EMBO J 28, 513–522 (2009); published online 1 March 2009
The ubiquitin-editing protein A20 limits the duration of NF-κB activation and is essential to control inflammatory responses. Recent experiments have established that A20 mediates its inhibitory function in a complex with two other proteins, TAX1BP1 and the E3 ligase Itch. In a paper published in this issue, it is shown that RNF11 is also a critical component of the A20 ubiquitin-editing complex.
NF-κB transcription factors regulate the expression of numerous genes involved in inflammation and immunity (Li and Verma, 2002). Activation of NF-κB is normally transient, and persistent NF-κB activation is associated with several autoimmune diseases and cancer. The duration of NF-κB activation is controlled by a number of inhibitory proteins, including A20, which functions in a negative feedback loop to limit NF-κB activation. A20 is essential for immune homeostasis, and A20−/− (Tnfaip3−/−) mice die prematurely from multi-organ inflammation caused by deregulated Toll-like receptor (TLR) signalling in response to commensal intestinal bacteria (Lee et al, 2000; Turer et al, 2008). Significantly, A20 has been identified as a disease-associated gene in rheumatoid arthritis, systemic lupus erythomatosus, Crohn's disease and coronary artery disease (Coornaert et al, 2008).
A20 is a deubiquitinating enzyme of the ovarian tumour (OTU) family and downregulates NF-κB activation by removing K63-linked ubiquitin chains from key target proteins (Coornaert et al, 2008). K63-linked ubiquitination, mediated by the tumor necrosis factor (TNF) receptor-associated factor (TRAF) protein family of E3 ligases, regulates interactions between signalling proteins required for receptor activation of inhibitor of κB (IκB) kinase (IKK). This kinase complex triggers IκB proteolysis, releasing NF-κB dimers to translocate into the nucleus and induce transcription of target genes, including A20 (Figure 1). A20 inhibition of TNF receptor 1 (TNFR1) signalling to NF-κB involves its removal of K63-linked ubiquitin chains from receptor-interacting protein (RIP) 1, an essential adaptor for NF-κB activation by TNFR1. A20 removal of K63-linked ubiquitin from TRAF6 and RIP2, similarly turns off activation of NF-κB by TLR4 and nucleotide-binding oligomerization domain containing-2 (NOD2), respectively.
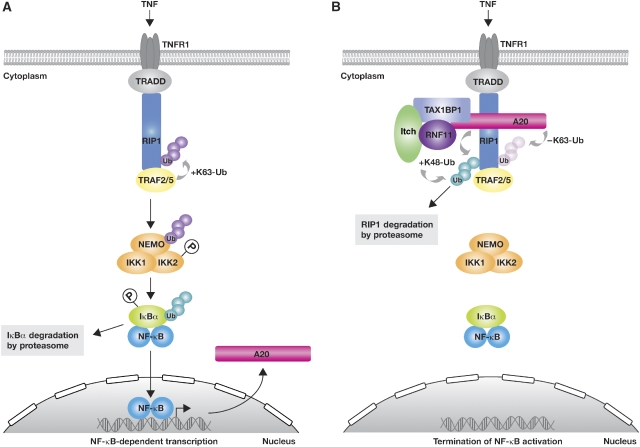
Figure 1.
Regulation of TNFR1 signalling by ubiquitination. (A) TNF stimulation induces binding of a TRADD–RIP1–TRAF2–TRAF5 complex to TNFR1. The TRAF proteins then catalyse the K63-linked polyubiquitination of RIP1, inducing RIP1 association with the IKK complex (IKK1/IKK2/NEMO), which is subsequently activated by a process that involves both its K63-linked ubiquitination and phosphorylation. IKK phosphorylates IκBα, triggering K48-linked polyubiquitination of IκBα, which induces its subsequent degradation by the proteasome. As a result, associated NF-κB dimers are freed to translocate into the nucleus and activate the transcription of NF-κB target genes, including A20. (B) The A20 ubiquitin-editing complex is critical to ensure that NF-κB activation induced by TNF is transient. This complex, comprising A20, TAX1BP1, Itch and RNF11, catalyses the removal of K63-linked ubiquitin chains from RIP1, utilizing the deubiquitinating activity of A20. Subsequently, A20 and Itch catalyse addition of K48-linked ubiquitin chains of RIP1, promoting its degradation by the proteasome and termination of NF-κB activation. K63-linked ubiquitin chains, purple; K48-linked ubiquitin chains, turquoise; P, phosphorylation; Ub, ubiquitin; +Ub, ubiquitination; −Ub, deubiquitination.
Negative regulation of NF-κB signalling by A20 requires two other proteins with which it associates, Tax1-binding protein 1 (TAX1BP1) and Itch (Heissmeyer and Rao, 2008). TAX1BP1 (also known as T6BP and TXBP151) functions as an adaptor protein recruiting A20 to its substrates, K63-ubiquitinated RIP1 and TRAF6, through a novel ubiquitin-binding domain (Shembade et al, 2007; Iha et al, 2008). TAX1BP1 also binds to the WW domain of HECT (homologous to E6-AP carboxyl terminus) domain E3 ligase Itch through its two conserved PPXY motifs (Pro-Pro-X-Tyr, where X is any amino acid), recruiting Itch to A20 in TNF-stimulated fibroblasts (Shembade et al, 2008). Both TAX1BP1 and Itch are required for the association of RIP1 with A20, the deubiquitination of RIP1 and TRAF6 by A20, and termination of NF-κB and Jun N-terminal kinase (JNK) activation after TNF stimulation of fibroblasts. TAX1BP1 and Itch, therefore, facilitate the targeting and deubiquitination of substrates by A20.
Removal of K63-linked ubiquitin chains by A20 is not sufficient to turn off the signalling activity of RIP1. This requires RIP1 degradation by the proteasome, induced by its K48-linked ubiquitination following prolonged TNF stimulation. K48-linked ubiquitination of RIP1 is mediated by A20, functioning as a ‘ubiquitin-editing' E3 ligase, and Itch. The K48-linked ubiquitin E3 ligase activities of both A20 and Itch are required to downregulate NF-κB activation by TNF. However it is not known why two K48-ubiquitin E3 ligases are needed to regulate RIP1 proteolysis, or whether K48-linked ubiquitination of TRAF6 and RIP2 is also required for A20 to block their activation of NF-κB.
The present study by Harhaj and colleagues shows that ring finger protein 11 (RNF11), which may also function as an E3 ligase, is another essential component of the A20 ubiquitin-editing complex (Shembade et al, 2009). RNF11 was identified as a gene overexpressed in breast cancer and has been implicated in tumour growth factor beta signalling (Azmi and Seth, 2005). The potential link with A20 was suggested in an earlier two-hybrid screen, which identified A20, TAX1BP1 and Itch as potential RNF11-binding partners (Colland et al, 2004). Shembade et al now show that endogenous RNF11 interacts with A20, TAX1BP1 and RIP1 in a TNF-dependent manner in 293T cells and primary murine macrophages. They also demonstrate that TNF-induced NF-κB and JNK activation in human THP-1 monocytes is prolonged after RNF11 knockdown, due to increased RIP1 and TRAF6 polyubiquitination. Furthermore, immunoprecipitation experiments indicate that RNF11 is required for A20 association with RIP1 after TNF stimulation. A PPXY motif of RNF11 is needed both to terminate NF-κB signalling and for complexing with A20, TAX1BP1 and RIP1. The PPXY motif could bind directly to the Itch WW domain, and one of the functions of RNF11 may be to facilitate Itch ubiquitination of specific target proteins, such as RIP1.
Earlier studies have clearly highlighted the role of associated proteins in mediating A20's inhibitory function in NF-κB signalling in fibroblasts (Shembade et al, 2007, 2008; Iha et al, 2008). Together with the present work from the Harhaj laboratory (Shembade et al, 2009), these genetic and immunoprecipitation experiments suggest that negative regulation of NF-κB is mediated by a quaternary A20–TAX1BP1–Itch–RNF11 complex. However, the role of TAX1BP1 in mediating A20's inhibitory function may be restricted to certain tissues, as Tax1bp1−/− mice develop inflammatory cardiac valvulitis (Iha et al, 2008), whereas inflammation is widespread in A20-deficient mice (Lee et al, 2000). Clearly, the generation and analysis of Rnf11−/− mice will be essential to determine the physiological roles of RNF11 in inflammatory responses, and whether RNF11 regulation of A20 function is tissue or stimulus specific. Such analyses will help to establish whether RNF11 is the last piece in the A20 ubiquitin-editing complex puzzle, or whether more remain to be discovered.
References
- Azmi P, Seth A (2005) RNF11 is a multifunctional modulator of growth factor receptor signalling and transcription regulation. Eur J Cancer 41: 2549–2560 [DOI] [PubMed] [Google Scholar]
- Colland F, Jacq X, Trouplin V, Mougin C, Groizeleau C, Hamburger A, Meil A, Wojcik J, Legrain P, Gauthier JM (2004) Functional proteomics mapping of a human signaling pathway. Genome Res 14: 1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert B, Carpentier I, Beyaert R (2008) A20: central gatekeeper in inflammation and immunity. J Biol Chem (e-pub ahead of print; doi:10.1074/jbc.R800032200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissmeyer V, Rao A (2008) Itching to end NF-κB. Nat Immunol 9: 227–229 [DOI] [PubMed] [Google Scholar]
- Iha H, Peloponese J-M, Verstrepen L, Zapart G, Ikeda F, Smith CD, Starost MF, Yedavalli V, Heyninck K, Dikic I, Beyaert R, Jeang K-T (2008) Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-κB activation. EMBO J 27: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A (2000) Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289: 2350–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2: 725–734 [DOI] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Liebl DJ, Harhaj EW (2007) Essential role for TAX1BP1 in the termination of TNF-α, IL-1- and LPS-mediated NF-κB and JNK signaling. EMBO J 26: 3910–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW (2008) The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol 9: 254–262 [DOI] [PubMed] [Google Scholar]
- Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW (2009) The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-κB signalling. EMBO J 28: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A (2008) Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J Exp Med 205: 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]