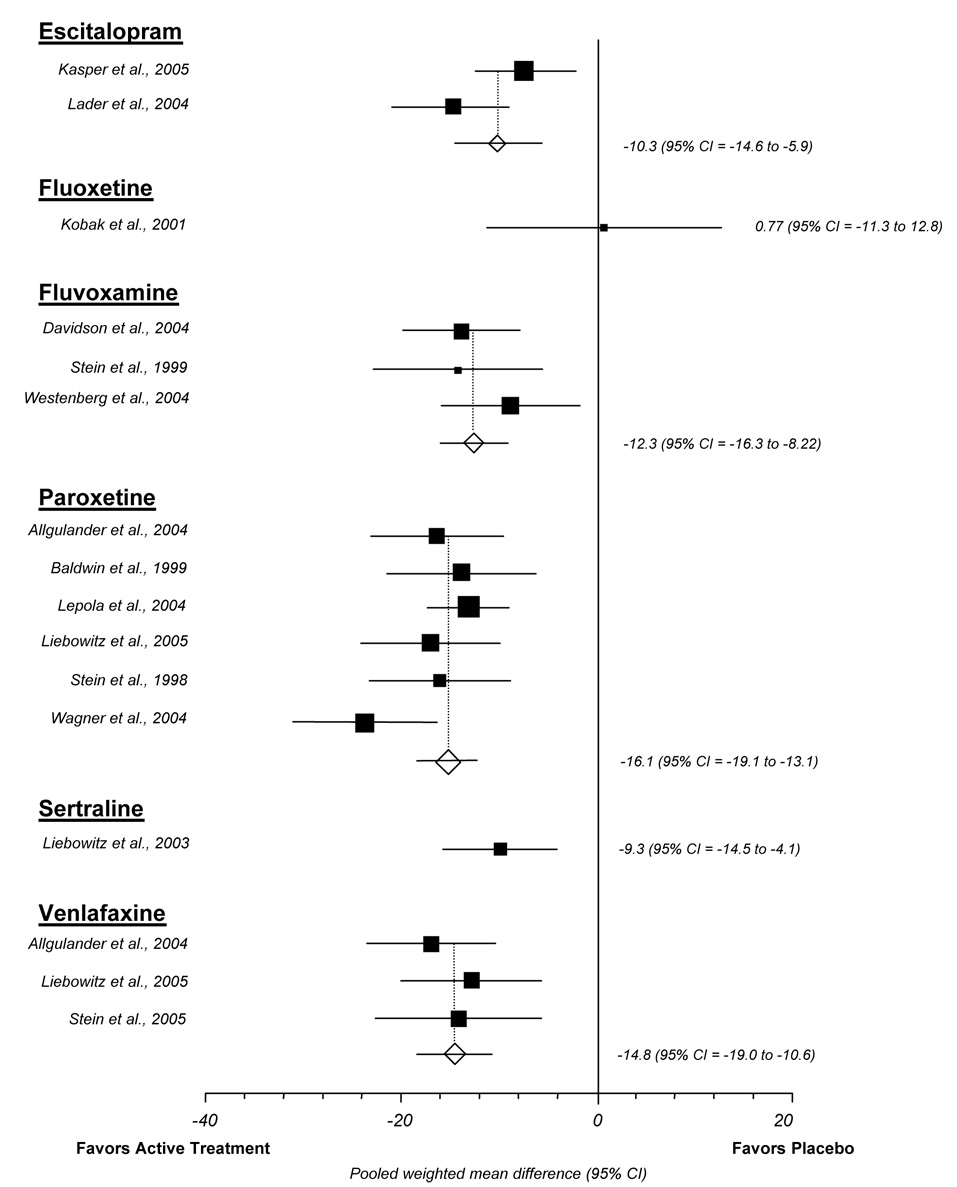
We abstracted usable data for the mean reduction in LSAS total score for 14 trials, representing 16 placebo-controlled comparisons. Data were sufficient to calculate a pooled weighted mean difference between placebo and escitalopram, fluvoxamine, paroxetine, and venlafaxine (Figure 1). Overall for these pooled data, active treatments lead to a 10 to 16 point greater LSAS reduction than placebo. The pooled weighted mean difference was 10.3 (95% CI 5.9 to 14.6) for escitalopram, 12.3 (95% CI 8.2 to 16.3) for fluvoxamine, 16.1 (95% CI 13.1 to 19.1) for paroxetine, and 14.8 (10.6 to 19.0) for venlafaxine. Only minimal heterogeneity was detected and tests for publication bias were not significant.
Figure 1.
Liebowitz social anxiety scale meta-analysis (random effects)
Clinical Response: Functional Impairment
Two trials that compared venlafaxine with paroxetine (Allgulander et al., 2004; Liebowitz et al., 2005) and one trial that compared escitalopram with paroxetine (Lader et al., 2004) assessed disability using the SDS. Active treatments did not differ significantly in reducing disability from SAD.
We abstracted data for the mean reduction in family, social, and work domains of the SDS from 12 trials including 13 different placebo-controlled comparisons (i.e., 1 head-to-head trial provided usable data); 2 trials of escitalopram, 3 of fluvoxamine, 5 of paroxetine, 2 of sertraline, and 1 of venlafaxine. Active treatment was statistically significantly better than placebo on the work domain of the SDS in all trials (P < 0.05). Compared with placebo, active treatment produced a 0.7 to 2.2 point greater reduction in the work domain (pooled difference 1.25; 95% CI 0.9 to 1.5). The social domain was significantly more improved for active treatment compared with placebo in all but one trial; the trial that failed to show statistically significant differences compared controlled-release fluvoxamine to placebo over 12 weeks (Westenberg et al., 2004). Overall, the weighted mean difference between active treatment and placebo for the reduction in the social domain of the SDS ranged from 0.5 to 1.9 (pooled difference 1.16; 95% CI 0.9 to 1.4). Trends toward greater improvement in the family domain for active treatment compared with placebo were observed in most trials, although this difference did not reach statistical significance in six trials (escitalopram (Kasper et al., 2005); fluvoxamine (Westenberg et al., 2004); paroxetine (Lader et al., 2004; Stein et al., 1998); sertraline (Blomhoff et al., 2001); and venlafaxine (Rickels et al., 2004)). The weighted mean difference between active treatment and placebo for the family domain ranged from 0 to 1.7 (pooled difference 0.86; 95% CI 0.6 to 1.1). Only minimal heterogeneity was detected and tests for publication bias were not significant.
Clinical Response: Global Impression
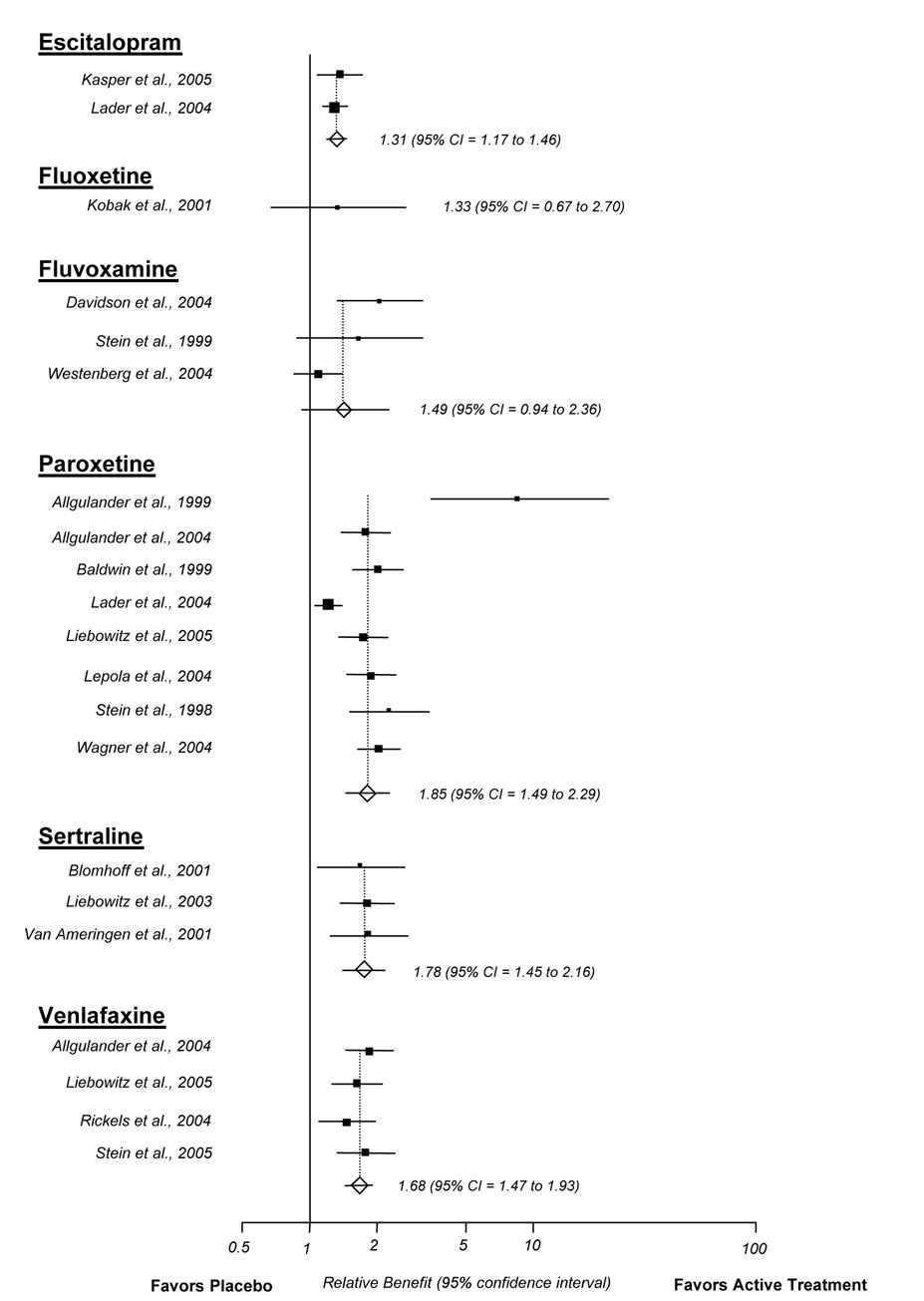
We abstracted CGI-I response data on active treatment and placebo from 18 trials. Responders were characterized by a rating of 1 “very much” or 2 “much” improved on the CGI-I. The pooled relative benefit of most drugs was positive; approximately 30 to 85 percent more patients that received active treatment achieved response than did those that received placebo (Figure 2). The pooled relative benefits for escitalopram (RB 1.31; 95% CI 1.17 to 1.46), paroxetine (RB 1.85; 95% CI 1.49 to 2.29), sertraline (RB 1.78; 95% CI 1.45 to 2.16), and venlafaxine (RB 1.68; 95% CI 1.47 to 1.93) were statistically significantly better than placebo. Tests for publication bias were not significant. Substantial heterogeneity was detected in the meta-analysis of placebo-controlled paroxetine trials (I2 = 82%). We explored whether this heterogeneity was related to a single trial that reported unusually large differences between paroxetine and placebo (Allgulander, 1999). Comparing this trial with other paroxetine trials, the inconsistency may be related to the fact that this trial was relatively small and, on average, patients had less severe anxiety (mean baseline LSAS = 74). As a sensitivity analysis, removing this trial did not significantly change the pooled relative benefit (RB without Allgulander et al. (1999) 1.77; 95% CI 1.62 to 1.92; RB with Allgulander et al. (1999) 1.85; 95% CI 1.49 to 2.29). The I2 statistic remained relatively large after removing this trial (I2 = 80.9%), suggesting additional inconsistencies among trials.
Figure 2.
Clinical global impression of improvement meta-analysis (random effects)
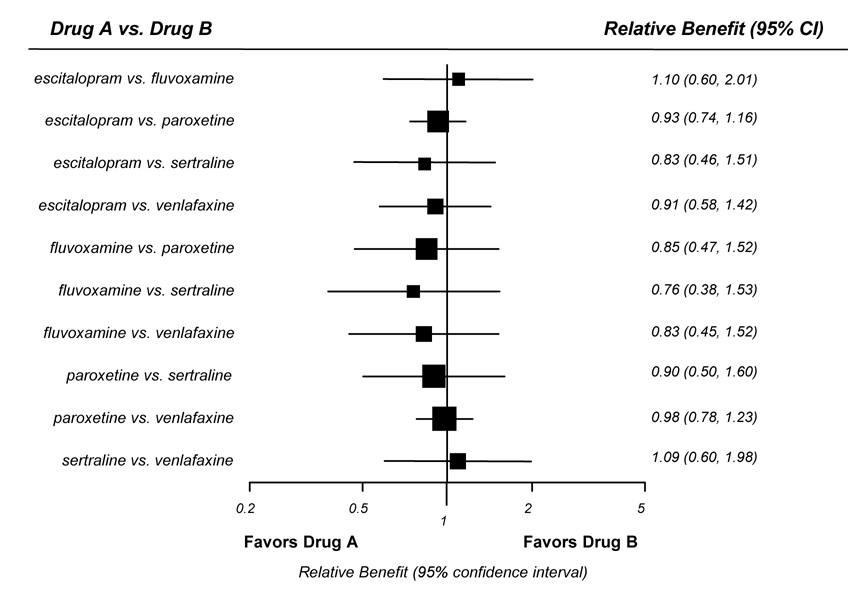
Two trials that directly compared venlafaxine with paroxetine (Allgulander et al., 2004; Liebowitz et al., 2005) and one trial that compared escitalopram with paroxetine (Lader et al., 2004) assessed CGI-I response. The numbers of responders did not differ significantly for either comparison. Our network meta-analysis (Figure 3) also found no significant differences for either of these comparisons or for any of the other possible comparisons among escitalopram, fluvoxamine, paroxetine, sertraline, or venlafaxine. However, confidence intervals for the indirect comparisons were large and likely are not able to detect small but potentially clinically significant differences.
Figure 3.
Indirect comparisons of second-generation antidepressants
* Outcome defined as “much” or “very much” improved on the clinical global impression of improvement scale
Adverse Events
Methods used to assess adverse events and the quality of reporting of specific events differed among studies. In most trials, open-ended questioning was used to elicit adverse events, leading to great variability in the quantity and quality of reporting. The pooled mean incidence and 95% CIs of common adverse events reported in randomized controlled trials are shown in Table 3. The most commonly reported adverse events were nausea, asthenia (i.e., loss of energy or strength) or fatigue, or changes in sleep. In general, the types of adverse events reported among patients with SAD are similar to those reported in patients with other psychiatric diseases such as major depressive disorder (Hansen et al., 2005), although the trends in the incidence of certain adverse events were generally higher in these patients. For instance, nausea was reported by 25% to 39% of patients with social anxiety while the observed rate was between 15% and 31% in patients with major depression. Similarly, as many as 47% of patients with SAD reported insomnia; the observed incidence for patients with major depression disorder was between 10% and 15% (Hansen et al., 2005).
Table 3.
Mean incidence of adverse events across randomized controlled trials in patients with Social Anxiety Disorder
| Drug | Adverse Event Mean Percentage (95% Confidence Interval) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Nausea | Asthenia* | Sweating | Somnolence | Insomnia | Dry Mouth | Abnormal Ejaculation |
Libido Decrease |
|
| Escitalopram | 25 (19–32) | 14 (13–15) | 9 (3–15) | 11 (9–12) | 9 (NA) | NR | 8 (4–12) | 6 (5–7) |
| Fluoxetine | NR | 30 (NA) | NR | NR | 47 (NA) | NR | NR | NR |
| Fluvoxamine | 39 (23–55) | 28 (NA) | NR | 27 (18–35) | 32 (31–33) | NR | 11 (9–13) | 9 (6–11) |
| Paroxetine | 25 (21–29) | 19 (16–21) | 13 (9–17) | 15 (9–21) | 15 (11–18) | 15 (7–24) | 18 (12–25) | 9 (7–11) |
| Sertraline | 27 (17–37) | 18 (17–19) | 11 (10–12) | 11 (NA) | 27 (22–33) | 14 (13–15) | 13 (10–16) | 7 (NA) |
| Venlafaxine | 31 (27–34) | 16 (8–24) | 15 (8–22) | 22 (14–29) | 22 (16–28) | 17 (13–21) | 14 (10–17) | 8 (6–11) |
NR = adverse event was not reported among included studies
NA = not applicable; only 1 trial reported this adverse event
Asthenia = lack of energy or strength
DISCUSSION
Fair evidence suggests that several second-generation antidepressants are effective treatments for patients with SAD. Currently, sertraline, paroxetine, and venlafaxine are approved by the FDA and other regulatory agencies for SAD; limited evidence from our review also supports the efficacy of escitalopram and fluvoxamine. Only one study assessed the efficacy and safety of fluoxetine (Kobak et al., 2002), and no study was identified for bupropion, citalopram, duloxetine, or mirtazapine.
Consistent with the meta-analyses by van der Linden and colleagues (van der Linden et al., 2000) and Hedges and colleagues (Hedges et al., 2007), our review found relatively strong evidence supporting clinical response (i.e., CGI-I) and improvement in social anxiety assessments. Across trials, 40% to 85% of patients randomized to escitalopram, fluvoxamine, paroxetine, sertraline, or venlafaxine were characterized as CGI-I responders. Thus, from the clinician’s perspective, 40% to 85% had a clinically significant improvement.
Similarly, LSAS scores improved by an average of 34 points across trials, representing roughly a 40% improvement. Reductions in LSAS scores of 20% to 30% from baseline have previously been defined as clinically significant (Liebowitz, 2005). Overall, the mean reduction in LSAS scores was roughly 10 to 15 points greater for escitalopram, fluvoxamine, paroxetine, sertraline, and venlafaxine than placebo, representing approximately a 20% greater reduction in LSAS scores than placebo.
Head-to-head trials (Allgulander et al., 2004; Lader et al., 2004; Liebowitz et al., 2005) did not identify significant differences in efficacy for escitalopram compared with paroxetine or venlafaxine compared with paroxetine. Although patients treated with the highest dose of escitalopram (20mg/day) had statistically significantly greater improvements in LSAS total scores than paroxetine-treated patients (20mg/day) in an observed cases analysis, these results were not statistically significant in the ITT analysis (Lader et al., 2004). Our network meta-analysis also found that escitalopram, fluvoxamine, paroxetine, sertraline, and venlafaxine do not differ with regard to clinician’s global assessment of improvement. Thus, given available evidence, these drugs do not appear to differ with regard to efficacy.
Although our intent was not to compare these drugs to other types of pharmacotherapy or cognitive-behavioral therapy, our results can be put in context of such studies. For example, a Cochrane Collaboration review (Stein et al., 2000) concluded that SSRIs were significantly more effective than moclobemide and brofaromine (RIMAs). Fedoroff and Taylor (Fedoroff and Taylor, 2001) found SSRIs and benzodiazepines to be better than psychotherapies in improving various outcome measures.
Our analysis of SDS scores found relatively consistent reduction (i.e., improvements) for the work and social domains for escitalopram, fluvoxamine, paroxetine, sertraline, and venlafaxine. Reductions observed in the family domain of the SDS were marginally significant, although the mean difference in this measure was more inconsistent across trials compared to the work and social domains. This is likely related to the nature of the disease. Patients with SAD generally have little dysfunction in the family domain of function and little room for improvement with treatment. In contrast, SAD symptoms often interfere with a person’s normal routine, occupational functioning, relationships, and social activities. These symptoms are likely to be reflected in the work and social domains of the SDS, but not in the family domain. This point is illustrated by the baseline domain-specific scores across trials included in our analysis; average scores were 3.3 for family, 6.8 for social, and 5.4 for work. Given better baseline scores for the family domain compared to the social and work domains, our finding that the family domain did not consistently improve makes sense.
We observed a general trend toward a higher incidence of certain adverse events among patients with SAD than has been previously reported among patients with major depressive disorder (Hansen et al., 2005). This observation may be spurious, however, because our analysis consisted of fewer component studies than the number of studies analyzed in patients with major depressive disorder. The nature of SAD itself or the patients prone to this disease may well differ from that for patients with major depressive disorder, and this finding needs to be investigated further. Side effects commonly reported in major depressive disorder, such as dizziness, headache, and weight gain, were not consistently assessed or reported in social anxiety trials, so conclusions in this regard are limited.
Although systematic reviews help eliminate some sources of bias, limitations must be considered. First, we are limited by the quantity and quality of evidence available. We found only three fair- or good-rated comparative trials, and placebo-controlled trials were too few in number to conduct reliable indirect comparisons. We used network meta-analysis (Glenny et al., 2005; Lumley, 2002) to pool head-to-head trials with placebo controlled trials so that we could compare different treatments indirectly. To date this method has not been commonly used for making drug-drug comparisons. Additionally, because of the small number of component studies, this analysis is imprecise – as reflected by our wide confidence intervals. Doses and dosing designs varied among studies included in quantitative analyses. All included studies represented a range of doses deemed to be clinically relevant. Among fixed dose studies, we analyzed only the highest dosing arm approved by regulatory authorities. Although this method avoids the risk of including sub-therapeutic doses, it may overestimate the actual treatment effect. In our quantitative analyses, we attempted to assess the degree of publication bias (higher publication rates among studies that show a statistically significant effect of treatment) but these methods are of low statistical power given the limited number of trials published for each drug. Another limitation is incomplete trial reporting and differences in outcome measures utilized in trials. For example, although 14 trials provided data for total LSAS score, only 6 of these trials reported complete data on the fear/anxiety and avoidance subscales. We requested unpublished data from the FDA and manufacturers of included drugs but had to exclude unpublished studies because abstracts or summaries did not provide enough information to allow critical appraisal of the study.
Overall, fair evidence supports statistically significant benefits for escitalopram, fluvoxamine, paroxetine, sertraline, and venlafaxine in patients with SAD. Aside from documented differences in the incidence of specific adverse events, existing evidence does not suggest differences in efficacy for these drugs.
Acknowledgments
Sources of Support
Funding for this research was provided to the Cecil G. Sheps Center for Health Services Research through a sub-contract with the Center for Evidence-Based Policy; Oregon Health & Science University. Dr. Hansen is supported by grant K12RR023248
REFERENCES
- Allgulander C. Paroxetine in social anxiety disorder: a randomized placebo-controlled study. Acta Psychiatr Scand. 1999;100:193–198. doi: 10.1111/j.1600-0447.1999.tb10845.x. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Mangano R, Zhang J, Dahl AA, Lepola U, Sjodin I, et al. Efficacy of Venlafaxine ER in patients with social anxiety disorder: a double-blind, placebo-controlled, parallel-group comparison with paroxetine. Hum Psychopharmacol. 2004;19:387–396. doi: 10.1002/hup.602. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Nilsson B. A prospective study of 86 new patients with social anxiety disorder. Acta Psychiatr Scand. 2001;103:447–452. doi: 10.1034/j.1600-0447.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Baldwin D, Bobes J, Stein DJ, Scharwachter I, Faure M. Paroxetine in social phobia/social anxiety disorder. Randomised, double-blind, placebo-controlled study. Paroxetine Study Group. Br J Psychiatry. 1999;175:120–126. doi: 10.1192/bjp.175.2.120. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Blanco C, Schneier FR, Schmidt A, Blanco-Jerez CR, Marshall RD, Sanchez-Lacay A, et al. Pharmacological treatment of social anxiety disorder: a meta-analysis. Depress Anxiety. 2003;18:29–40. doi: 10.1002/da.10096. [DOI] [PubMed] [Google Scholar]
- Blomhoff S, Haug TT, Hellstrom K, Holme I, Humble M, Madsbu HP, et al. Randomised controlled general practice trial of sertraline, exposure therapy and combined treatment in generalised social phobia. Br J Psychiatry. 2001;179:23–30. doi: 10.1192/bjp.179.1.23. [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. CRD report No. 4. 2nd ed. York, United Kingdom: Centre for Reviews and Dissemination; 2001. [Google Scholar]
- Davidson J, Yaryura-Tobias J, DuPont R, Stallings L, Barbato LM, van der Hoop RG, et al. Fluvoxamine-controlled release formulation for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:118–125. doi: 10.1097/01.jcp.0000106222.36344.96. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry. 2006;67 Suppl 12:20–26. [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Altman DG. Systematic reviews in health care. 2nd edition. London: BMJ; 2001. [Google Scholar]
- Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol. 2001;21:311–324. doi: 10.1097/00004714-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Fehm L, Pelissolo A, Furmark T, Wittchen HU. Size and burden of social phobia in Europe. Eur Neuropsychopharmacol. 2005;15:453–462. doi: 10.1016/j.euroneuro.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Fox DM. Evidence of evidence-based health policy: the politics of systematic reviews in coverage decisions. Health Aff (Millwood) 2005;24:114–122. doi: 10.1377/hlthaff.24.1.114. [DOI] [PubMed] [Google Scholar]
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134. iii–iv. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- Gould RA, Buckminister S, Pollack MH, Otto MW, Yap L. Cognitive-behavioral and pharmacological treatments of social phobia: a meta-analysis. Clin Psychol Science Prac. 1997;4:291–306. [Google Scholar]
- Guy W. ECDEU assessment manual for pscyhopharmacology. Revised DHEW pub. (ADM) Rockville: National Institutes of Mental Health; 1976. [Google Scholar]
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415–426. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- Hedges DW, Brown BL, Shwalb DA, Godfrey K, Larcher AM. The efficacy of selective serotonin reuptake inhibitors in adult social anxiety disorder: a meta-analysis of double-blind, placebo-controlled trials. J Psychopharmacol. 2007;21:102–111. doi: 10.1177/0269881106065102. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Stein DJ, Loft H, Nil R. Escitalopram in the treatment of social anxiety disorder: randomised, placebo-controlled, flexible-dosage study. Br J Psychiatry. 2005;186:222–226. doi: 10.1192/bjp.186.3.222. [DOI] [PubMed] [Google Scholar]
- Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW, Mantle JM, Serlin RC. Sertraline for social phobia: a double-blind, placebo-controlled crossover study. Am J Psychiatry. 1995;152:1368–1371. doi: 10.1176/ajp.152.9.1368. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Greist JH, Jefferson JW, Katzelnick DJ. Fluoxetine in social phobia: a double-blind, placebo-controlled pilot study. J Clin Psychopharmacol. 2002;22:257–262. doi: 10.1097/00004714-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Lader M, Stender K, Burger V, Nil R. Efficacy and tolerability of escitalopram in 12- and 24-week treatment of social anxiety disorder: randomised, double-blind, placebo-controlled, fixed-dose study. Depress Anxiety. 2004;19:241–248. doi: 10.1002/da.20014. [DOI] [PubMed] [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- Lepola U, Bergtholdt B, St Lambert J, Davy KL, Ruggiero L. Controlled-release paroxetine in the treatment of patients with social anxiety disorder. J Clin Psychiatry. 2004;65:222–229. doi: 10.4088/jcp.v65n0213. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Symposium presented at the 25th Annual Meeting of the Anxiety Disorders Association of America; Seattle, Washington. 2005. [Google Scholar]
- Liebowitz MR, DeMartinis NA, Weihs K, Londborg PD, Smith WT, Chung H, et al. Efficacy of sertraline in severe generalized social anxiety disorder: results of a double-blind, placebo-controlled study. J Clin Psychiatry. 2003;64:785–792. doi: 10.4088/jcp.v64n0708. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gelenberg AJ, Munjack D. Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry. 2005;62:190–198. doi: 10.1001/archpsyc.62.2.190. [DOI] [PubMed] [Google Scholar]
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–2324. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- Martins EA, Pigott TA, Bernstein SE, Doyle BB, Sunderland B, Smolka VM, et al. Sertraline in the treatment of patients with social phobia. Anxiety. 1994;1:291–297. doi: 10.1002/anxi.3070010609. [DOI] [PubMed] [Google Scholar]
- Muller JE, Koen L, Seedat S, Stein DJ. Social anxiety disorder: current treatment recommendations. CNS Drugs. 2005;19:377–391. doi: 10.2165/00023210-200519050-00002. [DOI] [PubMed] [Google Scholar]
- Rickels K, Mangano R, Khan A. A double-blind, placebo-controlled study of a flexible dose of venlafaxine ER in adult outpatients with generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:488–496. doi: 10.1097/01.jcp.0000138764.31106.60. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, et al. Functional impairment in social phobia. J Clin Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Balkom AJ. Pharmacotherapy for social phobia. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD001206.pub2. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Van Balkom AJ. Pharmacotherapy for social anxiety disorder. Cochrane Database Syst Rev. 2000 [Google Scholar]
- Stein DJ, Westenberg HG, Yang H, Li D, Barbato LM. Fluvoxamine CR in the long-term treatment of social anxiety disorder: the 12- to 24-week extension phase of a multicentre, randomized, placebo-controlled trial. Int J Neuropsychopharmacol. 2003;6:317–323. doi: 10.1017/S146114570300364X. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fyer AJ, Davidson JR, Pollack MH, Wiita B. Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. Am J Psychiatry. 1999;156:756–760. doi: 10.1176/ajp.156.5.756. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kean YM. Disability and quality of life in social phobia: epidemiologic findings. Am J Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- Stein MB, Liebowitz MR, Lydiard RB, Pitts CD, Bushnell W, Gergel I. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- Stein MB, Pollack MH, Bystritsky A, Kelsey JE, Mangano RM. Efficacy of low and higher dose extended-release venlafaxine in generalized social anxiety disorder: a 6-month randomized controlled trial. Psychopharmacology (Berl) 2005;177:280–288. doi: 10.1007/s00213-004-1957-9. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C. Pharmacotherapy of social anxiety disorder at the turn of the millennium. Psychiatr Clin North Am. 2001;24:783–803. doi: 10.1016/s0193-953x(05)70263-x. [DOI] [PubMed] [Google Scholar]
- Van Ameringen MA, Lane RM, Walker JR, Bowen RC, Chokka PR, Goldner EM, et al. Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:275–281. doi: 10.1176/appi.ajp.158.2.275. [DOI] [PubMed] [Google Scholar]
- van der Linden GJ, Stein DJ, van Balkom AJ. The efficacy of the selective serotonin reuptake inhibitors for social anxiety disorder (social phobia): a meta-analysis of randomized controlled trials. Int Clin Psychopharmacol. 2000;15 Suppl 2:S15–S23. doi: 10.1097/00004850-200008002-00004. [DOI] [PubMed] [Google Scholar]
- van Vliet IM, den Boer JA, Westenberg HG. Psychopharmacological treatment of social phobia; a double blind placebo controlled study with fluvoxamine. Psychopharmacology (Berl) 1994;115:128–134. doi: 10.1007/BF02244762. [DOI] [PubMed] [Google Scholar]
- Versiani M. A review of 19 double-blind placebo-controlled studies in social anxiety disorder (social phobia) World J Biol Psychiatry. 2000;1:27–33. doi: 10.3109/15622970009150563. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61:1153–1162. doi: 10.1001/archpsyc.61.11.1153. [DOI] [PubMed] [Google Scholar]
- Westenberg HG, Liebowitz MR. Overview of panic and social anxiety disorders. J Clin Psychiatry. 2004;65 Suppl 14:22–26. [PubMed] [Google Scholar]
- Westenberg HG, Stein DJ, Yang H, Li D, Barbato LM. A double-blind placebo-controlled study of controlled release fluvoxamine for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:49–55. doi: 10.1097/01.jcp.0000104906.75206.8b. [DOI] [PubMed] [Google Scholar]