Abstract
In metazoans, nuclear export of bulk mRNA is mediated by Tap-p15, a conserved heterodimeric export receptor that cooperates with adaptor RNA-binding proteins. In this article, we show that Thoc5, a subunit of the mammalian TREX complex, binds to a distinct surface on the middle (Ntf2-like) domain of Tap. Notably, adaptor protein Aly and Thoc5 can simultaneously bind to non-overlapping binding sites on Tap-p15. In vivo, Thoc5 was not required for bulk mRNA export. However, nuclear export of HSP70 mRNA depends on both Thoc5 and Aly. Consistent with a function as a specific export adaptor, Thoc5 exhibits in vitro RNA-binding activity and is associated with HSP70 mRNPs in vivo as a component of the stable THO complex. Thus, through the combinatorial use of an adaptor (e.g., Aly) and co-adapter (e.g., Thoc5), Tap-p15 could function as an export receptor for different classes of mRNAs.
Keywords: heat shock mRNA, nucleo-cytoplasmic transport, TREX complex
Introduction
Eukaryotic cells are organized in distinct subcellular compartments, each of which is surrounded by a specific organellar membrane. The nuclear membrane separates the nucleus from the cytoplasm, but nuclear pore complexes (NPCs) perforate the nuclear envelope to allow for nucleo-cytoplasmic transport between these two compartments. A huge variety of cargoes (proteins, RNAs, RNPs) is transported through the NPCs by binding to specific nucleo-cytoplasmic transport receptors, which decode the targeting signals (NLS or NES) in the various transport cargoes. Many of these receptors belong to the importin-β/karyopherin family of transport factors, which require the small GTPase Ran for the directionality of nucleo-cytoplasmic transport (for recent reviews, see Kohler and Hurt, 2007; Terry and Wente, 2007).
Nuclear export of mRNA is unique in the sense that the shuttling export receptor is not a member of the importin-β family and does not directly depend on RanGTP. However, the mRNA export receptor is evolutionarily conserved and is called Mex67-Mtr2 in the budding yeast Saccharomyces cerevisiae and Tap-p15 (or NXF1-NXT1) in metazoans (Segref et al, 1997; Gruter et al, 1998; Santos-Rosa et al, 1998; Kang and Cullen, 1999; Katahira et al, 1999; Herold et al, 2000). Although the transporters exhibit RNA-binding activity by themselves in vitro (Santos-Rosa et al, 1998; Katahira et al, 1999; Liker et al, 2000), they predominantly select their mRNA cargoes through interactions with adaptor RNA-binding proteins. Tap exhibits a modular domain organization, in which the region including the amino terminal (N-) and leucine-rich repeat (LRR-) domains, and the middle (M-) and carboxyl terminal (C-) domains, are assigned as binding sites of adaptor RNA-binding proteins and FG-nucleoporins, respectively (see Figure 1A) (Katahira et al, 1999; Bachi et al, 2000; Strasser et al, 2000; Stutz et al, 2000; Fribourg et al, 2001; Huang et al, 2003).
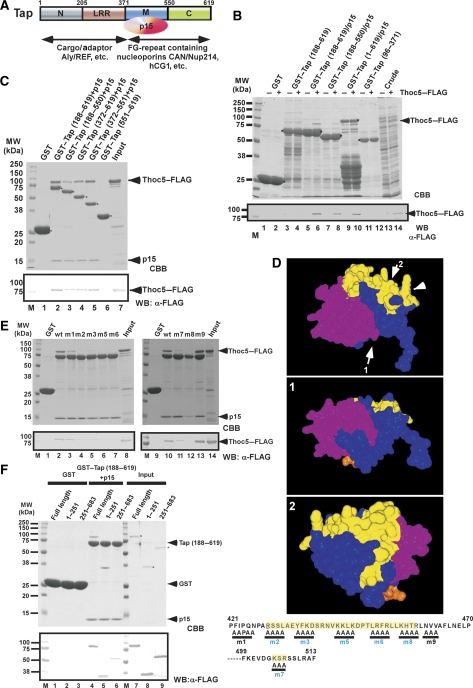
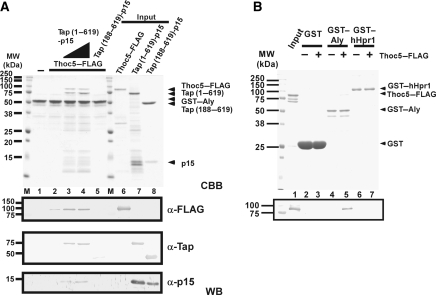
Figure 1.
Thoc5 is a novel interaction counterpart of Tap. (A) Domain organization and the known interaction counterparts of the Tap-p15 heterodimer. Numbers on each rectangle indicate amino-acid positions of human Tap (for details, see Segref et al, 1997; Liker et al, 2000; Rodrigues et al, 2001; Reed and Hurt, 2002). (B) Crude lysates of E. coli with (indicated by +) or without (indicated by −) Thoc5–FLAG expression were added to GSH beads pre-adsorbed to GST or GST fused to various fragments of Tap. Bound proteins were analysed by SDS–PAGE followed by CBB staining (upper panel) and western blot using anti-FLAG antibody (lower panel). In lanes 13 and 14, aliquots of 10% of each input were loaded. Arrowheads indicate the positions of Thoc5–FLAG. Positions of molecular weight markers are indicated on the left in kDa. Note that in this particular gel, p15 migrated to the dye front. (C) Purified Thoc5–FLAG was added to GSH beads pre-adsorbed to GST (lane 1) or GST fused to various fragments of Tap (lanes 2–6). Aliquots of 25% of each bound fraction were separated by SDS—PAGE, and protein bands were detected by CBB staining (upper panel) and western blot using anti-FLAG antibody (lower panel). In lane 7, a total of 10% of input was loaded. Arrowheads indicate the positions of p15 and Thoc5–FLAG, whereas asterisks indicate the positions of each GST-fusion protein. Positions of molecular weight markers are indicated on the left in kDa. (D) Surface representation showing the Ntf2-like domain of Tap (blue) complexed with p15 (magenta) and FG-containing peptide (orange) (Fribourg et al, 2001). Different surfaces are also viewed from orientations indicated by the arrows in the upper-most figure. The regions of Tap that are critical for Thoc5 binding are coloured in yellow. An arrowhead indicates the short loop (aa 505–507) of Tap. The positions of alanine-scan mutations are indicated at the bottom of the figures as a single-letter code. The numbers on top of the sequence indicate the amino-acid positions of Tap. The residues shaded in yellow correspond to the region coloured in yellow in the 3D model. (E) Same as in (C), but Tap (188–619) containing the alanine-scan mutations complexed with p15 were used. Arrowheads show the positions of p15 and Thoc5–FLAG on the gel. Positions of molecular weight markers are indicated on the left in kDa. (F) Full-length and different domains (aa 1–251 and 251–683) of Thoc5 were expressed in E. coli as His6- and FLAG double-tagged proteins. Purified proteins (5% of input, lanes 7–9) were pulled down by GST (lanes 1–3) or GST-Tap (188-619)-p15 (lanes 4–6). Proteins in bound fractions (25%) were analysed by SDS–PAGE followed by CBB staining (upper) and western blot using anti-FLAG antibody (lower). The positions of Thoc5–FLAG and its derivative are indicated by asterisks, whereas those of Tap (188–619), GST and p15 are indicated by arrowheads. Positions of molecular weight markers are shown on the left in kDa.
The evolutionarily conserved TREX complex is required for coupled transcription elongation and nuclear export of mRNAs, and provides an example of an mRNA-specific adaptor (Reed and Hurt, 2002; Aguilera, 2005; Reed and Cheng, 2005; Kohler and Hurt, 2007). The yeast complex is composed of the THO transcription elongation complex (Hpr1, Tho2, Mft1 and Thp2), Tex1, Sub2 and Yra1 (Strasser et al, 2002). The complex is loaded onto mRNAs co-transcriptionally, facilitating assembly of nascent transcripts into export competent mRNPs. Yra1, which is recruited onto mRNAs through interaction with the DEAD-box type RNA helicase Sub2, physically interacts with Mex67-Mtr2 and functions as an adaptor (Strasser and Hurt, 2000, 2001; Abruzzi et al, 2004). Accordingly, yeast TREX mutants show a nuclear export defect for bulk poly(A)+ RNAs and are synthetically lethal with many mutants of the mRNA export machinery (Strasser et al, 2002).
An equivalent complex exists in metazoans (Reed and Hurt, 2002; Reed and Cheng, 2005; Kohler and Hurt, 2007). The human TREX complex is composed of the hTHO subcomplex (hHpr1, Thoc2, Thoc5, Thoc6 and Thoc7), TEX1, UAP56 and Aly. A related complex has also been identified in the fruit fly Drosophila melanogaster (Strasser et al, 2002; Rehwinkel et al, 2004; Masuda et al, 2005; Reed and Cheng, 2005). Aly, similar to its yeast orthologue Yra1 (Strasser and Hurt, 2000), is recruited to mRNA through the interaction with UAP56 (the Sub2 orthologue) and directly interacts with Tap-p15. In Xenopus oocytes, Aly was shown to be a limiting factor for nuclear export of mRNAs (Stutz et al, 2000; Zhou et al, 2000; Luo et al, 2001; Rodrigues et al, 2001). Despite these structural and functional conservations, gene knockdown experiments performed with cultured fruit fly cells have shown that only the UAP56 orthologue, but not the other THO/TREX components (including Drosophila Aly), is essential for bulk poly(A)+ RNA export (Gatfield et al, 2001; Herold et al, 2001; Gatfield and Izaurralde, 2002; Farny et al, 2008). It has also been shown that nuclear export of only a subset of mRNAs is affected by depletion of the TREX components (Rehwinkel et al, 2004; Farny et al, 2008). These data suggest that various nuclear mRNA export pathways, which may be dictated by different adaptor RNA-binding proteins, exist in higher eukaryotes.
In this study, we show that the human TREX component Thoc5 binds directly to the middle domain of Tap, which exhibits an Ntf2-like fold. Our data further indicate that Thoc5, along with the other THO components, although not required for bulk mRNA export, is crucial for nuclear export of a specific mRNA (HSP70) in conjunction with the adaptor protein Aly. In vitro, Thoc5 exhibits RNA-binding activity and can shuttle between the nucleus and cytoplasm. Biochemical studies showed that Thoc5 binds to the Ntf2-like domain of Tap, whereas Aly is recruited to the N- and LRR-domain of Tap. Together, these findings suggest that Thoc5 functions in the nuclear export of HSP70 mRNA as a co-adaptor in close overlap with the general adaptor protein Aly. Thus, by the recruitment of an adaptor (Aly) and co-adaptor (Thoc5) to non-overlapping binding sites, Tap-p15 could be involved in the nuclear export of different classes of mRNAs.
Results
The TREX component Thoc5 binds to the Ntf2-like (middle) domain of the Tap-p15 heterodimer
Several interacting proteins of the Tap mRNA export receptor have been identified in yeast two-hybrid screens, including FG-nucleoporins and hCG1 (Katahira et al, 1999). Notably, one of the functionally unknown Tap-interacting factors found in these screens turned out to be Thoc5, a component of the human TREX complex (this protein was previously designated as ‘anonymous gene product' (Xle et al, 1993). The insert of the two-hybrid prey plasmid interacting with the Tap bait protein contained a sequence of human Thoc5 that encompassed amino acids 164–683. Further two-hybrid analyses suggested that the M-domain of Tap, which has an Ntf2-like fold, interacts with Thoc5 (Supplementary Figure S1).
To show a direct interaction between Tap and Thoc5, both proteins were expressed in Escherichia coli and GST pull-down assays were performed. The full-length Tap-p15 heterodimer, as well as fragments containing the middle (M) domain, effectively enriched Thoc5 from E. coli whole cell lysates (Figure 1B, lanes 6, 8, 10), whereas a fragment consisting of the LRR- and part of the N-domain (residues 96-371) known to be involved in adaptor binding (e.g., Aly, 9G8 or SRP20; Rodrigues et al, 2001; Huang et al, 2004) did not interact with Thoc5 (Figure 1B, lane 12; for domain organization, see Figure 1A). Further truncation analyses showed that the Ntf2-like domain of Tap (residues 372–551) bound to p15 is necessary and sufficient for binding to Thoc5 (Figure 1C, lanes 2–5).
The M-domain of Tap contains hydrophobic patches on the surface, which bind the phenylalanine residues that are part of the FG repeats (Fribourg et al, 2001). To examine whether the binding site of Thoc5 on the Ntf2-like domain of Tap overlaps with the FG-binding sites, a series of alanine-scan mutations were generated in the M-domain of Tap (Figure 1D). The alanine substitutions (m2–m7), which mapped to the surface of Tap-p15 that is opposite the FG-repeat binding site (Fribourg et al, 2001; Yao et al, 2007) (see Figure 1D for 3D structure), effectively inhibited binding to Thoc5, but FG repeat binding was unaffected (Figure 1E, lanes 4–7, 11 and 12; and data not shown). These data suggest that Thoc5 is recruited to the Ntf2-like domain of Tap-p15 and not to a region on Tap, which binds to FG repeats of nucleoporins. Notably, the way Thoc5 binds to a specific region on the Ntf2-like domain of Tap is reminiscent of the binding of pre-ribosomal particles and the Nup84p complex to a related surface on the homologous Mex67-Mtr2 heterodimer (Yao et al, 2007, 2008).
To determine the region in Thoc5 that binds to Tap-p15, bacterially expressed N- (1–251) and C-terminal (251–683) parts of Thoc5 were subjected to GST pull-down assays. Thoc5 (1–251), but not Thoc5 (251–683), showed a robust interaction with Tap-p15, which was comparable with the binding of full-length Thoc5 (Figure 1F, lanes 4 and 5). The N-terminal domain of Thoc5 is composed of a series of predicted α-helices, which by homology modelling are thought to form an α-solenoid structure (Supplementary Figures S2A and S2B). Such a structure may be crucial for interaction with Tap-p15 (see Discussion).
Thoc5 and Aly are required for HSP70 mRNA export in mammalian cells
To further characterize Thoc5 in mammalian cells, a polyclonal antibody was raised against human Thoc5, which recognized Thoc5 on western blots (Supplementary Figure S3A, lane 2) and in HeLa cells by indirect immunofluorescence. As anticipated, Thoc5 was found concentrated in the splicing factor-rich nuclear compartment that contains the SC35 marker protein as well as Aly and hHpr1 (Supplementary Figure S3B, upper panels) (Zhou et al, 2000; Masuda et al, 2005).
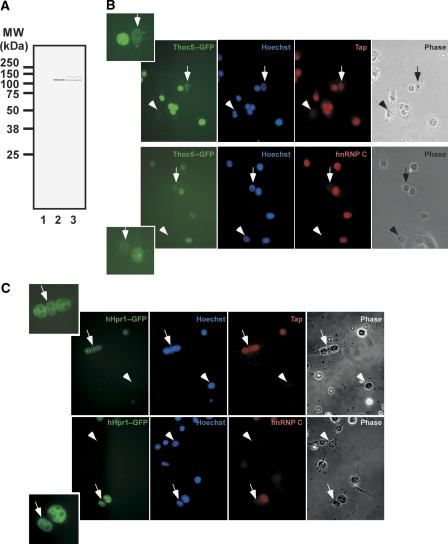
To find out whether Thoc5 can shuttle between the nucleus and the cytoplasm, we performed an inter-species heterokaryon assay using a mouse L929 cell line stably expressing Thoc5–GFP (Figure 2A, lane 2). hnRNP C, a non-shuttling protein, and human Tap were used as controls to show shuttling between the mouse and Xenopus nucleus (Figure 2B). Apparently, Thoc5–GFP, similar to Tap or Aly (Katahira et al, 1999; Zhou et al, 2000; Rodrigues et al, 2001), can shuttle in the heterokaryon assay. As shown in Figure 2C, hHpr1–GFP also shuttles, suggesting that Thoc5 may rapidly shuttle in and out of the nucleus as part of the THO–TREX complex.
Figure 2.
Thoc5 shuttles between the nucleus and the cytoplasm. (A) Total cell extracts prepared from parental L929 cells (lane 1) or L929 cell lines stably expressing Thoc5–GFP (lane 2) and hHpr1–GFP (lane 3) were subjected to western blot using anti-GFP antibody. Positions of molecular weight markers are indicated on the left in kDa. (B, C) Heterokaryon formation was performed using Xenopus A6 cells and the L929 cell lines stably expressing Thoc5–GFP (B) or hHpr1–GFP (C) in the presence of CHX. After incubation for 3 h at 30°C in the presence of CHX, the cells were fixed and immunostained with anti-Tap (shuttling; upper panels) and anti-hnRNP C (non-shuttling; lower panels) antibodies. Nuclei were stained with Hoechst 33342 dye. Arrows indicate Xenopus nuclei in fused cells, whereas arrowheads indicate those in unfused cells. Insets show magnified views of the GFP signals in the fused cells.
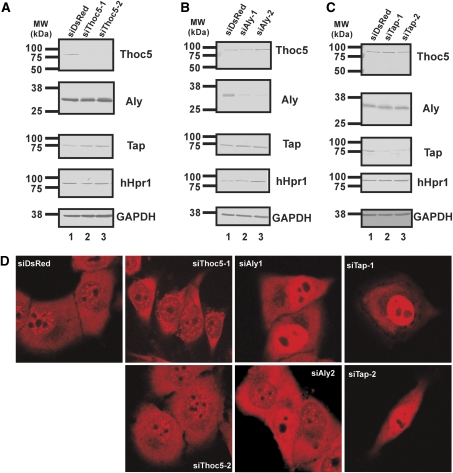
To examine whether Thoc5 has an important function in mRNA export, siRNA knockdown experiments were performed. Expression of Thoc5, Aly and Tap was efficiently blocked by siRNA transfection (Figures 3A–C). However, depletion of Thoc5 did not affect nuclear export of poly(A)+ RNAs, which was examined by in situ hybridization using Cy-3 labelled oligo-dT probes. In contrast, depletion of Tap-p15 resulted in a strong nuclear accumulation of poly(A)+ RNA. Upon depletion of Aly, ∼70% (in the case of siAly-1) and ∼20% (in the case of siAly-2), respectively, of the siRNA transfected cells exhibited a strong nuclear accumulation of poly(A)+ RNA (Figure 3D). These data indicate that in cultured mammalian cells, Tap-p15 and Aly, but not Thoc5, has a crucial function in nuclear export of poly(A)+ RNA.
Figure 3.
Depletion of Thoc5 does not affect bulk poly(A)+ RNA export in mammalian cells. (A) HeLa cells were treated with the indicated siRNAs for 72 h. Total cell extracts were subjected to western blot using the indicated antibodies. For a negative control, siRNA against DsRed protein was used. Positions of molecular weight markers are indicated on the left in kDa. (B) HeLa cells were treated with the indicated siRNAs for 72 h. Total cell extracts were subjected to western blot using the indicated antibodies. For a negative control, siRNA against DsRed protein was used. Positions of molecular weight markers are indicated on the left in kDa. (C) HeLa cells were treated with the indicated siRNAs for 45 h. Total cell extracts prepared from the cells were subjected to western blot using the indicated antibodies. For a negative control, siRNA against DsRed protein was used. Positions of molecular weight markers are indicated on the left in kDa. (D) HeLa cells grown on glass-bottomed dishes were treated with the indicated siRNAs for either 45 h (siRNAs against Tap) or 72 h (others). The cells were fixed and subjected to in situ hybridization using Cy3-labelled oligo-dT50 probe.
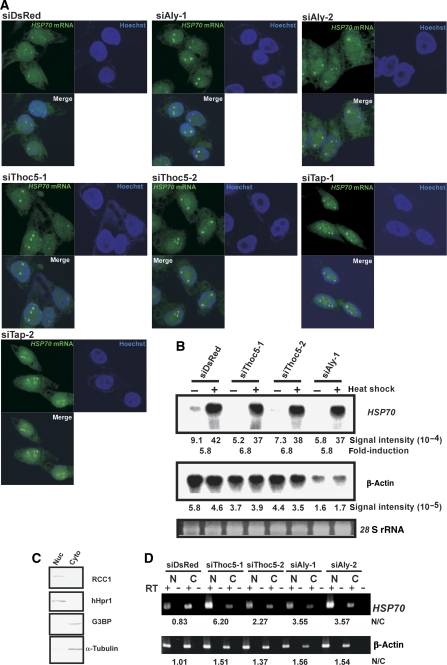
Previously, it was reported that the Drosophila THO–TREX complex is required for nuclear export of heat-shock mRNAs but is dispensable for nuclear export of bulk poly(A)+ RNA (Rehwinkel et al, 2004). Therefore, we tested whether Thoc5 is required for the export of HSP70 mRNA. HSP70 mRNA was induced in cells upon heat shock and detected in a few nuclear foci by in situ hybridization, which were shown to be transcription sites adjacent to the heat-shock genes (Jolly et al, 1997, 1999). Our FITC-labelled oligonucleotide probes allowed specific detection of HSP70 mRNAs in nuclear foci after heat shock (Supplementary Figures S4A and B). Notably, upon depletion of Thoc5, a robust increase in signal intensity of the nuclear HSP70 mRNA-containing foci was observed (Figure 4A, see also Supplementary Figure S5; 94 and 87% of cells treated with siThoc5-1 and siThoc5-2, respectively, showed larger nuclear foci). A similar increase in signal intensity of the nuclear foci was observed when the expression of Tap was inhibited by RNAi. Depletion of Aly with the two different siRNAs also resulted in enlargement of nuclear HSP70 mRNA containing foci in almost all the cells (Figure 4A; 89 and 94% of cells treated with siAly-1 and siAly-2, respectively, showed enlarged nuclear foci). This was in contrast to the effect of Aly depletion on bulk poly(A)+ RNA export. The nuclear foci were not observed in siRNA-treated cells under unstressed condition (Supplementary Figure S4C). This excludes a possibility that the nuclear foci are caused by the RNAi treatment. Moreover, we did not observe nuclear dot-like accumulation of β-actin transcripts upon siRNA treatment (Supplementary Figures S4D and E). As shown by northern blot analysis, the degree of induction of HSP70 mRNA expression under heat stress was similar in each of the siRNA-transfected cells, whereas β-actin mRNA expression was severely impaired in Aly-depleted cells (Figure 4B, middle panel). These findings indicate that Thoc5 and Aly have a minor function in the transcription of HSP70 mRNA and that HSP70 mRNA retained in close proximity to the gene loci does not undergo significant degradation. A strong increase in nuclear HSP70 mRNA levels was also observed by cell fractionation (see also Figure 4C for efficiency of fractionation procedure) followed by RT–PCR, when expression of either Thoc5 or Aly was repressed (Figure 4D upper), whereas distribution of β-actin mRNA was affected to a much lesser extent (Figure 4D lower) as expected from the results of in situ hybridization. Taken together, these data indicate that Thoc5 and Aly are required for efficient nuclear export of HSP70 mRNAs in mammalian cells.
Figure 4.
HSP70 mRNA export requires both Thoc5 and Aly. (A) siRNAs against DsRed (siDsRed: negative control), Aly (siAly-1 and -2), Thoc5 (siThoc5-1 and -2) or Tap (siTap-1 and -2) were transfected to HeLa cells. At 48 h (for siTap-1 and -2) or 72 h (for other siRNAs) post-transfection, the cells were subjected to heat shock at 43°C for 1 h, and in situ hybridization using FITC-labelled human HSP70 oligonucleotide probes was performed. (B) HeLa cells treated with indicated siRNAs for 72 h were left untreated (indicated by −) or temperature shifted at 43°C for 1 h (indicated by +). Total RNAs (5 μg per lane) prepared from the cells were subjected to northern blot analysis using [32P]-labelled HSP70 and β-actin cDNA probes. 28 S rRNA detected by ethidium bromide staining served as loading control. (C) HeLa cells were fractionated into nuclear (left) and cytoplasmic (right) fractions. Each fraction was subjected to SDS–PAGE followed by western blot using anti-RCC1 and anti-hHpr1 (nuclear markers) or anti-G3BP and anti-α-tubulin (cytoplasmic markers) antibodies. (D) HeLa cells treated with the indicated siRNAs for 72 h were temperature-shifted at 43°C for 1 h. Total RNA was isolated from nuclear (N) and cytoplasmic (C) fractions separated as in (C). HSP70 and β-actin mRNAs in each fraction was detected by RT–PCR using specific primers. Relative nuclear signals (N/C) are shown at the bottom of the panels.
Thoc5 and Aly bind concomitantly to the Tap-p15 heterodimer
The data indicate that Tap-p15, Thoc5 and Aly are required for nuclear export of HSP70 mRNA in mammalian cells. Although Thoc5 and Aly bind to different domains within Tap-p15, it is possible that they bind concomitantly to the mRNA export receptor. To find out whether these different factors can assemble into a complex, Thoc5 was pre-incubated with increasing amounts of Tap-p15 and added to GST-Aly immobilized on GSH beads. When equimolar amounts of Tap-p15 and Thoc5 were added to GST–Aly, a stoichiometric complex was formed between Thoc5, Aly and Tap-p15 (Figure 5A, lanes 3 and 4). In the absence of Tap-p15, much weaker binding of Thoc5 to GST–Aly, but not GST–hHpr1 or GST, was observed (Figure 5A, lane 2; Figure 5B, lane 3, 5, 7). When a stoichiometric amount of Thoc5 was pre-incubated with a mutant Tap-p15 that lacked a part of the N-domain of Tap (Tap188–619) and was then added to GST–Aly, neither Thoc5 nor Tap-p15 was bound to immobilized GST–Aly (Figure 5A, lane 5). The decrease in binding of Thoc5 to GST–Aly in the presence of the Tap mutant was probably due to competition between ‘free' versus Tap-p15-bound Thoc5 for binding to GST–Aly. Thus, Aly and Thoc5 most probably do not have direct contact in the hetero-tetrameric complex. These data suggest that a hetero-tetrameric complex was formed, in which Thoc5 was bound to the M-domain and Aly to the N- and LRR-domain of Tap. Taken together, these biochemical data imply that Thoc5 and Aly can simultaneously bind to the Tap-p15 export receptor.
Figure 5.
Tap-p15, Thoc5 and Aly constitute a hetero-tetramer. (A) Purified Thoc5–FLAG (25 μg) along with increasing amounts of full-length Tap-p15 (lanes 2–4; 0, 8, 16 μg) was incubated with GSH-beads pre-adsorbed to purified GST-Aly (10 μg). In lane 5, Tap (188–619)-p15 (20 μg) was used instead of full-length Tap-p15. The beads were washed and aliquots of the bound fractions (25%) were analysed by SDS–PAGE followed by CBB staining. In lane 1, buffer alone was added in binding reaction for comparison. In lanes 6–8, purified Thoc5–FLAG (1.5 μg), Tap-p15 (2 μg) and Tap (188–619)-p15 (2.5 μg) were run as markers. The bound fractions (10%) were also subjected to western blot using the indicated antibodies. Positions of molecular weight markers are indicated on the left in kDa. (B) Purified Thoc5–FLAG (lanes 3, 5, 7) or buffer alone (lanes 2, 4, 6) was incubated with GSH-beads pre-adsorbed to GST (lanes 2 and 3), GST–Aly (lanes 4 and 5) and GST–hHpr1 (lanes 6 and 7). The beads were washed and aliquots of the bound fractions (25%) were analysed by SDS–PAGE followed by CBB staining and western blot using anti-FLAG antibody. In lane 1, 10% of input was loaded.
Thoc5, a novel adaptor RNA-binding protein, is associated with HSP70 mRNP in vivo as a component of THO complex
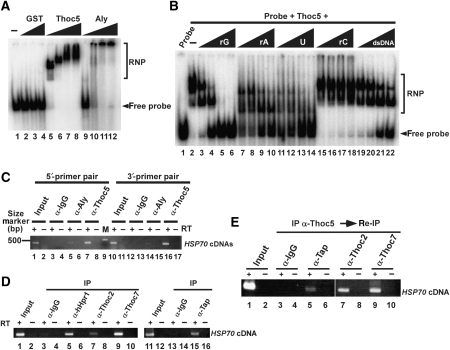
To find out whether Thoc5 can function as an adaptor RNA-binding protein after recruitment to the Tap-p15 mRNA export receptor, we performed RNA band shift assays. This analysis showed that Thoc5, similar to Aly, caused a robust RNA shift in the assay, showing that Thoc5 exhibits in vitro RNA-binding activity (Figure 6A, lanes 5–8). Competition experiments using ribo-homopolymers showed the relative affinities of Thoc5 to different RNAs. The results indicate that Thoc5 exhibits certain preference for different RNA sequences (i.e., poly(rG), poly(U), poly(rA) ≫poly(rC)). Moreover, binding of Thoc5 to the RNA probe was inhibited by a double-stranded DNA, although less efficiently (Figure 6B).
Figure 6.
Thoc5 and Aly are components of the HSP70 mRNP. (A) RNA-binding assay was performed using a 91 bp [32P]-labelled RNA encoding pBluescript SK polylinker sequence. Increasing amounts of purified recombinant GST (lanes 2–4: 160, 320, 480 pmol), Thoc5–FLAG (lanes 5–8: 2, 4, 12, 20 pmol) and GST–Aly (lanes 9–12: 0.3, 0.6, 2, 3 pmol) proteins were added to total 10 μl of each binding reaction. Probe alone was run in lane 1. The binding reactions were separated by 6% polyacrylamide gel electrophoresis and visualized by autoradiography. A bracket indicates the positions of the protein–RNA complexes. (B) Competition assay of the RNA-binding activity of Thoc5 was performed using increasing amounts (10, 25, 100, 300 ng each) of ribo-homopolymers (lanes 3–18) and a dsDNA (lanes 19–22) as competitors. (C) Heat-shocked HeLa cells were subjected to RNA co-immunoprecipitation assay. Co-precipitated RNAs were then subjected to PCR using the indicated primer pairs. RNA isolated from the crude cell extract was also subjected to RT–PCR (lanes 1, 2, 10 and 11; input). In lanes indicated by RT, reverse transcriptase was omitted from the first-strand synthesis reactions. Amplified cDNA fragments were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. DNA size markers were run on lane 9 (indicated by M) and the position of the 500 bp marker is indicated on the left. (D) Heat-shocked HeLa cells were subjected to RNA co-immunoprecipitation assay using the indicated antibodies as in (C). (E) Heat-shocked HeLa cells were subjected to RNA co-immunoprecipitation assay using anti-Thoc5 antibody. mRNPs released from the anti-Thoc5 beads were re-immunoprecipitated using the indicated antibodies. Co-precipitated RNAs were analysed as in (C).
To test the possibility that Thoc5 is associated with mRNA in vivo, we analysed whether HSP70 mRNA could be co-immunoprecipitated by anti-Thoc5 antibody. To generate HSP70 mRNA, a HeLa cell culture was heat-shocked followed by cross-linking with formaldehyde to stabilize protein–protein and protein–RNA interactions. Thoc5 was then immunoprecipitated by specific antibodies from whole cell lysates. Antibodies against Aly and an unrelated antigen (mouse IgG) were used for positive and negative controls, respectively. The co-immunoprecipitated RNA was then extracted and subjected to RT–PCR using human HSP70-specific primer sets. HSP70 mRNA was specifically immunoprecipitated with either anti-Thoc5 or anti-Aly antibodies, but not with control antibodies (Figure 6C, lanes 3, 5, 7, 12, 14, 16). These data indicate that Thoc5, similar to Aly, is associated with HSP70 mRNA.
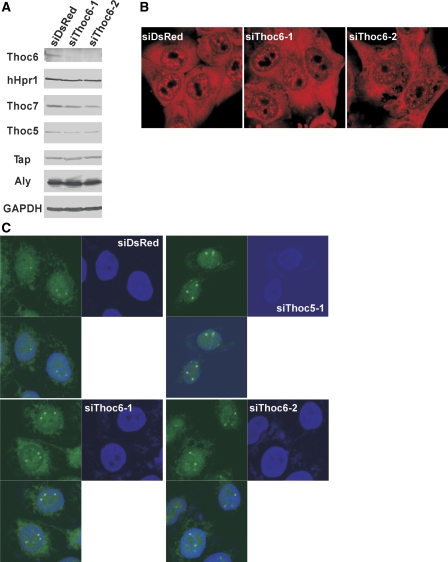
To further explore the nature of the HSP70 mRNP, antibodies against different factors were used in immunoprecipitation. Under heat-shock condition, the THO complex remained stable and the components were efficiently co-immunoprecipitated by the different anti-THO antibodies (Supplementary Figure S3C; see also Figures S3A and B for specificity of the antibodies). HSP70 mRNA was also co-immunoprecipitated by these antibodies (Figure 6D, lanes 5, 7, 9) as well as by the antibody against Tap (Figure 6D, lane 15). Strikingly, Thoc5-containing HSP70 mRNP could be re-immunoprecipitated by anti-Tap, anti-Thoc2 and anti-Thoc7 antibodies (Figure 6E, lanes 5, 7, 9). These data strongly suggest that HSP70 mRNA forms a complex with a novel adaptor Thoc5 and the exporter Tap-p15 in vivo and that Thoc5 in HSP70 mRNP is a part of stable THO complex. In good agreement with the latter conclusion, depletion of Thoc6, another metazoan-specific component of the THO complex, by siRNA, also induced modest, but still significant, nuclear accumulation of HSP70 mRNA (Figure 7C; see also Supplementary Figure S6) without affecting the expression of the other THO components, including Thoc5 (Figure 7A) and cellular localization of bulk poly(A)+ RNAs (Figure 7B). Taken together, these data support a model that Thoc5 has an adaptor function as a component of stable THO complex.
Figure 7.
Depletion of Thoc6 also blocks nuclear export of HSP70 mRNA. (A) HeLa cells were treated with the indicated siRNAs for 72 h. Total cell extracts prepared from the cells were subjected to western blot using the indicated antibodies. For a negative control, siRNA against DsRed protein was used. Positions of molecular weight markers are indicated on the left in kDa. (B) HeLa cells grown on glass-bottomed dishes were treated with the indicated siRNAs for 72 h. The cells were fixed and subjected to in situ hybridization using Cy3-labelled oligo-dT50 probe. (C) HeLa cells grown on glass-bottomed dishes were treated with the indicated siRNAs against Thoc6 for 72 h. The cells were fixed and subjected to in situ hybridization using the FITC-labelled HSP70 oligonucleotide probes. Cells treated with siThoc5-1 (upper right panel) are shown for comparison of the size of the nuclear foci.
Discussion
Genetic and biochemical studies have linked the subunits of the TREX complex to the mRNA export machinery in yeast and metazoans (Stutz et al, 2000; Zhou et al, 2000; Luo et al, 2001; Rodrigues et al, 2001; Jimeno et al, 2002; Strasser et al, 2002; Rehwinkel et al, 2004; Masuda et al, 2005). However, the specific function(s) of the individual TREX components have remained largely unknown. Our results indicate that Thoc5, a subunit of the metazoan TREX complex with no apparent orthologue in yeast, can directly bind to the nuclear export receptor Tap-p15. Notably, Thoc5 can interact with Tap-p15 even in the presence of another bound adaptor protein Aly due to the presence of non-overlapping binding sites on the export receptor. In vivo, Thoc5 is not required for bulk mRNA export, but nuclear export of a specific mRNP (HSP70) depends on Thoc5, which also involves the general adaptor protein Aly and the exporter Tap. Despite the fact that Thoc5 does not exhibit known RNA-binding motifs, it can bind to RNA in vitro. Our data also suggest that Thoc5 is involved in HSP70 mRNP during nuclear export as a component of stable THO complex. Thus, Thoc5 is a novel adaptor RNA-binding protein in mammalian cells that, in concert with the other adaptor Aly, functions in Tap-p15-mediated export of a specific mRNA.
To date, only the N- and LRR-domains of human Tap (residues 1–371) have been described as binding sites for adaptor proteins involved in mRNA export (Stutz et al, 2000; Fribourg et al, 2001; Huang et al, 2003). However, the yeast mRNA export receptor Mex67-Mtr2 can interact with pre-ribosomal particles or with a nucleoporin (Nup85) through a loop-confined surface on the Ntf2-like domain of Mex67 (Yao et al, 2007, 2008). Our studies identified a related surface on the Tap-p15 heterodimer, although with shorter loops, which is opposite the FG-repeat binding domain (Figure 1D) and is involved in binding to Thoc5 and possibly other targets. As suggested for ribosomal subunit export by the Mex67-Mtr2 heterodimer (Yao et al, 2007), Tap-p15 could simultaneously bind to FG-nucleoporins and Thoc5 through different surfaces on the Ntf2-like fold, which could be crucial for nuclear export of HSP70 mRNA. It has been shown that p15 is required for maintenance of the structure of the M-domain (Fribourg et al, 2001). Our in vitro binding data using Tap monomer and Tap point mutants complexed with p15 also suggest that the structural integrity of the M-domain, but not the presence of p15 per se, is important for Thoc5 binding, although we cannot exclude a possibility that p15 also physically interacts with Thoc5.
Through homology modeling, we noted that the N-terminal domain of Thoc5, which is predicted to be rich in α-helices, could adopt an α-solenoid fold. It was suggested previously that an elongated Nup85 molecule, which also has a series of predicted antiparallel α-helices that could form an α-solenoid (Devos et al, 2006), binds through its longitudinal axis to an extended surface on the Mex67-Mtr2 heterodimer (Yao et al, 2008). Thus, it is possible that Thoc5 binds in a similar way to a surface on the Tap-p15 heterodimer that could correspond to the loop-defined surface on the Mex67-Mtr2 export receptor.
In some strains of yeast, heat shock rapidly induces inhibition of nuclear export of bulk mRNA (Saavedra et al, 1996). In metazoans, heat shock attenuates the expression of non-heat-shock genes, mainly by blocking their splicing and changing the mode of translation (Storti et al, 1980; Yost and Lindquist, 1986; Bond, 1988; Shukla et al, 1990; Joshi-Barve et al, 1992). Consistent with this observation, most of the heat-shock mRNAs are devoid of introns, which could enable them to by-pass the splicing-dependent inhibition of mRNA export under heat shock (Yost and Lindquist, 1986). On the other hand, the mammalian TREX complex has been reported to be recruited to pre-mRNAs during splicing (Cheng et al, 2006). Moreover, TREX subunits are located in splicing factor-rich nuclear speckles and co-enriched with spliceosomes during purification (Zhou et al, 2000; Masuda et al, 2005; Chen et al, 2007; Merz et al, 2007). However, our data have showed that both the human THO complex and Aly are associated with naturally intron-less HSP70 mRNA and hence could have an important function in transcription-dependent loading of the mRNA export machinery to these nascent transcripts (Nojima et al, 2007; Yoh et al, 2007; Taniguchi and Ohno, 2008).
Another unexpected finding of this study is that nuclear export of HSP70 mRNA in mammalian cells strictly depends on Aly, but its requirement for bulk poly(A)+ RNA is less pronounced. This finding is similar to the earlier conclusions made in different organisms (Gatfield and Izaurralde, 2002; Longman et al, 2003) and may indicate that relative requirement of certain adaptor proteins in nuclear export of different mRNA may differ in higher eukaryotes. In addition to Aly and Yra1, the nucleo-cytoplasmic shuttling Ser/Arg-rich (SR) proteins, such as mammalian 9G8 and SRP20 and yeast Npl3, have been shown to interact with Tap-p15 or Mex67-Mtr2, and thus could function as alternative adaptors (Huang et al, 2003, 2004; Gilbert and Guthrie, 2004). Although the relationship between the SR proteins and the TREX complex in metazoans is unknown, in yeast it is thought that the SR-like proteins Gbp2 and Hrb1 are recruited to nascent mRNAs by their physical interaction with the TREX complex (Hurt et al, 2004). Thus, it would be interesting to analyse the relationship between Thoc5 and SR proteins, which interact with the N-terminal adaptor binding site on Tap (Huang et al, 2004), for nuclear export of different classes of mRNAs.
Another surprising observation in this study was that HSP70 mRNA requires the co-adaptor Thoc5 for efficient nuclear export. There are several explanations for this observation. One possibility is that due to the absence of introns, HSP70 mRNA requires a specific adaptor RNA-binding protein for efficient export, which can be recruited by a splicing-independent (e.g., transcription-dependent) mechanism. Another scenario could be that efficient recruitment and stable binding of Aly to HSP70 mRNA require a second adaptor protein, such as Thoc5, that could stabilize the export receptor-cargo complex under heat shock, which may tend to destabilize protein complexes (e.g., Shukla et al, 1990). Finally, in light of the recent findings that transcription and translation can be coupled in eukaryotic cells (Hampsey and Kinzy, 2007; Marr II et al., 2007; Rother and Strasser, 2007), it is tempting to speculate that Thoc5 and/or components of the THO complex could have an additional function in HSP70 gene expression. The HSP70 mRNA is translated by a cap-independent mechanism due to an IRES (-like) activity within the 5′-untranslated region in higher eukaryotes (Klemenz et al, 1985; McGarry and Lindquist, 1985; Hultmark et al, 1986; Joshi and Nguyen, 1995; Hernandez et al, 2004). Thus, the recruitment of the TREX complex to the 5′-part of the mRNA could be used not only for nuclear export (Cheng et al, 2006), but also for subsequent steps in gene expression, including translation. Genome-wide identification of mRNA species exported through TREX-dependent pathway in mammalian cells will be one of the most important subject of future investigations to further understand the biological functions of the export pathway.
Materials and methods
Reagents
Antibodies against Aly (Abcam), GAPDH (Ambion), hHpr1/Thoc1 (GeneTex), Thoc6 (Abnova), splicing factors (SC35, Sigma), G3BP (BD Biosciences Pharmingen), GFP (Molecular Probes), α-tubulin (Sigma), FLAG peptide tag (Sigma) and mouse IgG (Zymed Laboratories) were commercially acquired. A mouse monoclonal antibody against hnRNP C was a gift from Dr G Dreyfuss. A rabbit polyclonal anti-Thoc5 antibody was raised against the full-length N-terminally His-tagged Thoc5 protein. Rabbit polyclonal antibodies against Thoc2 and Thoc7 were raised against GST-fusion proteins. Rabbit polyclonal antibodies against Tap and p15 have been described (Katahira et al, 1999).
Plasmids
The construction of E. coli expression plasmids for GST–Tap (188–619), GST–Tap (96–371), GST–Tap (188–550) and untagged p15 (pET9d-p15) has been described elsewhere (Katahira et al, 1999, 2002, 2007). A two-hybrid prey plasmid (pACT2-hCG1) has been reported (Katahira et al, 1999). Construction of the human Tap prey plasmids is described in the supplementary data. An E. coli expression vector for GST–Aly was a gift from Dr Mutsuhito Ohno (Kyoto University) (Taniguchi and Ohno, 2008). Details of construction of the expression vectors for Thoc5, Aly, hHpr1, Thoc2, Thoc7 and various fragments of Tap are given in the Supplementary data. Point mutations were introduced by the Quick Change kit (Stratagene).
Yeast two-hybrid screening
The methods of yeast two-hybrid screening and in vivo β-galactosidase plate assay have been described previously (Katahira et al, 1999, 2007).
Protein expression and purification
Recombinant proteins were expressed in E. coli BL21(DE3) CodonPlus strain (Stratagene). Expression and purification of GST- or 6 × His-fusion proteins were performed as described previously (Katahira et al, 1999, 2002, 2007; Taniguchi and Ohno, 2008). Untagged full-length Tap-p15 and Tap (188–619)-p15 were prepared by on-column cleavage of GST-tag by PreScission Protease (GE Healthcare).
Cell culture, transfection and establishment of stable cell lines
HeLa and L929 cells were grown in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum at 37°C in 5% CO2 atmosphere. Transfection of plasmid DNAs was performed by the Effectene Transfection Reagent Kit according to the manufacturer's protocol (Qiagen). L929 cell lines stably expressing Thoc5–GFP and hHpr1–GFP fusion proteins were established as reported previously (Zhou et al, 2000).
siRNA treatment
Transfection was performed using the Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's protocol. Double-stranded siRNAs were synthesized by Eurogentec through NIPPON EGT. The sequences of siRNAs are given in the Supplementary data. At 72 h after the initial transfection, the cells were harvested for western and northern blot analysis or subjected to in situ hybridization. We found that siRNA-mediated depletion of Tap impaired cell viability more severely than that of Thoc5 or Aly, and Tap-depleted cells detached from culture dishes almost completely at 72 h post-transfection. Subsequent experiments with Tap-depleted cells were therefore done at an earlier time point (45–48 h post-transfection), when 70–80% of the cells were still viable.
In situ hybridization
In situ hybridization was performed by the method of Singer et al. (http://www.singerlab.org/protocols). Anti-sense oligodeoxynucleotides internally labelled with FITC were synthesized and purified by JBIOS Co. Ltd. The sequences of each oligodeoxynucleotide are indicated in the Supplementary data.
RNA co-immunoprecipitation assay
A protocol for chromatin immunoprecipitation for mammalian cells (Horie-Inoue et al, 2004) was adapted for RNA co-immunoprecipitation assay. The detailed procedure is described in the Supplementary data.
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Drs Mutsuhito Ohno and Ichiro Taniguchi for discussion and for providing the human REF expression plasmid. We also thank Dr Wei Yao for a critical reading of the manuscript, and Dr Gideon Dreyfuss for providing the anti-hnRNP C antibody. We are grateful to Dr Yoshinari Yasuda for sharing the protocol of the ChIP experiment. This work was supported, in part, by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Takeda Science Foundation and the Human Frontier Science Program.
References
- Abruzzi KC, Lacadie S, Rosbash M (2004) Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. EMBO J 23: 2620–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A (2005) Cotranscriptional mRNP assembly: from the DNA to the nuclear pore. Curr Opin Cell Biol 17: 242–250 [DOI] [PubMed] [Google Scholar]
- Bachi A, Braun IC, Rodrigues JP, Pante N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Gorlich D, Carmo-Fonseca M, Izaurralde E (2000) The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6: 136–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U (1988) Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J 7: 3509–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Moore RE, Ge HY, Young MK, Lee TD, Stevens SW (2007) Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors. Nucleic Acids Res 35: 3928–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R (2006) Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 127: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A (2006) Simple fold composition and modular architecture of the nuclear pore complex. Proc Natl Acad Sci USA 103: 2172–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farny NG, Hurt JA, Silver PA (2008) Definition of global and transcript-specific mRNA export pathways in metazoans. Genes Dev 22: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg S, Braun IC, Izaurralde E, Conti E (2001) Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear exprot factor. Mol Cell 8: 645–656 [DOI] [PubMed] [Google Scholar]
- Gatfield D, Izaurralde E (2002) REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol 159: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield D, Le Hir H, Schmitt C, Braun IC, Kocher T, Wilm M, Izaurralde E (2001) The DExH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Curr Biol 11: 1716–1721 [DOI] [PubMed] [Google Scholar]
- Gilbert W, Guthrie C (2004) The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell 13: 201–212 [DOI] [PubMed] [Google Scholar]
- Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E (1998) TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell 1: 649–659 [DOI] [PubMed] [Google Scholar]
- Hampsey M, Kinzy TG (2007) Synchronicity: policing multiple aspects of gene expression by Ctk1. Genes Dev 21: 1288–1291 [DOI] [PubMed] [Google Scholar]
- Hernandez G, Vazquez-Pianzola P, Sierra JM, Rivera-Pomar R (2004) Internal ribosome entry site drives cap-independent translation of reaper and heat shock protein 70 mRNAs in Drosophila embryos. RNA 10: 1783–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Klymenko T, Izaurralde E (2001) NXF1/p15 heterodimers are essential for mRNA nuclear export in Drosophila. RNA 7: 1768–1780 [PMC free article] [PubMed] [Google Scholar]
- Herold A, Suyama M, Rodrigues JP, Braun IC, Kutay U, Carmo-Fonseca M, Bork P, Izaurralde E (2000) TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol Cell Biol 20: 8996–9008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie-Inoue K, Bono H, Okazaki Y, Inoue S (2004) Identification and functional analysis of consensus androgen response elements in human prostate cancer cells. Biochem Biophys Res Commun 325: 1312–1317 [DOI] [PubMed] [Google Scholar]
- Huang Y, Gattoni R, Stevenin J, Steitz JA (2003) SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell 11: 837–843 [DOI] [PubMed] [Google Scholar]
- Huang Y, Yario TA, Steitz JA (2004) A molecular link between SR protein dephosphorylation and mRNA export. Proc Natl Acad Sci USA 101: 9666–9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D, Klemenz R, Gehring WJ (1986) Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell 44: 429–438 [DOI] [PubMed] [Google Scholar]
- Hurt E, Luo MJ, Rother S, Reed R, Strasser K (2004) Cotranscriptional recruitment of the serine-arginine-rich (SR)-like proteins Gbp2 and Hrb1 to nascent mRNA via the TREX complex. Proc Natl Acad Sci USA 101: 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno S, Rondon AG, Luna R, Aguilera A (2002) The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J 21: 3526–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mongelard F, Robert-Nicoud M, Vourc'h C (1997) Optimization of nuclear transcript detection by FISH and combination with fluorescence immunocytochemical detection of transcription factors. J Histochem Cytochem 45: 1585–1592 [DOI] [PubMed] [Google Scholar]
- Jolly C, Vourc'h C, Robert-Nicoud M, Morimoto RI (1999) Intron-independent association of splicing factors with active genes. J Cell Biol 145: 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CP, Nguyen HT (1995) 5′ Untranslated leader sequences of eukaryotic mRNAs encoding heat shock induced proteins. Nucl Acids Res 23: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Barve S, De Benedetti A, Rhoads RE (1992) Preferential translation of heat shock mRNAs in HeLa cells deficient in protein synthesis initiation factors eIF-4E and eIF-4 gamma. J Biol Chem 267: 21038–21043 [PubMed] [Google Scholar]
- Kang Y, Cullen BR (1999) The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev 13: 1126–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Miki T, Takano K, Maruhashi M, Uchikawa M, Tachibana T, Yoneda Y (2007) Nuclear RNA export factor 7 is localized in processing bodies and neuronal RNA granules through interactions with shuttling hnRNPs. Nucleic Acids Res 36: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Strasser K, Saiwaki T, Yoneda Y, Hurt E (2002) Complex formation between Tap and p15 affects binding to FG-repeat nucleoporins and nucleocytoplasmic shuttling. J Biol Chem 277: 9242–9246 [DOI] [PubMed] [Google Scholar]
- Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung JU, Hurt E (1999) The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J 18: 2593–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R, Hultmark D, Gehring WJ (1985) Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J 4: 2053–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Hurt E (2007) Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol 8: 761–773 [DOI] [PubMed] [Google Scholar]
- Liker E, Fernandez E, Izaurralde E, Conti E (2000) The structure of the mRNA export factor TAP reveals a cis arrangement of a non-canonical RNP domain and an LRR domain. EMBO J 19: 5587–5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman D, Johnstone IL, Caceres JF (2003) The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA 9: 881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R (2001) Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 413: 644–647 [DOI] [PubMed] [Google Scholar]
- Marr MT II, D'Alessio JA, Puig O, Tjian R (2007) IRES-mediated functional coupling of transcription and translation amplifies insulin receptor feedback. Genes Dev 21: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R (2005) Recruitment of the human TREX complex to mRNA during splicing. Genes Dev 19: 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry TJ, Lindquist S (1985) The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell 42: 903–911 [DOI] [PubMed] [Google Scholar]
- Merz C, Urlaub H, Will CL, Luhrmann R (2007) Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. RNA 13: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T, Hirose T, Kimura H, Hagiwara M (2007) The interaction between cap-binding complex and RNA export factor is required for intronless mRNA export. J Biol Chem 282: 15645–15651 [DOI] [PubMed] [Google Scholar]
- Reed R, Cheng H (2005) TREX, SR proteins and export of mRNA. Curr Opin Cell Biol 17: 269–273 [DOI] [PubMed] [Google Scholar]
- Reed R, Hurt E (2002) A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 108: 523–531 [DOI] [PubMed] [Google Scholar]
- Rehwinkel J, Herold A, Gari K, Kocher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E (2004) Genome-wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster. Nat Struct Mol Biol 11: 558–566 [DOI] [PubMed] [Google Scholar]
- Rodrigues JP, Rode M, Gatfield D, Blencowe B, Carmo-Fonseca M, Izaurralde E (2001) REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc Natl Acad Sci USA 98: 1030–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother S, Strasser K (2007) The RNA polymerase II CTD kinase Ctk1 functions in translation elongation. Genes Dev 21: 1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra C, Tung KS, Amberg DC, Hopper AK, Cole CN (1996) Regulation of mRNA export in response to stress in Saccharomyces cerevisiae. Genes Dev 10: 1608–1620 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E (1998) Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol 18: 6826–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E (1997) Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J 16: 3256–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla RR, Dominski Z, Zwierzynski T, Kole R (1990) Inactivation of splicing factors in HeLa cells subjected to heat shock. J Biol Chem 265: 20377–20383 [PubMed] [Google Scholar]
- Storti RV, Scott MP, Rich A, Pardue ML (1980) Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell 22: 825–834 [DOI] [PubMed] [Google Scholar]
- Strasser K, Basler J, Hurt E (2000) Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG, and FG repeat nucleoporins is essential for nuclear mRNA export. J Cell Biol 150: 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Hurt E (2000) Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J 19: 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Hurt E (2001) Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature 413: 648–652 [DOI] [PubMed] [Google Scholar]
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E (2002) TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417: 304–308 [DOI] [PubMed] [Google Scholar]
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E (2000) REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6: 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi I, Ohno M (2008) ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol 28: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry LJ, Wente SR (2007) Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex. J Cell Biol 178: 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xle YG, Han FY, Peyrard M, Ruttledge MH, Fransson I, DeJong P, Collins J, Dunham I, Nordenakjold M, Dumanski J (1993) Cloning of a novel, anonymous gene from a megabase-range YAC and cosmid contig in the neurofibromatosis type 2/meningioma region on human chromosome 22q12. Hum Mol Genet 2: 1361–1368 [DOI] [PubMed] [Google Scholar]
- Yao W, Lutzmann M, Hurt E (2008) A versatile interaction platform on the Mex67-Mtr2 receptor creates an overlap between mRNA and ribosome export. EMBO J 27: 6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Roser D, Kohler A, Bradatsch B, Bassler J, Hurt E (2007) Nuclear export of ribosomal 60S subunits by the general mRNA export receptor Mex67-Mtr2. Mol Cell 26: 51–62 [DOI] [PubMed] [Google Scholar]
- Yoh SM, Cho H, Pickle L, Evans RM, Jones KA (2007) The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev 21: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S (1986) RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45: 185–193 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Luo MJ, Strasser K, Katahira J, Hurt E, Reed R (2000) The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407: 401–405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information