Abstract
Many inner ear disorders cannot be adequately treated by systemic drug delivery. A blood-cochlear barrier exists, similar physiologically to the blood-brain barrier, which limits the concentration and size of molecules able to leave the circulation and gain access to the cells of the inner ear. However, research in novel therapeutics and delivery systems has led to significant progress in the development of local methods of drug delivery to the inner ear. Intratympanic approaches, which deliver therapeutics to the middle ear, rely on permeation through tissue for access to the structures of the inner ear, whereas intracochlear methods are able to directly insert drugs into the inner ear. Innovative drug delivery systems to treat various inner ear ailments such as ototoxicity, sudden sensorineural hearing loss, autoimmune inner ear disease, and for preserving neurons and regenerating sensory cells are being explored.
Keywords: Cochlea, Intracochlear, Intratympanic, Hearing, Local drug delivery, Device, Passive, Active, Pharmacokinetics
1. Introduction
The inner ear provides a unique opportunity for local drug delivery. Access to the inner ear is limited by the presence of a blood-cochlear barrier, which is anatomically and functionally similar to the blood-brain barrier [1, 2]. Due to tight junctions between cells, substances in systemic circulation encounter substantial physical barriers to entry, preventing many molecules with potentially therapeutic effect from gaining access to their inner ear targets. Additionally, the cochlea is a closed space, and cochlear function is sensitive to small changes in fluid volume. Therefore, delicate approaches are required to avoid possible damage from the delivery method itself.
Otologic practice requires drug delivery to the inner ear, but current methods of delivery utilize inefficient routes. Drugs are commonly delivered systemically, with the expectation that they will reach their intended inner ear targets in the necessary form and concentration, and without deleterious side effects. Systemic corticosteroids, for example, are used in the otologic management of sudden sensorineural hearing loss (SSNHL) and autoimmune inner ear disease (AIED) [3–5]. Their clinical usefulness, however, is limited by undesirable side effects arising from the high systemic doses required to achieve therapeutic concentration in the cochlea [6].
Additional applications include otoprotection from a variety of sources toxic to the inner ear. An active area of research is focused on preventing hearing loss due to cisplatin chemotherapy, radiation for cancer treatments and aminoglycoside antibiotics. Noise induced hearing loss (NIHL) affects millions of people and represents another target for local therapy.
Recent advances in the pharmacology and molecular biology of hearing have revealed new possibilities for preventing or minimizing hearing loss. Sensorineural hearing loss results from death of the cochlear sensory and neural cells. In humans, these cells do not regenerate, and loss is often followed by secondary degeneration of auditory neurons. Scientists and clinicians are making progress in understanding the molecular mechanisms associated with cochlear and auditory nerve degenerative processes [7] and in finding agents that can minimize degeneration and facilitate repair [8, 9]. These insights offer the potential for development of novel and precise drug treatments. Moreover, the extraordinary progress that has been made in defining genes and protein activities offers hope for gene transfer and cell-based approaches to treat these diseases [10, 11].
For current applications and new therapies based on these discoveries to be clinically relevant, it is necessary to develop safe and reliable mechanisms for the direct delivery of compounds into the inner ear. Methods for local delivery can be categorized as either intratympanic or intracochlear approaches.
Intratympanic delivery can be accomplished via perfusion of the middle ear with the goal of diffusion through the round window membrane (RWM) into the fluid spaces of the inner ear. This method, introduced over 50 years ago [12], remains in common use in the treatment of inner ear diseases. Technological advances beyond the simple middle ear injection method include the use of hydrogels, nanoparticles, and microcatheter systems. These systems aim to maintain therapeutic levels of drug on the round window for extended periods of time.
Direct intracochlear drug delivery involves placement of drugs within cochlear perilymphatic spaces via a cochleostomy in the surrounding bone or RWM [13, 14]. This mode of delivery allows drugs to reach their intended targets more directly than with systemic delivery. Molecules perfused into a perilymph compartment (ideally the scala tympani) have direct access to the cells of the inner ear [15]. Methods of delivery include direct perfusion using micropumps and osmotic pumps. Research is being conducted on modifying the electrodes of cochlear prostheses to integrate drug delivery components, while yet others are developing novel implantable delivery devices [16–19].
In this review, we provide a brief overview of the anatomy and physiology of the inner ear as it relates to local drug delivery. Current and horizon applications for conditions that may be treated via direct inner ear delivery are discussed. Finally, methods to locally deliver drugs to the inner ear for both clinical and research applications are presented. These approaches are categorized by the location of delivery and by the passive versus active mode of distribution.
2. Anatomy and Physiology
The inner ear, which contains both the organ of hearing, the cochlea, and the organ of balance, the vestibular system, is embedded deep within the skull near the brainstem in the petrous bone, one of the hardest bones in the body. The extreme inaccessibility of the cochlea, coupled with its very small size, creates unique though tractable problems for cochlear drug delivery. The complexity of the cochlear structures and their extreme sensitivity to the changes in fluid volume also must be considered.
The cochlea can be thought of as a long coiled tube, 31–33 mm in human, [20] looking much like the snail shell from which it derives its name. It varies in diameter along its length from apex to base. Stretched across the middle of the tube is the organ of Corti, which is the highly organized basilar membrane that contains the mechano-sensory cells of the inner ear. The basilar membrane moves in response to sound waves that enter the inner ear. The structure of the organ of Corti is tonotopically organized so that high frequency sounds produce the greatest motion in the base of the tube and low frequency sounds move the organ most at the apex.
The mechano-sensory cells of the ear are called hair cells because early microscopists considered the stereocilia at the apical surfaces to look like tiny hairs. Hair cells are organized into rows that run the length of the coil from its base to its apex and are organized tonotopically in their response to sound. There are two types of hair cells: a single row of inner hair cells responds to sound by releasing a neurotransmitter to excite afferent auditory neurons, and three rows of outer hair cells serve to amplify the sound-evoked motion of the basilar membrane to more effectively deliver frequency-specific stimulation to the inner hair cells.
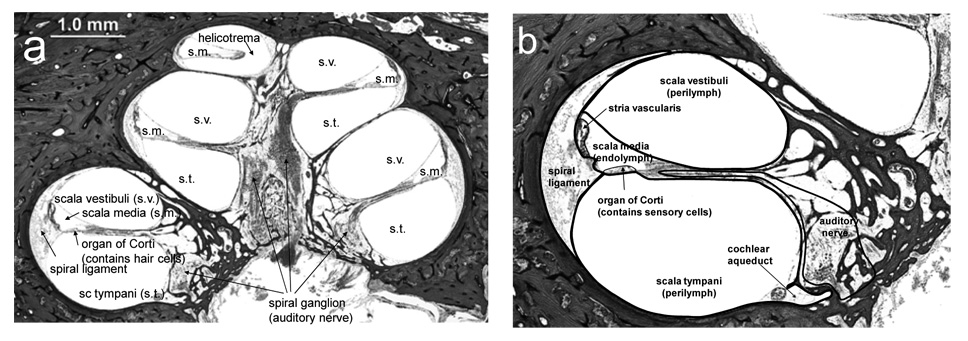
If one looks at a cross section through the cochlea, as shown in Figure 1, a distinctive feature is the presence of three relatively large fluid-filled compartments. These three compartments coil up the cochlea along with the organ of Corti. The middle compartment is the scala media, which is filled with endolymph (discussed below). The scala media forms a border of the organ of Corti, and the apical surfaces of cells in the organ of Corti, including the hair cells, project into the scala media. The lower and upper fluid compartments respectively are the scala tympani and scala vestibuli, both of which are filled with perilymph. These two compartments communicate with each other at the apex of the cochlea through the helicotrema. There are two orifices in the surface of the cochlear bone, both of which are located at the base of the cochlea. The round window is a membranous opening in the bone within the scala tympani. It sits at the base of the scala tympani and is very compliant, capable of bulging into the middle ear. It separates perilymph from the middle ear space. The oval window, in the scala vestibuli, contains the footplate of the stapes, one of the middle ear bones, that transmits acoustic vibrations from eardrum to the inner ear.
Figure 1.
(a) A mid-modiolar section from a human temporal bone indicates the coiled structure of the cochlea as it winds around a central modiolus, which contains the spiral (auditory) ganglion and its associated auditory nerve fibers. (b) Each segment contains 3 fluid filled structures, the scala tympani (s.t.), scala media (s.m.), and scala vestibuli (s.v.). The organ of Corti, which contains the sensory cells of the cochlea (hair cells), separates the scala media and the scala tympani with the apical surface of the epithelium facing the fluid space of the scala media and all other cell surfaces bathed in perilymph of the scala tympani. The scala vestibuli is the fluid space at the upper portion of each turn. The outer edge of each turn is occupied by the spiral ligament, which is in diffusional continuity with perilymph. The outer edge of the scala media is bordered by the stria vascularis. The scala tympani and scala vestibuli are joined at the apical extreme of the cochlea at the helicotrema. The cochlear aqueduct connects the fluid space of the scala tympani with the cerebrospinal fluid space.
Perilymph is the primary fluid of the cochlea. It bathes most of the cells within the cochlea including the basolateral surfaces of the sensory cells, the neurons, and most of the other specialized structures within the cochlea. Perilymph is similar to cerebrospinal fluid (CSF), with which it is in diffusional continuity, though protein concentration in perilymph is significantly (10 to 20 times) higher than in CSF [21–26]. The volume of perilymph in the human cochlea is about 70 µL, with around 40 µl in the scala tympani [26].
The scala media is filled with endolymph, about 8 µL in human, which has an ionic composition similar to the intracellular environment, high in potassium and low in sodium. The fluid space provides a highly unique environment for the apical surface of the hair cells. The cells that form the borders of the scala media are all connected by tight junctions. The primary function of the scala media is to provide an electrochemical environment that supports the hair cells’ ability to transduce mechanical motion into electrical potentials. It contains a relatively large (100 mV) positive electrical potential. This unique electrochemical environment is created by a structure called the stria vascularis, which is, as its name implies, highly vascularized. It runs along the outside of the scala media and extracts energy from the blood that is needed to create the electrochemical battery of the scala media.
The cochlea can be viewed as a set of membranous tubes within a coiled bony tunnel with the organ of Corti, containing the hair cells, stretched across the middle, the scala media providing the electrochemical battery for transduction forming the apical border of the organ of Corti, and tubes containing perilymph and bathing most of the cochlear structures on either side of the organ of Corti and scala media. The stria vascularis runs along the outside of the scala media. Finally, the coil of the cochlea winds around the modiolus, into which are packed the cell bodies of the auditory neurons, appropriately termed the spiral ganglion neurons (SGN). These bipolar neurons send dendrites to the inner hair cells and axons to the brain.
Airborne sound passes through the external auditory canal and moves the tympanic membrane. Motion of the membrane by the sound waves is transmitted through the ossicles (3 tiny bones: the malleus, the incus, and the stapes) to transfer the airborne sound into the movement of the footplate of the stapes, which is inserted in the oval window of the inner ear. The piston-like motion of the stapes transfers a pressure wave to the fluid-filled space of the inner ear behind the oval window. Thus the middle ear functions to match the acoustic impedance of the airborne sound to that of fluid environment of the inner ear.
Motion of the basilar membrane ultimately produces movement of the stereocilia at the apical surfaces of the hair cells. As the stereocilia move, mechanically sensitive ion channels on the stereocilia open and shut in synchrony with the motion. As a result, the hair cells depolarize and repolarize with an electrical waveform that is similar to the mechanical motion. The outer hair cells possess resonance properties that are tonotopically organized to correspond to the maximum sensitivity of the basilar membrane, so that they in essence amplify the motion of the basilar membrane over a narrow range of frequencies, enhancing the frequency discrimination of the system. The inner hair cells respond to the outer hair cell-enhanced basilar membrane motion by releasing neurotransmitter from the base of the hair cell. This release activates the auditory nerve fibers.
There are a number electrophysiological parameters one can monitor to assess cochlear function, and some of these (distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs)) are often used clinically. Current flowing through the transduction channels in the hair cells will generate a cochlear microphonic (CM) potential that mimics the acoustic waveform. One can record the CM potential to get an indication of hair cell function or to assess the effects of drugs on the hair cells. The CM is dominated by response of the outer hair cells. Another monitor of hair cell function, DPOAEs, takes advantage of the mechanical motion induced in the basilar membrane by the outer hair cell resonance. This mechanical response by the outer hair cell is propagated back out through the middle ear as an acoustic signal that can be measured in the auditory canal. This mechanical response is inherently nonlinear and will generate distortion products that can be detected noninvasively in the ear canal. The distortion product corresponding to a particular frequency is quite robust and is commonly used as a metric of cochlear function, and in particular, outer hair cell function.
Responses of the auditory neurons can be monitored individually with microelectrodes, but it is also feasible to monitor the synchronous activity of groups of nerve fibers. The compound action potential (CAP) is measured with a ball electrode placed near the RWM in response to a click or a tone pip. In this case, a group of fibers discharge synchronously in response to the stimulus, producing a signal large enough to measure even at a distance. Tone pips of varying frequency allow the investigator to assess specific regions along the length of the cochlea, e.g. responses to high frequency tone pips are generated in the basal portion of the organ of Corti. This measure is surgically invasive but useful in animal experiments. Clinically, it is possible to monitor these responses with electrodes placed on the surface of the scalp by averaging responses to a large number of stimuli. These ABRs are clinically useful in providing objective assessment of cochlear function noninvasively.
3. Pharmacokinetics
3.1. Absorption
3.1.1. Fluid compartments
Most of the structures of the cochlea are protected from the systemic circulation by the presence of a blood-cochlear barrier (or blood-labyrinthine barrier), similar to the blood-brain barrier. The extracellular fluid spaces of the ear can be considered pharmacologically as comprising four components: (1) the systemic circulation - largely confined to the stria vascularis and to fluid contained within the blood vessels and capillaries; (2) perilymph, a fluid similar in composition to CSF that provides a favorable milieu for sensory cell and neuronal function; (3) endolymph, a fluid high in potassium ions which provides the electrochemical drive for sensory cell function; and (4) the extracellular fluid of cochlear bone, which seems to be a transitional region between the systemic circulation and the perilymph.
A small portion of the inner ear, the stria vascularis, is readily accessible from the systemic circulation. It is surrounded by cells connected with tight junctions, which provide a part of the blood-cochlear barrier [27]. Drugs that act in the stria vascularis can be administered systemically, and the most likely action is a reduction of the endocochlear potential. Because the endocochlear potential is the battery that drives the cochlear transduction process, a reduction in the endocochlear potential will decrease hearing capability. The vascularization of the rest of the cochlea is relatively sparse, though the modiolus and walls of the scala tympani are exceptions [28]. The endothelial cells that line the vasculature are connected with tight junctions and no fenestrations [27, 29], and this network of tightly coupled endothelial cells is the dominant component of the blood-cochlear barrier.
Perilymph, the primary fluid of the inner ear, is in diffusional continuity with CSF via the cochlear aqueduct in most animals, though the transfer of fluid between the two spaces is limited [30]. In humans, the aqueduct is usually not patent [31], though diffusional continuity is achieved via other pathways, most notably the spaces around and within the auditory nerve [32].
Perilymph is primarily produced locally via the vascular supply to the cochlea (reviewed in [33, 34]), though a minor contribution from the CSF is possible. The site of production of perilymph is not known, though the vascular supply to the modiolus and walls of the scala tympani have been suggested [32, 35]. Perilymph resembles CSF in composition, and this similarity likely arises from a similar mode of production of perilymph and CSF.
Endolymph, the highly specialized extracellular fluid that fills the scala media, bathes the apical surfaces of the sensory cells. Endolymph is primarily produced in the stria vascularis and is secreted by the marginal cells of the stria vascularis, which face the endolymph. Endolymph contains high concentrations of potassium, low concentrations of sodium, and very low concentrations of calcium. The scala media is lined with cells that contain tight junctions. The tight junctions help the scala media retain its unusual electrical potential – around +100 mV, called the endocochlear potential. The endocochlear potential and the high potassium concentration of the scala media provide the electrochemical drive to the hair cells to allow their transduction of mechanical motion.
The cochlea is surrounded by petrous bone. This bone normally does not remodel and is very poorly vascularized. The bone is accessible to perilymph via lacuna canaliculi which are canals or holes in the bone in free communication with the scala tympani [32, 36, 37]. However, the bone is also accessible to the systemic circulation via fenestrated blood vessels. The bone has not been characterized with regard to its presence in or out of the blood-cochlear barrier.
3.1.2. Barrier mechanisms
The prime component of the blood-cochlear barrier is a continuous capillary endothelium lining blood vessels in the cochlea. The endothelial cells are connected with tight junctions and no fenestrations [27]. Thus drugs entering the perilymph from the systemic circulation must either be transported across, or dissolve into and out of, the capillary endothelia.
The scala media is also surrounded by cells connected by tight junctions. Chemicals entering the scala media from the vasculature must either enter via the marginal cells of the stria vascularis, or by first entering the perilymph. Either way, the drugs must be transported across the cell lining to enter the scala media. Because the scala media has a relatively high positive charge due to the endocochlear potential, the charge a drug carries will be a significant factor in its ability to enter the scala media, with positively charged drugs at a disadvantage.
As with the blood-brain barrier, there are other elements of the barrier as well. The relatively high protein content of perilymph [23, 24, 38] will tend to bind drugs that do enter, buffering the concentration of such drugs. There are also likely to be other components to the blood-cochlear barrier, such as enzymes and cellular uptake systems that can reduce the concentration of chemicals that enter the cochlea, but these are not well characterized in the cochlea.
3.1.3. Chemical properties of drugs that can cross the barrier
As with the blood-brain barrier, drugs that are large or highly charged have difficulty crossing passively. Drugs with high lipid solubility cross more readily. Drugs with high protein binding are less likely to cross. The unusual positive potential in the scala media poses an additional constraint for drug entry. Drugs that are positively charged tend not to enter the scala media since the electrical gradient works against them.
3.2. Distribution
Once a drug enters the cochlear fluids, its distribution is governed by many of the same pharmacokinetic variables seen in the bloodstream. Most of the structures of the cochlea are in diffusional continuity with the perilymph. Thus a drug placed into the perilymph can diffuse into the organ of Corti, which contains hair cells, neuronal terminals, and other specialized cells. The perilymph is also in diffusional continuity with the spiral ganglion and structures within the modiolus of the cochlea. Access to structures in the modiolus is enhanced by the presence of numerous lacuna canaliculi on the surface of the osseous spiral lamina [37]. These perforations are on the order of 0.2 to 23 microns in diameter [36]. Finally, the spiral ligament and spiral limbus are also in diffusional continuity with perilymph.
Cochlear structures that are not accessible from the perilymph include the stria vascularis and the scala media, and thus the surfaces of cells that line the scala media. Both of the structures are lined with cells that are connected via tight junctions [27].
The protein composition of perilymph in the human has a number of elements in common with that of blood, though perilymph concentrations are lower [23], and protein interactions with drugs are as important in the perilymph as in blood. Albumin levels are high and can bind acidic drugs, and acid glycoproteins can bind basic drugs. Partition coefficients of drugs with these proteins will determine free concentration of the drug. These proteins can provide a reservoir for accumulation of the drug in perilymph over time.
The distribution of drugs within the inner ear is dependent upon the point of entry of the agents. There are 3 practical entry points: through or near the RWM (at the base of the cochlea on the scala tympani side), through or near the oval window (at the base of the cochlear on the scala vestibuli side), or through the bone of the cochlea via application in the middle ear.
The cochlea is a long set of coiled tubes. The perilymph of the scala tympani can be thought of as beginning at the base of the cochlea near the RWM, continuing apically to the end of the tube where it then connects with the perilymph of the scala vestibuli and continues back down to the base to the oval window, where the footplate of the stapes is located. Drug transport via simple diffusion along the length of the perilymphatic space, because of the large length relative to cross section would require very long times (hours to days) for significant drug distribution throughout the cochlea.
Salt, Plontke, and colleagues have extensively modeled the distribution of drugs applied at the RWM [39, 40]. They suggest that in addition to diffusion along the length of the cochlea, diffusion through the tissue of the cochlea from one scala to another must be considered as well [41, 42]. The magnitude of the interscalar distribution will be highly dependent upon the drug [43]. Highly lipid soluble drugs, like steroids, will have a greater component of its distribution through tissue than would a drug with a high rate of cellular uptake via carrier systems.
Many drugs are delivered into the middle ear space with the hope that they will diffuse through the RWM into the scala tympani of the basal turn. The round window is an Achilles heel in the blood-cochlear barrier. It is permeable to water and many drugs [44, 45]. The major problems with this mode of entry from a pharmacokinetic perspective are that it relies on simple diffusion to pass drug from the middle to the inner ear, and once in the scala tympani, the only mode of distribution is simple diffusion in a fluid space with little turnover. Using implanted electrodes and tracer ions, the longitudinal volume flow in the scala tympani was determined to be negligible, approximately 1.6 nL/min directed apically [46]. The RWM can vary greatly in thickness [44] which can lead to variability in absorption of drug into the scala tympani.
Delivery of agent into the scala vestibuli does not provide as favorable access to the structures of the organ of Corti as application via the scala tympani. In theory, access to the auditory neurons should be possible via canal perforantes in the scala vestibuli portion of the osseous spiral lamina [32]. Also, there are possible diffusion pathways to the scala tympani via entry into and exit from the modiolus as well as into and out of the spiral ligament.
3.3. Metabolism and Clearance
Issues of metabolism and clearance of drugs are specific for each drug. Some of the same barriers that limit drug entry, e.g. tight junctions, will also limit exit of drugs once they enter. Uptake systems to remove agents from the perilymph and either metabolize or transport agents to the systemic circulation will be important. Plasma has enzymes, such as esterases, which are likely to be present in perilymph as well and can alter the structure of drugs that enter the perilymph.
4. Inner ear drug delivery applications
Current applications for inner ear drug delivery are grouped into three main categories, otoprotection, SSNHL, and AIED. The sensory cells of the cochlea must be protected from noise and surgical trauma, ototoxic drugs such as cisplatin and aminoglycoside antibiotics, and head and neck radiation. In addition, drugs are used to minimize or reverse hearing loss due to sudden events of unknown nature and to immune reactions within the cochlea.
Future application research is focused on maintenance of spiral ganglion cells after hearing loss, and regeneration of hair cells. A variety of techniques including gene transfer and stem cell transplantation are being explored.
4.1. Current applications
4.1.1. Noise induced hearing loss
Hearing loss is associated with both acute and chronic noise exposure. The overwhelming of the natural antioxidant system by the formation of free radicals is a hypothesized cause of NIHL [47]. Many types of steroids, antioxidants, and nerve and growth factors have been studied to protect the ear from trauma or to minimize or reverse damage [48].
The effectiveness of steroids in reducing hearing loss due to noise has been inconclusive. In one study, dexamethasone was administered in an intracochlear manner using an osmotic pump, and the animal was exposed to noise trauma on day four. A dose dependent reduction in outer hair cell loss was seen seven days after exposure, and the highest dose tested, 100 ng/mL, resulted in less ABR shifts than control [49]. Using a different methodology, Sendowski et al. directly infused methylprednisone for seven days into the cochlea after exposing the guinea pig to impulse noise trauma. The steroid accelerated the recovery of temporary hearing shifts during the period and reduced hair cell loss. However, the drug did not limit permanent threshold shifts [50]. Both cyclosporine A and immunosuppressant, FK506, delivered intraperitoneally immediately prior to sound exposure showed decreased ABR threshold shift after both one and two weeks [51]. Steroids, if proven effective, may be excellent candidates for local delivery as patients can experience significant side effects when given at high doses systemically. High doses are likely necessary to achieve a therapeutic concentration within the cochlea.
Other researchers have used antioxidants such as D-methionine and N-acetylcysteine to prevent NIHL [52–54]. Caroverine, a glutamate antagonist and antioxidant, was delivered to counteract excess glutamate and reactive oxygen species released during noise trauma. An osmotic pump was implanted to deliver subcutaneously, and the guinea pig was pretreated for 48 hours prior to noise exposure. The protective properties of the caroverine resulted in 5–10dB shifts after four weeks compared to 30dB shifts seen in the controls [55].
A variety of new therapies, such as growth factors and peptides, are being introduced to combat the effects of NIHL. Insulin-like growth factor-1 (IGF-1) applied in a hydrogel to the RWM reduced ABR shifts and outer hair cell loss at seven and thirty days post noise exposure [56]. Likewise, neurotrophic factor-3 (NT-3) protected the inner ear from noise induced damage, but brain-derived neurotrophic factor (BDNF) showed no protection [57]. Researchers attempting to inhibit the apoptotic cascade initiated by noise trauma have met with promising results using AM-111 and D-JNKI-1 peptides, among others [58–60]. Large and complex molecules such as peptides will likely require local delivery to the inner ear as they may have difficulty in traversing the blood-cochlear barrier or may be quickly inactivated by enzymes.
4.1.2. Cisplatin ototoxicity
Cisplatin, a chemotherapy agent commonly used in cancer treatment, is ototoxic, and compounds such as antioxidants and platinum binders are being investigated for co-administration to mitigate the hearing loss associated with cisplatin treatment [61, 62]. A study of the dose dependency of cisplatin ototoxicity in guinea pigs revealed that a sudden drop in CAP of 40 dB occurred from one to five days after cisplatin was administered, depending on dose, which varied from 3 to 300 µg/mL [63]. The drug resulted in loss of outer hair cells, especially in the basal turn of the cochlea. Cisplatin generates reactive oxygen species by activating the NOX-3 enzyme, unique in the cochlea, and leads to apoptosis of outer hair cells [64]. With damage occurring first at high frequency, some reports show hearing thresholds elevated in 75–100% of patients taking cisplatin. Ototoxicity is both dose and age dependent with elderly and children affected to a greater extent [61, 65].
Antioxidants, such as methionine, are useful in scavenging free radicals from the inner ear [66]. When delivered with cisplatin, hearing loss is reduced [67]. Unfortunately, a study where L-methionine was delivered systemically with the cisplatin resulted in hearing protection but also made the cisplatin ineffective [68]. Locally delivered, antioxidants may protect hair cells without inactivating the chemotherapy treatment. Two studies have proven inconclusive, though. D-methionine was delivered to the RWM via osmotic pump in combination with systemic cisplatin. Chemotherapy was given for five days, and the D-methionine treated ears had better DPOAEs on days 3 and 4. The threshold shifts were not significant by days 5 and 6 [69]. A different antioxidant, thiourea was also investigated by delivering it directly through a cochleostomy via osmotic pump. Five days after starting thiourea, cisplatin was started. The study resulted in less outer hair cell loss but threshold shifts were not affected [70].
Thiols neutralize cisplatin since the sulfur has a high affinity for the platinum. However, sodium thiosulfate (STS), if administered systemically, will inactivate the cisplatin and reduced its anti-tumor activity [71]. In order to utilize the otoprotective aspects of STS, it must be contained within the inner ear, making it an excellent candidate for local delivery. Wang, et al. used an osmotic pump for intracochlear delivery and showed that six days after cisplatin the thiol protected 93% of inner and outer hair cells [72]. Guinea pigs were treated with STS, half via osmotic pump in the middle ear and half via daily injections into the middle ear. Cisplatin was started two days later, and both delivery methods resulted in less hearing loss [73].
4.1.3. Aminoglycoside ototoxicity
Another possible application for inner ear drug delivery is in the prevention of aminoglycoside antibiotic ototoxicity due to the generation of oxygen free radicals [74]. Some reports show the incidence of hearing loss associated with these antibiotics is as high as 33% [61, 65]. There is a dose dependent effect, and patients experience high frequency hearing loss due to the loss of outer hair cells in the basal turn. Hypotheses that reactive oxygen species leading to apoptosis are involved have led to testing protective agents such as antioxidants and iron chelators. The assumptions are that the antioxidants scavenge free radicals, and the iron chelators bind iron in the cochlea so it cannot react with aminoglycosides to generate reactive oxygen species [61, 65]. A study by Sergi et al. showed that antioxidants delivered with gentamicin resulted in less hearing loss [67].
Steroids have also been tested for their otoprotective attributes during antibiotic treatment. The intracochlear infusion of dexamethasone before and after kanamycin delivery protected hearing. More outer hair cells survived, and shifts in ABR were decreased [75].
4.1.4. Radiation ototoxicity and trauma
Hearing loss due to head and neck radiation and intracochlear surgical trauma represents a possible area that would benefit from local delivery of otoprotective agents. During radiation treatment of head and neck tumors, the cochlea is often exposed. Hearing loss is dependent on both dose and tumor site and can result in sudden or progressive hearing loss [76]. This side effect occurs in approximately one third of patients and results from loss of hair cells, degeneration of the stria vascularis, and loss of spiral ganglion cells [77]. Patients are commonly given steroids to reduce inflammation, but local delivery would reduce the side effects associated with systemic steroid treatment. Inflammation often results from inner ear surgical trauma, as well. The application of corticosteroid by syringe following cochleostomy enhanced the recovery of CAP thresholds over four weeks [78].
4.1.5. Sudden sensorineural hearing loss
SSNHL is defined as the loss of more than 30 dB of hearing in three consecutive frequencies in less than three days [79]. There are two hypothesized causes: viral infections and vascular events resulting in a local decrease in blood flow [80]. Steroids have some effectiveness in treating SSNHL, and the best indicators of treatment success are the severity of hearing loss and the time before treatment is started [80].
This disorder is an excellent candidate for local delivery because the high systemic doses of steroids needed for treatment cause significant side effects. The Microwick, a device for facilitating diffusion through the RWM has been utilized as one method of local delivery [81, 82]. In a human study of ten patients treated intratympanically, hearing improved in 80% of participants [83]. Often, patients who have failed to tolerate systemic steroids or could not take them have been treated locally. Dexamethasone or methylprednisone delivered intratympanically has shown varied success [84, 85]. Hearing improvement in 44% to 75% of patients has been reported [86–88].
Results of treatment have varied, and some studies point to a strong correlation between success and time to commencing treatment [89]. A retrospective study of a single intratympanic injection of dexamethasone showed no improvement if treatment began more than 36 days after SSNHL onset. If begun sooner, 40% of patients show improvement [90]. Battista reported no significant hearing recovery for 25 patients given dexamethasone intratympanically. The mean time until treatment was 28 days, but there was evidence of improvement for those patients whose treatment started within eleven days [91].
4.1.6. Autoimmune inner ear disease
AIED results in the loss of hearing when the immune regulation system is compromised [92]. Treatment results from high dose systemic steroids and locally delivered steroids have been inconclusive [81, 93]. Encouraging results have been shown with methotrexate [94]. Ryan et al. suggested a treatment of high dose prednisone with the addition of methotrexate if relapse occurred when the steroid was tapered [8]. Improvements in treatment may be facilitated by local delivery for both SSNHL and AIED.
4.2. Horizon Applications
Hearing loss is associated with progressive degeneration of the cochlea, loss of the sensory hair cells, and their associated neural connections. In the human, hair cells and neurons do not regenerate spontaneously after they have been lost. Thus, clinical treatments to date have focused mainly on functional improvement, such as cochlear implants. While cochlear implantation can provide sensory function for individuals with impairment, the effectiveness of application inherently depends upon the residual function of surviving sensory cells and neurons. As a first pass effort, the ability to preserve existing sensory cells and their functions can be used to enhance therapeutic efficacy of cochlear prostheses. However, with a better understanding of the inner ear cell cycle machinery, emphasis can also be placed on discovering therapies for the regeneration of new sensory hair cells, and ultimately a clinical treatment for restoring functional hearing. Ideally, concurrent advances in therapeutic development and drug delivery technologies to the auditory system will be developed in order to realize a clinically relevant treatment of progressive hearing loss.
4.2.1. Neurotrophin therapy
The loss of hair cells in hearing loss is followed by the degeneration of spiral ganglion cells. This degeneration can be attributed to the loss of hair cell activity as well as survival factors, such as neurotrophins. Neurotrophic factors have the ability to influence cell growth, differentiation, division, and survival. Application of neurotrophic factors as a clinical therapeutic for hearing loss has been limited due to their short half-life in vivo, as well as poor pharmacokinetics, proteolytic degradation, and the potential side-effects of high-dose, systemic delivery. However, the advent of novel drug delivery systems, as described below, has allowed for localized neurotrophin delivery to the inner ear and the decrease in chances of non-specific and undesirable side-effects.
Neurotrophins can be delivered to the inner ear by direct injection or longer-term continuous delivery by methods such as polymer release, cannula microinfusion systems, or osmotic pump [95]. Delivery of exogenous neurotrophic factors to the inner ear has been shown to preserve both hair cells and SGN. The neurotrophic factors NT-3, glial cell-derived neurotrophic factor (GDNF), BDNF, and fibroblast growth factor (FGF) have all been shown to protect hair cells from cell death in models of ototoxicity [95–99]. Nerve growth factor, NT-3, BDNF, GDNF, and ciliary neurotrophic factor promote SGN survival in numbers similar to pre-deafened states, individually and in combination [95, 100–102]. BDNF and NT-3, in particular, have been implicated as the principal neurotrophins involved in the neuronal development of the cochlea, and consequently, the in vivo survival and rescue of adult auditory neurons [11]. More recently, it has been shown that rescue can occur even after delayed neurotrophin delivery. Miller et al. [102] examined the effects of chronic infusion of BDNF and FGF initiated 4 days, 3 weeks, and 6 weeks after de-afferentiation from deafening and found that maintenance and regrowth of spiral ganglion cells could occur with delayed treatment out to 6 weeks. However, relatively little is known about the longevity of effects after treatment is withdrawn. There is evidence that SGN survival is maintained well after cessation of neurotrophin administration [103]. However, it has also been demonstrated that as early as 2 weeks after cessation of BDNF treatment, a rapid decline in neuron survival could be seen in that the number surviving was not significantly different from deafened, untreated cochleae [104, 105]. Furthermore, the rate of neuronal degeneration after cessation of treatment was more rapid than in the deafened, untreated cochleae. The exact mechanism behind loss of survival effects is unclear. It is thought that this accelerated degeneration may be due to the loss of direct trophic support to the auditory neurons from BDNF, plus a loss of endogenous survival factors from supporting cells that were also preserved by BDNF. It has also been shown that BDNF increases nitric oxide synthase expression, leading to an increase in nitric oxide production, which can initiate a neurotoxic cascade. Furthermore, the reaction of NO with superoxide radicals associated with certain ototoxic agents leads to the formation of even stronger oxidative agent, which accumulates and only elicits a response after BDNF withdrawal [104].
However, the effect of neurotrophins can be enhanced when administered in combination with other forms of therapy, such as electrical stimulation. in vivo studies have demonstrated enhanced SGN survival in deafened cochleae treated with concurrent exogenous neurotrophin delivery and electrical stimulation [106, 107]. More importantly, continued chronic electrical stimulation after cessation of neurotrophin delivery significantly reduced the rate of SGN loss, although this effect was localized to the region of the cochlea proximal to the electrode array [105]. Therefore, a system which can provide electrical simulation as well as concurrent delivery of antioxidants would be synergistic to continuous exogenous neurotrophin treatment.
4.2.2. Gene Therapy
Gene therapy is the addition or transfer of a segment of genetic material into a cell that results in expression of transgenic protein, and ultimately a change in the function or behavior of the cell. Although the development of gene therapy for the human ear to date has been limited due to technical and ethical issues, it still remains a promising technique for treating both genetic and acquired forms of hearing loss [108, 109]. In the inner ear, gene therapy is generally targeted at three main application areas: the introduction of protective factors to enhance spiral ganglion and hair cell survival, the regeneration of functional hair cells by transdifferentiation of supporting cells, and the treatment of hereditary disease by insertion of a functional wild-type gene.
Local delivery to the inner ear of genes that can express therapeutic products is an alternative to the delivery of exogenous molecules for spiral ganglion and hair cell protection and survival. Transfer of BDNF [110, 111], GDNF [112, 113], or NT-3 [114] genes in the cochlea gave comparable outcomes to conventional exogenous delivery of neurotrophic factors in terms of SGN and hair cell survival in the injured inner ear. Gene therapy to deliver GDNF in conjunction with chronic electrical stimulation has also been applied. Individually, both treatments exhibited significant rescue of SGN compared with deafened controls, with GDNF being more effective than chronic electrical stimulation [106]. However, as with exogenous neurotrophin delivery, combining treatments was significantly more effective than either treatment alone. Aside from long term delivery of neurotrophins, gene therapy has also been used to provide overexpression of antioxidants. Overexpression of catalase and manganese superoxide dismutase was able to protect hair cells and hearing thresholds in a guinea pig aminoglycoside ototoxicity model [115]. The overexpression of antioxidant genes may prove beneficial in protecting the cochlea against oxidative stress due to other forms of ototoxicity, such as noise, as well as aging.
Because the mature cochlear sensory epithelium has only sensory hair cells and non-sensory supporting cells, regeneration in the inner ear sensory epithelium must depend on the transdifferentiation of one cell type to another. Many of the genes and factors which determine cell fate in the inner ear have been identified. By re-expressing these developmental genes in the injured tissue, supporting cells could potentially be forced into a hair cell phenotype. One such gene is Atoh1 (also known as Math1, or the human homolog Hath1). Atoh1 is a basic helix-loop-helix transcription factor which is necessary to induce cell differentiation in the epithelial ridge of the developing organ of Corti. Atoh1 has also been shown to be successful in transdifferentiation of supporting cells into new hair cells in the deafened adult guinea pig by demonstration of increased hair cell counts and lower thresholds on ABR relative to untreated, deafened controls [116, 117].
Genes whose dysfunction or mutation is associated with hearing loss are also possible targets for inner ear gene therapy. Advances in molecular biology and genetics have enabled the identification of over 75 different genes contributing to hereditary hearing loss; each of these is a potential target for gene therapy [118, 119]. The largest proportion of hereditary sensorineural hearing loss cases are due to mutations in the GJB2 gene, which encodes the gap junction channel protein connexin 26 (cx26). Connexins are transmembrane proteins that assemble to form the gap junction channel of a cell, which in turn regulates intracellular signaling. In the inner ear, cx26 is widely expressed throughout non-sensory epithelial and connective tissue cells, allowing these cells to communicate in regulating fluid and ion balance. Potassium ions are transported by gap junctions to maintain high levels of the endocochlear potential for hair cell excitation [120, 121]. Alterations in cx26 cause a disturbance in potassium homeostasis by preventing potassium ions from being recycled by the hair cells back to the endolymph, ultimately leading to hair cell death by potassium intoxication. However, because GJB2 knockout mice are embryonic-lethal, the pathogenesis of deafness due to mutations of this gene has been difficult to examine. Therefore, several groups are working towards viable mouse models to study the pathological role of GJB2 [120, 122, 123]. Relevant mouse models for studying abnormalities of GJB2 in the pathogenesis of deafness will be needed before clinical applications of gene therapy can be considered for such cases of hereditary hearing loss.
The final common pathway in any case, acquired or genetic hearing loss, and inner ear dysfunction, is hair cell injury and loss. By targeting hair cells, supporting cells, or neurons, gene therapy can be used to change cellular phenotype for preservation, rescue, and even regeneration of hair cells. The various cell types of the inner ear can be selectively transfected by viral vectors, including adenovirus, adeno-associated virus, herpes simplex virus, vaccinia virus, lentivirus, and nonviral cationic liposomes. However, cytotoxicity and short-term expression of gene products remain problematic. Reviews regarding the practicality of utilizing these transfer agents can be found in the literature [119, 124, 125].
The ear itself is considered a particularly attractive target organ for the study and potential application of gene therapy. The bony housing of the sensory epithelium isolates the organ system, thereby limiting unwanted systemic diffusion of transfer agents. However, delivery of vectors into the inner ear requires increasingly sophisticated methods of delivery. Although liposomes and some viral vectors can follow pathways for small molecules, such as diffusion across the RWM, most vectors cannot. Therefore, efforts in delivery of transfer agents have focused on delivery to the basal turn of the cochlea through a cochleostomy, or by injection directly through the RWM. Vector delivery can also be achieved by accessing the inner ear through the stapes footplate or the semicircular canal [125]. However, similar to the delivery of other therapeutic agents to the inner ear, diffusion of vectors through the cochlea shows a gradient of transfection with the greatest at the site of injection. Therefore, one important consideration in moving towards clinical applications will be determining a method to provide the uniform distribution of transfer throughout the entire cochlea. Another consideration will be the duration of expression of the transgene. Currently, expression is often short-lived, lasting only days to months. A method of delivery which ensures continued expression of the therapeutic gene over an extended period of time will also be crucial in the successful applications of gene therapy.
4.2.3. RNA Interference
In certain situations, it is desirable to decrease or eliminate the expression and function of a particular gene. Studies on mouse cochleae have shown that a large number of negative cell growth genes are upregulated in later development, including cyclin-dependant kinase inhibitors (p27kip1 and p19inkd)[126] and tumor suppressor genes (pRb)[127]. Their expression coincides with cell cycle exit, as well as establishment and maintenance of the quiescent status of cochlear sensory epithelia. Certain genes may also be upregulated as a result of injury. For example, ototoxic insult from aminoglycosides, cisplatin, and noise can all lead to a common cascade of cell death genes leading to cell cycle arrest and apoptosis. Potential targets in this cascade include caspases, the Bcl-2 family of proteins, p53, and the c-jun NH2-terminal kinases [128].
The effects of these genes have been well characterized by creating null cells in culture and in knockout animal models. Targeted inhibition of gene expression can be carried out utilizing homologous recombination, antisense vectors, ribozymes, and DNAzymes. However, recently, a new method of gene manipulation known as RNA interference (RNAi) has shown to be a promising therapeutic approach in shutting off gene expression. RNAi is a method used to silence gene expression post-transcriptionally through the introduction of double stranded RNA. The double stranded RNA induces the sequence-specific degradation of complementary messenger RNA. RNAi provides a rapid means to assess the loss of gene. However, double stranded RNA longer than 30 base pairs provokes the antiviral/interferon pathway in mammals and results in the global shutdown of protein synthesis. Therefore, the use of RNAi as a clinical therapy did not become apparent until the advent of chemically synthesized small interfering RNAs (siRNAs) of 21–22 nucleotides, which could be used to target mammalian genes by RNAi while evading the interferon response [129, 130].
In order to realize RNAi as a therapeutic modality for gene silencing, several obstacles need to be addressed. SiRNAs are relatively small and are rapidly excreted when administered through the blood stream. They are also relatively unstable in a serum environment with a half-life of approximately 0.8 hours, and can be degraded by RNase activity within a short window of time [131]. As with other molecules, when administered systemically, a nonspecific distribution occurs, which in turn decreases the local concentration at the site of action. Even at the target site, cellular uptake requires efficient endocytosis of the double stranded siRNA. The duration of siRNA effects is also limited, lasting only 3–5 days in rapidly proliferating cells and up to three weeks in non-dividing cells [131]. To increase stability, techniques to chemically modify siRNA oligonucleotides are being explored including changes in backbone structure, replacement of nucleotides with analogs, and addition of conjugates. Both viral and non-viral carrier systems are also being examined as a means to efficiently transfect cells. However, the development of systems for local delivery will be highly dependent on the accessibility of the target tissue.
Several siRNA-based drug candidates are in pre-clinical commercial development for applications in the inner ear, and recently, lipocomplexed siRNA has been effectively delivered intact by Gelfoam to the RWM in rats with successful silencing in the cochlea [132]. Also, siRNA complexes perfused directly to the scala media of the guinea pig have shown uptake in strial cells along the length of the cochlea [133]. However, before a clinical development program can be initiated for siRNA in the inner ear, basic information such as residence time of siRNA in the inner ear cells and duration of therapeutic effect will need to be examined. With long-term expression of siRNAs, RNAi may become a potential therapeutic strategy to preserve hearing.
4.2.4. Cell-Based Therapy
Recent advances have also made possible the potential for cell-based approaches to preserve hearing as well as for regeneration [124, 134, 135]. Cells have been transplanted to the inner ear as an answer to the need for long term stable delivery of neurotrophins, which is not possible with current methods of exogenous neurotrophin delivery and gene transfection. Fibroblasts transduced with BDNF transgene have been encapsulated in a matrix of agarose and delivered via a modified electrode through a cochleostomy site [136]. These cells were shown to express transgene and secrete BDNF in vivo. The BDNF-excreting electrodes were also able to preserve significantly more spiral ganglion cells than control electrodes, though this effect decreased with distance from the basal turn. Although, this approach combines the electrode stimulation with a possible method for long term growth factor delivery, a method would need to be set in place to ensure that these transplanted cells would not proliferate, invade or obliterate the cochlea. Another cell-based method for neurotrophin delivery is the transplantation of neural stem cells [137]. Neural stem cells injected by infusion pump into the lateral semicircular canal were found attached to the cochlea in every turn after 28 days. Most of these cells expressed markers for glial cells and were positive for GDNF, while approximately 50% of the cells were positive for BDNF. If injected at the time of cochlear implant surgery, the spontaneous production of neurotrophin by these transplant cells could provide an effective mechanism for spiral ganglion preservation.
Although these neural stem cells are able to survive in the inner ear environment, they have not been found to migrate into the sensory epithelium in normal ears, but have had limited success in migration and transdifferention into hair cells in ears subjected to traumatic injury. Parker et al. [138] found that neuronal stem cells from a clonal cell line were able to migrate as well as receive signals from the microenvironment to upregulate genetic cell fate programs expressed by local endogenous cells - either neural, glial, hair cell or supporting cell types. Therefore, though the inner ear cannot regenerate sensory cells from its own endogenous population, it is still able to influence stem cell differentiation among particular cochlear phenotypes.
The cell type with the greatest capacity for differentiation into multiple cell types is the pluripotent embryonic stem cell. Survival of embryonic stem cells in the inner ear after transplantation has been well documented, and migration can occur in the auditory nerve fibers of the rat cochlea [139–142]. However, undifferentiated cells grafted into the inner ear also showed no potential to differentiate into hair cell phenotype [142]. Embryonic stem cells can, however, be directed by applying cues such as in normal development. It has been shown that, from embryonic stem cells, it is possible to enrich a population of inner ear progenitor cells which express marker genes that define the developing inner ear by culture in the presence of endothelial growth factor and IGF-1 [143]. Injection of these cells into chick embryonic ear resulted in integration into cochlear sensory epithelia and a hair cell phenotype. Recently it has been shown that endogenous stem cells exist and can be isolated from the adult ear and expanded as neurospheres in vitro in the presence of mitogenic factors to obtain sufficient numbers of cells for transplantation [144, 145]. Injection of these inner ear progenitor cells into the chick inner ear resulted in similar results to the injection of embryonic stem cell derived progenitors in terms of survival, integration, and differentiation into hair cells.
Another option for cell regeneration is bone-marrow derived cells, which can be obtained from the recipient patient, enabling autologous transplant [146, 147]. When introduced into the chinchilla inner ear without selection for inner ear progenitors, the cells showed the upregulation of neural and glial markers [147]. However, the number is limited, and a method to induce neuronal differentiation would be needed in order to induce regeneration of SGN from transplants.
Stem cell therapy is a promising step in the regeneration of sensory cells in the inner ear and the restoration of hearing. However, it is difficult to predict how this technology can be translated into clinical practice. Functional restoration will depend not only on the introduction of new cells, but also differentiation of cells to the correct phenotype, and the formation of appropriate three-dimensional tissue structures. This will rely heavily on the ability to control the local microenvironment of the cells once transplanted. The derived cells and tissues will also need to be mechanically and structurally compliant with the native tissue, and able to integrate into the host without immunological rejection. Before stem cells can be considered for therapeutic applications, these issues of sustainability and functionality must be addressed. In this relatively young field, it is currently unclear what environment is needed to induce functional cells and tissues of the inner ear. It is possible that a treatment for hearing loss does not exist in any one therapy, and the ability to restore hearing will ultimately rely on an intricate combination of drug treatment, gene manipulation, as well as transplantation in conjunction with implanted devices, such as integrated delivery systems and cochlear prostheses.
5. Inner ear drug delivery methods
Inner ear drug delivery methods can be divided into two main categories based on the location of entry of the drug. Intratympanic delivery involves depositing the therapeutic agent in the middle ear, relying primarily on diffusion through the RWM for access to the scala tympani. The second method, intracochlear, depends on a cochleostomy with direct delivery into the inner ear space, completely bypasses the middle ear.
Intratympanic delivery must rely solely on diffusion through the round window and for dispersion throughout the scala tympani. Therefore, access to the apical regions of the scala tympani can be limited, and large concentration gradients may develop. Also, the RWM presents a physical barrier to delivery. A great deal of variability of condition and thickness of the membrane across population has been observed [148, 149], limiting dosage control from patient to patient. Alzamil reports, for example, round window niche obstruction in a third of human ears [150]. Also, due to the anatomy of the round window, substances may be more or less likely to transverse the membrane based on size and constitution [45, 151]. Because it is applied in the middle ear, drug can be lost through the Eustachian tube, which leads from the middle ear to the pharynx. These factors, along with the inability to control specific delivery parameters, combine for a lack of precise control of dosage in intratympanic delivery. This method of delivery does offer less opportunity for surgical trauma or damage than the intracochlear method, and the middle ear is relatively easy to access. Many of these implantation procedures do not require general anesthesia.
Intracochlear delivery provides direct access to neural and sensory cells and does not necessarily rely solely on diffusion for drug distribution. Fluid flow may augment diffusion which could provide access to more apical regions of the cochlea. Because drug is deposited directly into the perilymph, the types of molecules and/or particles are not limited by RWM transit. While dosage may be precisely controlled, a greater possibility of surgical trauma exists. Additionally, indwelling devices are at risk of biofouling due to tissue growth and protein build-up in the scala tympani.
Both intratympanic and intracochlear delivery offer opportunities for inner ear drug delivery and can be utilized as required for optimal delivery parameters.
5.1. Intratympanic drug delivery
For intratympanic delivery, multiple passive and active drug delivery approaches are being explored, with applications ranging from protection from NIHL to delivery of regenerative compounds aimed at arresting sensorineural hearing loss. This research is often aimed at establishing extended release profiles with controllable and predictable kinetics using compounds within the middle ear. In the following, examples of passive drug delivery to the cochlea will be described for several principal approaches: biodegradable polymers, hydrogelbased systems, and nanoparticles. In addition, examples of active intratympanic delivery are described using a variety of components and devices, including microcatheters, microwicks, and osmotic pumps. Here, we define active as any device used for intratympanic dosing of therapeutics, although in some cases, these devices by themselves do not provide a mechanism for changing dosing.
5.1.1. Passive Intratympanic delivery
Passive drug delivery systems are designed to deliver drug compounds with specific kinetic profiles using local triggers for release, such as hydrolysis in the in vivo environment, or in response to local stimuli such as temperature or pH. Drug may be dispersed within a matrix or contained within a reservoir encapsulated by a shell of bulk polymer, depending upon the specific application.
5.1.1.1. Biodegradable polymer intratympanic delivery
Early biodegradable polymer drug delivery systems, developed for a wide range of therapeutic applications, were based upon poly (lactic) co-glycolic acid (PLGA), and this approach has also been employed for the inner ear. Biodegradable polymer delivery has been successfully employed for a number of clinical applications, including implantation of wafers to treat glioblastoma multiforme, depot systems for contraception and treatments for endometriosis or estrogen replacement therapy, and inserts for patients with glaucoma. Biodegradable polymer devices for delivery may take the form of degradable microparticles or nanoparticles, hydrogels or larger microfabricated structures with specific release profiles.
Sustained release structures based on siloxane have been investigated as fast- and slow-release vehicles for delivery of compounds of interest for inner ear disease therapies and tinnitus. Release rates for devices on the order of 1mm in dimension ranged from 15 – 50 µg/day, and were filled with glucocorticoid or beclomethasone and placed on the RWM to study effects on hearing over the 28 day duration of the guinea pig studies. Monitoring of auditory function via ABR, assays of perilymph following delivery, and histology were all conducted. Histology appeared normal in the guinea pig cochleae, and assays demonstrated the presence of beclomethasone in the perilymph of the scala tympani of virtually all implanted animals. Auditory function over a range of frequencies from 3 – 12 kHz was altered as monitored by ABR thresholds, and by day 28 there were significant differences in hearing for all but the placebo groups [152].
The specific nature of the biodegradable polymer delivery vehicle can determine whether efficacious therapy is achieved, such as in the targeting of SGN [95]. Meniere’s disease patients have been treated using a biodegradable gelatin polymer matrix known as Gelfoam, with high success rates for elimination of vertigo and tinnitus following gentamicin-soaked Gelfoam material placement on the RWM [153]. When Gelfoam was applied in an animal model as a means to reverse hearing loss and promote SGN survival in deafened guinea pigs, however, there was no evidence of enhanced response. Specifically, SGN survival was not observed when neurotrophin-soaked Gelfoam was administered to deafened guinea pigs, in spite of the fact that radiolabeling confirmed that release of the drug was sustained. When alginate polymer was substituted for Gelfoam, SGN survival was maintained in the implanted ear, suggesting that the kinetics and maintenance of drug concentration is dependent upon the specific polymer chemistry and degradation characteristics of the delivery vehicle [154].
5.1.1.2. Hydrogel-based intratympanic delivery
Hydrogel-based delivery for therapeutic applications can enable precise targeting of tissues and organs through the use of triggering mechanisms. These triggers may be chemical, including pH or other ionic factors or the presence of specific molecular species, or may be physical, such as temperature, pressure or electrical potential. Triggering will cause the hydrogel to swell and release its payload of drug in a controlled fashion. Many of the investigations of hydrogel-based delivery for treatment of inner ear diseases have employed biodegradable hydrogels, often with a hyaluronic acid-based chemistry. In one such study, a rescue agent was delivered locally onto the RWM following impulse noise trauma. The JNK inhibitor AM-111 was prepared in a hyaluronic acid gel formulation and applied to the RWM in a chinchilla, following exposure to acute acoustic trauma. The gel-based delivery approach was observed to provide significant and rapid recovery from the original insult, presumably due to fast degradation of the gel and subsequent transport of the drug across the RWM and into the cochlea [58].
Cochlear protection from NIHL has also been investigated using IGF. In one study, recombinant human insulin-like growth factor -1 (rhIGF-1) was loaded into a biodegradable hydrogel produced by glutaraldehyde cross-linking of collagen, and delivered by placement on the RWM. Auditory response was monitored using ABR measurements following noise exposure, and immunostaining was conducted to monitor outer hair cell degeneration. These evaluations concluded that significant protective benefit to the outer hair cells was conferred by the biodegradable hydrogel treatment with rhIGF-1 [56].
In another set of studies, BDNF was delivered to assess protection of auditory neurons, using the same biodegradable hydrogel carrier. Concentrations of BDNF in the perilymph were seen to be much higher in the cochleae of guinea pigs from the hydrogel group than in either control animals or a group that received an injection through the RWM. Analysis using ELISA confirmed sustained delivery of growth factor for periods of 7 days after placement of the hydrogel on the RWM [155–157].
5.1.1.3. Nanoparticle delivery
Nanoparticle delivery to the inner ear has emerged as a promising new avenue for delivering compounds to the cochlea in a sustained and controllable manner. The nanoparticles may be comprised of biodegradable or nondegradable materials, depending upon the application and the desired pharmacokinetic profile. In one study, Tamura et al. [158] encapsulated traceable fluorescent dye into PLGA nanoparticles and compared delivery profiles with transport of the unencapsulated rhodamine molecule in a guinea pig model. Nanoparticles with diameter of roughly 150 nm were prepared using a 50/50 ratio of lactic / glycolic acid and with rhodamine B contained within the matrix. For both systemic application and placement on the RWM, nanoparticles containing rhodamine B produced significant levels of rhodamine in the cochlea, as assessed by counting of fluorescent particles. The presence of rhodamine was not observed in the cochlea following either systemic application or RWM placement of the free molecule.
Nanoparticles have been investigated as an alternative to viral vector systems, which have shown promise for treatment of inner ear diseases but carry concerns about safety as well as precision of targeting of specific cells and tissues [159]. As a model system, Praetorius and co-workers delivered silica nanoparticles labeled with the fluorescent cyanine dye, Cy3, to the cochlea by placement on the RWM of mice. The silica particles had a diameter of 20 nm and their transport was observed via immunohistochemistry and histology, accompanied by ABR testing to ensure that hearing thresholds were not altered. No evidence of cytotoxicity was observed, and silica nanoparticles were observed in inner hair cells, vestibular hair cells and in the spiral ganglia. An interesting observation stemming from this work was the occurrence of retrograde axonal transport [159].
In another study, PLGA nanoparticles were prepared with a uniform dispersion of superparamagnetic iron oxide nanoparticles within the PLGA matrix and delivered in a chinchilla. The PLGA nanoparticles ranged from 100 – 300 nm in diameter, while the embedded magnetic materials were in the 5 – 15 nm range. Nanoparticles were placed upon the RWM for duration of 40 minutes. Either with or without magnetic field exposure, magnetite and PLGA-coated nanoparticles were observed throughout all turns of the cochlea, within the perilymph and within the inner and outer hair cells, as well as in the stria vascularis and the spiral ligament [160]. It is well known that low molecular weight compounds such as gentamicin can cross the RWM quite readily [44], either through tight junctions or via an intracellular pathway. The application of magnetic fields and modulation of degradation profiles of the PLGA can provide additional levels of control over long-term drug delivery to the inner ear structures.
Liposomes represent another particle-based mode for delivery to the inner ear, and these vehicles have been explored as a means for gene therapy. Cationic liposomes can be mixed with DNA for transfection of numerous cell types, and introduced into the cochlea via microinjection. In one such study, Wareing et al. [161] reported that 14 days of transgene expression was observed with an absence of toxicity or inflammatory response. Guinea pigs were treated with either injection or an osmotic pump delivery of cationic liposomes containing DNA complexes, and tissue sections were evaluated using immunohistochemistry and PCR analysis. From the base to the apex, beta-galactosidase was observed in virtually all tissue types, with highest concentrations in the spiral ligament; PCR-based assays confirmed these observations. Persistence of the transgene expression for two weeks was observed for both microinjection and osmotic pump infusion; however the osmotic pump delivery was associated with local trauma and inflammatory responses [161]. This suggests that liposome delivery is a viable approach for intratympanic delivery but that the means to transport the liposomes to the cochlea is a critical element of the strategy.
5.1.2. Active intratympanic drug delivery
Active intratympanic drug delivery was explored at least as early as the 1950’s for streptomycin ablation treatment of vertigo associated with Meniere’s disease [162]. This study showed that vestibular function could be attenuated, but a majority of test cases lost all cochlear function. It appears that interest in intratympanic drug delivery did not substantially accelerate until the 1990’s, although there were earlier large-scale efforts underway, e.g. Sakata’s study of dexamethasone treatment for tinnitus [163]. Intratympanic delivery is now the most frequently used first-line treatment for the vertigo of Meniere’s disease [82]. The methods for and uses of intratympanic drug delivery are sufficiently numerous that review articles have been dedicated to the topic [43, 79, 82, 164]. Treatments have expanded beyond those initially performed for vertigo, and now include perfusion methods to treat SSNHL, AIED, tinnitus, and the hearing loss associated with Meniere’s Disease. The compounds tested are numerous and include aminoglycosides, corticosteroids, glutamate receptor antagonists, protease inhibitors, antioxidants, and neurotrophins [95, 165]. The specific methods for intratympanic infusion are varied. Direct infusion via syringe was successfully used to treat patients 3–5 times per day in a study where gentamicin resolved vertigo in Meniere’s Disease as long as 25 years after therapy started in greater than 90% of cases [166]. The residence time of drugs delivered by this method of direct infusion can be increased by placing Gelfoam [164], fibrin glue[167], or other compounds at the round window, thereby increasing the likelihood of sustained diffusion into the cochlea across the membrane. Device development efforts have focused on at least two goals: the ability to improve the likelihood of delivering compound directly to the round window niche and the capability for either continuous or multi-dose drug administration, in some cases self-administered. Concentration of drug delivered and residence time at the niche site are affected not only by the exact placement of the infusion line but also by residual fluid in the middle ear and the patency of the Eustachian tube. Numerous devices which can be used for sustained release or multi-dose delivery have been developed in attempts to overcome the shortcomings of direct transtympanic injection. These include the Silverstein MicroWick (Micromedics, Eaton, MN), the Round Window Microcatheter or µCath™ (Durect Corp. Cupertino, CA) in conjunction with an electronically controlled pump, the Alzet osmotic pump (Durect Corp. Cupertino, CA) and other devices still in earlier stages of development. While these devices are sufficiently promising to warrant further study and development, the clinical results achieved by intratympanic delivery have to date been mixed. Despite demonstrations that higher concentrations of medication can be delivered to the cochlea, and that side effects are either completely alleviated or dramatically reduced as compared with systemic delivery methods [6], there are still major impediments to controlled, repeatable intratympanic drug delivery. As described earlier, anatomical variations can significantly alter the pathway from the delivery site to the round window. These include extraneous or false membranes, fibrous tissue and fatty plugs, and may be present in as high as one third of all patients [168]. In addition, there is a need for better characterization of pharmacokinetics in both the inner and middle ear. While substantial progress has been made on this front, particularly by Salt and Plontke [169][170][171], the inability to define consistent and accurate methods (or the lack of broad acceptance of such methods) for withdrawal and analysis of fluid samples taken from the inner ear during preclinical animal studies substantially impedes progress [43]. Certainly most reported clinical results do not include measurements of drug concentration at the round window or other quantities like middle ear fluid clearance rate that might be used to infer an approximate concentration. Even in preclinical animal experiments, measurement of cochlear concentrations is difficult. The need for continued development of improved methods for quantifying drug concentration in both the middle and inner ear is clear. Combined approaches, for example Hashimoto’s combination of hearing test and subsequent morphologic examination of hair cell death [172] appear promising. Ultimately it appears as though wide acceptance of a particular device or method may require the development of measurement techniques that provide data which permits evaluation of drug delivery and treatment outcome against a pharmacokinetic model.
5.1.2.1 Round Window Microcatheter
The Round Window Microcatheters (µ-Cath™ and e-Cath™) were developed by IntraEAR and later acquired by Durect in 1999. While they have been used for a wide variety of applications in both human and animal studies, Durect discontinued sales of these devices in September 2003 due to a change in business focus (2003 Form 10-K SEC filing) and subsequently entered into a licensing agreement with Neurosystec in the area of local drug delivery to the middle or inner ear for tinnitus (2004 Form 10-Q Ex.-10.39 SEC filing). The ẽCath™ has two lumens, one for infusion and one for fluid withdrawal, and the ẽCath™ has a third lumen with electrode for monitoring ear signals. The microcatheter has a bulbous tip of 1.5, 2.0 or 2.5 mm diameter. General anesthesia is typically required to produce a tympanomeatal flap through which the round window niche is exposed. The tip of the catheter system is compressible and is designed specifically to lock in place in the round window bony niche. In theory, a variety of pumping methods can be used to infuse the catheter, but frequently pumps developed for wearable insulin dispensing (e.g. Disetronics pumps from Roche) are used because their size provides the portability required for extended treatment protocols [85]. As described earlier, anatomical variation can produce variability in the quantity of drug actually delivered to the cochlea. At least one effort has been made to formalize the surgical procedure for Microcatheter implantation, advise the use of a transtympanic endoscope, and identify likely complications [148, 173].
Numerous animal studies have also been performed in an effort to understand pharmacokinetics using this delivery method. Two radioactively labeled antioxidants (D-methionine and thiourea) were administered for 1 hr in rat ears and elimination half-lives in the cochlea were found to be less than 1 hr for both compounds [176]. Ototoxic kanamycin was used in guinea pigs with differing concentration and delivery time intervals [172]. Outer hair cell disappearance rates were quantified as a function of position through the basal and second turn of the cochlea. As expected, large outer hair cell disappearance rates correlated with high delivery concentrations and longer delivery intervals, and much lower total quantities of compound were required to achieve a specific level of outer hair cell disappearance as compared to systemic delivery.
5.1.2.2 Silverstein MicroWick ™
The primary use for the MicroWick in humans since its first description [177] has been the treatment of Meniere’s related vertigo by perfusion of gentamicin. The polyvinyl acetate wick (1 mm diameter by 9 mm length) absorbs medication administered external to the ear and transports it directly to the RWM. The wick is inserted through a ventilation tube which passes through the ear drum via myringotomy or laser tympanostomy. The insertion procedure requires only local anesthesia and a noncertified office surgery room. Patients are able to self-administer medication, and hearing and vestibular functional tests are used for appropriate medication titration and therapy discontinuation. A typical administration protocol requires three fifteen minute sessions per day using from 5 to 10 mg/ml gentamicin for one to three weeks. The MicroWick has also been used to administer dexamethasone for the treatment of SSNHL and AIED [177, 178]. While quantitative studies of drug concentration in the cochlea have not been published to our knowledge, therapeutic effect has been shown [82]. Complications include the removal of middle ear plugs or extraneous membranes prior to wick insertion, extrusion of the vent tube, and the administration of topical antibiotics (in the case of steroid infusion). Finally, the MicroWick has also been implemented as a delivery tool in numerous animal studies aimed at understanding pharmacokinetics. Chinchilla ears were perfused with gentamicin (10 mg/ml) to characterize hearing loss over time [179]. However, we know of no reported quantification of the MicroWick’s effective delivery rate of drug as a function of time.
5.1.2.3 Preclinical Alzet osmotic pump
The implantable Alzet osmotic pump provides the means for continuous sustained delivery in a very small volume with simple form factor. This preclinical device was developed by ALZA Corporation and is now commercially available through Durect Corporation. The pump has reservoir volumes of 100 µL, 200 µL or 2 mL and flow rates ranging from 0.11 to 10 µL/hr. The device can be configured with a microcatheter or other infusion cannula and implanted in small animals such as guinea pigs and other rodents. Typically, access to the RWM occurs through a drilled hole in the bulla, and the pump is implanted subcutaneously [180]. Depending on the model, these pumps provide continuous infusion ranging from 1 day to 6 weeks; however, they provide no capability to change delivery rates in situ. The pump operates with a semi-permeable outer membrane surrounding an osmotic driving agent. Within the agent compartment, an impermeable reservoir holds the drug. As water flows into the compartment holding the osmotic agent, pressure is exerted on the flexible drug reservoir, and the drug is forced out through the delivery port [181]. These systems have been widely used for middle and inner ear drug delivery as described in Section 4.
5.1.2.4 Other systems
A miniaturized fully-implanted device for discontinuous drug delivery has been evaluated for animals. The Totally Implantable Drug Delivery System (TI-DDS®) is implanted subcutaneously and dispenses 5 to 10 µL of fluid via push button and can be refilled transcutaneously [182, 183]. We have not found publicized descriptions dated later than 2001 and are thus unclear on the status of development of this promising device.
5.2. Intracochlear drug delivery
A second local delivery approach for the inner ear is intracochlear delivery. A variety of tools including syringes, osmotic pumps, and other newer devices have been employed. Access is created via a cochleostomy through the RWM or directly through the otic capsule.
5.2.1. Syringe delivery
A simple method of delivering therapeutics directly into the inner ear is with a syringe. Direct injections and perfusions have been used in the clinical setting as well as in research. They allow for study of pharmaceutical effects without the added complications of more advanced delivery systems. However, direct injections do not allow for prolonged delivery.
Currently the only clinical use of direct intracochlear injection is in conjunction with cochlear prosthesis implantation. Cochlear prosthesis implantation has been show to elicit an inflammatory response. As reported by De Ceulaer et al., at the time of prosthesis insertion, a single dose of steroid was placed in the cochlea with a syringe. The treatment resulted in reduced reactive fibrosis that is usually caused by tissue insult. In general, fibrosis leads to increased impedance of the electrode which decreases electrode performance. The steroid injection was done to maintain optimal performance of the implant through reduced tissue growth [184]. Similarly, a direct injection of triamcinolone immediately prior to insertion of the electrode array reduced electrode impedance for 5–10 days post-surgery. The improvement was more pronounced at basal contacts than apical contacts showing an uneven distribution of steroid along the scala tympani [185].
5.2.2. Direct injection
Direct injection is a useful tool for researchers to study the effects of novel therapies for the inner ear [186, 187]. Stover et al. tested the use of adenovirus for gene transfer of reporter gene though both the round window and cochleostomy. The round window was injected with a 5 µL bolus using a 30 gauge needle, and a cannula was sealed in the cochleostomy to facilitate delivery. The cochleostomy delivery route resulted in better distribution throughout the cochlea and a higher degree of transfection [188]. Similarly, liposomes with plasmid DNA were injected via the RWM to test them as alternatives to viral vectors. Gene expression persisted for 14 days without evidence of inflammation [161]. Other work has been done to deliver agents to reduced damage in the inner ear, such as D-JNKI-1 to block the apoptotic cascade or glutamate antagonists to block firing of inner hair cell afferents [187, 189].
5.2.3. Syringe pump delivery
A variation on the direct injection method involves sealing a cannula into the cochlea and employing a syringe pump for drug infusion, usually with an outlet hole placed in the apex [190]. While the pump allows for continued delivery and can be easily programmed for constant infusion, it can only be used for acute animal experiments while the subject remains under anesthesia. In developing a surgical technique for delivery to the mouse cochlea, a syringe pump was used to deliver agents to confirm maintenance of hearing during surgery [191]. Also useful in pharmacokinetic studies, ions and microspheres can be delivered for tissue labeling and concentration studies [192, 193].
5.2.4. Osmotic pump delivery
Initial tests were used to show the effectiveness and safety of delivery by osmotic pump. The device delivered the toxin, TTX, for three days [180], and later experiments improved on cannula materials and sealing [194]. Carvalho et al. used the pump to deliver artificial perilymph to show the effect of cochleostomy and infusion on hearing. ABR was tested at 3 and 7 days after implantation, and results showed some threshold shifts at high frequency while low frequency hearing was preserved [195].
The osmotic pump has proven useful in testing a variety of compounds for many applications including otoprotection and neural preservation, as it offers a controlled delivery profile and proven surgical technique. Steroids have been administered at the time of cochlear prosthesis implantation to protect the cochlea from electrode insertion trauma [196] and at the time of ototoxic antibiotic exposure to prevent hair cell death [75].
Osmotic pumps were employed to test the effectiveness of steroid treatment to prevent hearing loss from noise trauma. The devices allowed for a dosage study of pre- and post-treatment with dexamethasone for noise exposure, and histology showed less hair cell loss than in untreated cochleae [49]. A similar study showed that 7 day infusion of steroid following impulse noise trauma resulted in no significant threshold difference after 14 days [50].
In order to study the ototoxicity of cisplatin chemotherapy treatment, researchers have utilized osmotic pumps to infuse various doses of cisplatin and record the increase in hearing thresholds [63]. To counter the damage to hair cells inflicted by cisplatin, STS was infused using the Alzet pump with a glass cannula [72]. STS protects the hair cells from death when given in combination with cisplatin. However, the local delivery afforded by the osmotic pump is essential, as the STS would inactivate the cisplatin throughout the body if dosed systemically. Other compounds have been investigated to block signaling events in the apoptotic pathway started by cisplatin ototoxicity [197].
Osmotic pumps allow testing of novel therapies such as growth factors for various applications. Neurotrophins, such as BDNF, have been studied as treatments to preserve neurons in deafened ears. This has implication in the degree of success a patient would achieve using a cochlear implant. Several studies continued to deliver neurotrophic factors for two to four weeks after deafening, and these therapies show increased survival of SGN in the inner ear [102, 104, 198–200].
The pump is being used to test other compounds such as D-JNKI-1, a peptide inhibitor of the apoptotic pathway, and BN82270, a peroxidation inhibitor to reduce free radical production, for their protective effects on the cochlea. BN82270, delivered into the cochlea via osmotic pump prior to trauma, was used to prevent damage from NIHL [201]. The application of D-JNKI-1 to rescue hair cells from aminoglycoside antibiotic ototoxicity or NIHL resulted in protection from both types of damage. In this study, the cochlea was perfused for seven days beginning two days after exposure [60].
5.2.5. Cochlear prosthesis-based delivery
Since the introduction of cochlear prostheses, researchers have worked to continuously improve their effectiveness in providing enhanced auditory function to patients. Cochlear implants stimulate the auditory neurons in the cochlea through an electrode inserted into the scala tympani. Hearing loss that causes patients to receive implants causes the continued degradation of neurons which can reduce the effectiveness of the electrodes. Evidence suggests the application of drugs in combination with the electrode can lead to better outcomes (reviewed in [202, 203]). By combining a drug delivery platform with the implant electrode, drugs such as neurotrophic factors may prevent further auditory degradation and maintain the number of surviving nerve fibers, thus improving the effectiveness of cochlear implants for long-term use [204]. In addition, steroids and other compounds could be used to prevent tissue growth on the electrode to keep impedance and, thus, power requirements low [205].
Med-El is a cochlear implant company investigating the combination of drug delivery with a prosthesis. Their development effort includes an electrode with a fluid channel connected to an infusion pump with outlets that allow delivery of drug into the basal turn. Cross-turn diffusion would supply the apical regions of the cochlea [205]. Cochlear, Ltd. is another cochlear implant company investigating drug delivery combined with the prosthesis [206].
Significant work has gone into testing these devices on the bench top prior to study in a guinea pig. Dyes were used to visualize the flow and delivery through the electrode platform and ensure patency, and various numbers and locations of openings in the fluid channel were tested to optimize the delivery profile [205–207]. Multiple openings led to the fastest distribution in a plastic cochlea model [208].
Testing in guinea pigs began with a standard electrode having polyimide tubing running through its center to an osmotic pump. The electrode was inserted through the round window and delivered 200 µL of drug over 28 days. Using neomycin, an ototoxic antibiotic, as a model compound, the assembly remained operational for the full delivery duration and showed minimal tissue response [209]. In a study using therapeutic agent BDNF designed to protect neurons for 28 days, results showed reduced hearing thresholds when compared to control [107].
5.2.6. Other delivery devices
Multiple pumping devices are in development, including a micro-injector and a reciprocating drug delivery device. Kingma et al. created a micro-injector from a syringe fitted with a screw to regulate the infused volume of drug for a chronic animal study. The cannula remained implanted allowing for daily injections of a toxin for 8 to 14 days [210].
Chen et al. have reported on a reciprocating drug delivery system [19]. The microfluidic device is being developed for use as an implantable long-term infusion system for a variety of drug types. The device uses a reciprocating fluidic system with one cannula directly implanted in the scala tympani that acts as both the inlet and outlet. The drug concentrated in this fluid diffuses and mixes with the perilymph in the scala tympani. During withdrawal, the same volume of less concentrated fluid is returned to the device. This pulsatile method may protect the sensitive hair cells from damaging changes in cochlear volume and pressure while also creating a net increase in drug concentration. The electronically-controlled device provides for precise control of delivery volumes, delivery of multiple therapeutics, and timed, sequenced delivery that can be altered as indicated [19, 211].
6. Concluding remarks
As treatment options improve for many inner ear diseases and injuries, methods for delivering precise and controlled doses become vital. Researchers in the field of inner ear drug delivery will continue to advance new and existing techniques that will support the arrival of better and better therapeutic compounds. Those suffering from hearing related disorders can look forward to improved quality of life as the field progresses.
Acknowledgments
This publication was made possible by Grant Number 5 R01 DC 006848-02 from NIH and Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the U.S. government. The authors are grateful to Charles Stark Draper Laboratory for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juhn S. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- 2.Juhn S, Rybak L. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 1981;91:529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- 3.McCabe BF. Autoimmune inner ear disease: Therapy. Am J Otol. 1989;10:196–197. [PubMed] [Google Scholar]
- 4.Moskowitz D, Lee KJ, Smith HW. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope. 1984;94:664–666. [PubMed] [Google Scholar]
- 5.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772–776. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- 6.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: An animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 7.Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–169. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Ryan AF, Pak K, Low W, Battaglia A, Mullen L, Harris JP, Keithley EM. Immunological damage to the inner ear: Current and future therapeutic strategies. Adv Otorhinolaryngol. 2002;59:66–74. doi: 10.1159/000059242. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Lalwani AK, Jero J, Mhatre AN. Current issues in cochlear gene transfer. Audiol Neurootol. 2002;7:146–151. doi: 10.1159/000058300. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF. Ablation therapy for the relief of meniere's disease. Laryngoscope. 1956;66:859–870. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Salt, Stopp The effect of cerebrospinal fluid pressure on perilymphatic flow in the opened cochlea. Acta Otolaryngol. 1979;88:198–202. doi: 10.3109/00016487909137160. [DOI] [PubMed] [Google Scholar]
- 14.Nuttall AL, LaRouere MJ, Lawrence M. Acute perilymphatic perfusion of the guinea pig cochlea. Hear Res. 1982;6:207–221. doi: 10.1016/0378-5955(82)90055-7. [DOI] [PubMed] [Google Scholar]
- 15.Tonndorf J, Duvall AJ, Reneau JP. Permeability of intracochlear membranes to various vital stains. Ann Otol Rhinol Laryngol. 1962;71:801–841. doi: 10.1177/000348946207100317. [DOI] [PubMed] [Google Scholar]
- 16.Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 17.Kingma GG, Miller JM, Myers MW. Chronic drug infusion into the scala tympani of the guinea pig cochlea. J Neurosci Methods. 1992;45:127–134. doi: 10.1016/0165-0270(92)90050-n. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Kujawa SG, McKenna MJ, Fiering JO, Mescher MJ, Borenstein JT, Swan EEL, Sewell WF. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J Control Release. 2005;110:1–19. doi: 10.1016/j.jconrel.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuknecht H. Pathology of the ear. Malvern, PA: Lea and Febiger; 1993. [Google Scholar]
- 21.Medina J, Drescher D. The amino-acid content of perilymph and cerebrospinal fluid from guinea-pigs and the effect of noise on the amino-acid composition of perilymph. Neuroscience. 1981;6:505–509. doi: 10.1016/0306-4522(81)90142-1. [DOI] [PubMed] [Google Scholar]
- 22.Scheibe F, Haupt H. Biochemical differences between perilymph, cerebrospinal fluid and blood plasma in the guinea pig. Hear Res. 1985;17:61–66. doi: 10.1016/0378-5955(85)90131-5. [DOI] [PubMed] [Google Scholar]
- 23.Thalmann I, Kohut R, Ryu J, Comegys T, Senarita M, Thalmann R. Protein profile of human perilymph: In search of markers for the diagnosis of perilymph fistula and other inner ear disease. Otolaryngol Head Neck Surg. 1994;111:273–280. doi: 10.1177/01945998941113P117. [DOI] [PubMed] [Google Scholar]
- 24.Thalmann I, Kohut R, Ryu J, Thalmann R. High resolution two-dimensional electrophoresis: Technique and potential applicability to the study of inner ear disease. Am J Otol. 1995;16:153–157. [PubMed] [Google Scholar]
- 25.Thalmann R, Comegys T, Thalmann I. Amino acid profiles in inner ear fluids and cerebrospinal fluid. Laryngoscope. 1982;92:321–328. doi: 10.1288/00005537-198203000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi M, Ohashi K, Ishii M. Morphometric comparison of endolymphatic and perilymphatic spaces in human temporal bones. Acta Otolaryngol. 1986;101:161–164. doi: 10.3109/00016488609132823. [DOI] [PubMed] [Google Scholar]
- 27.Jahnke K. The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl. 1975;336:1–40. [PubMed] [Google Scholar]
- 28.Axelsson A, Ryan AF. Circulation of the inner ear: I. Comparative dtudy of the vascular anatomy in the mammalian cochlea. In: Santos-Sacchi, editor. Physiology of the ear. New York: Raven Press; 1988. pp. 295–316. [Google Scholar]
- 29.Kimura R, Ota C. Ultrastructure of the cochlear blood vessels. Acta Otolaryngol. 1974;77:231–250. [PubMed] [Google Scholar]
- 30.Hara A, Salt AN, Thalmann R. Perilymph composition in scala tympani of the cochlea: Influence of cerebrospinal fluid. Hear Res. 1989;42:265–272. doi: 10.1016/0378-5955(89)90150-0. [DOI] [PubMed] [Google Scholar]
- 31.Gopen Q, Rosowski J, Merchant S. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 32.Rask-Andersen H, Schrott-Fischer A, Pfaller K, Gluekert R. Perilymph/modiolar communication routes in the human cochlea. Ear Hear. 2006;27:457–465. doi: 10.1097/01.aud.0000233864.32183.81. [DOI] [PubMed] [Google Scholar]
- 33.Sterkers O, Ferrary E, Amiel C. Production of inner ear fluids. Physiol Rev. 1988;68:1083–1128. doi: 10.1152/physrev.1988.68.4.1083. [DOI] [PubMed] [Google Scholar]
- 34.Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper A, Fay R, editors. The cochlea. New York: Springer-Verlag; 1996. pp. 130–185. [Google Scholar]
- 35.Zou J, Pyykko I, Counter S, Klason T, Bretlau P, Bjelke B. In vivo observation of dynamic perilymph formation using 4.7 t mri with gadolinium as a tracer. Acta Otolaryngol. 2003;123:910–915. doi: 10.1080/00016480310000548. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd R, Colreavy M. Surface microstructure of the perilymphatic space: Implications for cochlear implants and cell- or drug-based therapies. Arch Otolaryngol Head Neck Surg. 2004;130:518–523. doi: 10.1001/archotol.130.5.518. [DOI] [PubMed] [Google Scholar]
- 37.Schuknecht H, Seifi A. Experimental observations on the fluid physiology of the inner ear. Ann Otol Rhinol Laryngol. 1963;72:687–712. doi: 10.1177/000348946307200308. [DOI] [PubMed] [Google Scholar]
- 38.Thalmann I, Comegys T, Liu S, Ito Z, Thalmann R. Protein profiles of perilymph and endolymph of the guinea pig. Hear Res. 1992;63:37–42. doi: 10.1016/0378-5955(92)90071-t. [DOI] [PubMed] [Google Scholar]
- 39.Salt AN. Pharmacokinetics of drug entry into cochlear fluids. Volta Rev. 105:277–298. [PMC free article] [PubMed] [Google Scholar]
- 40.Salt AN. Simulation of methods for drug delivery to the cochlear fluids. Adv Otorhinolaryngol. 2002;59:140–148. doi: 10.1159/000059251. [DOI] [PubMed] [Google Scholar]
- 41.Salt AN, Ohyama K, Thalmann R. Radial communication between the perilymphatic scalae of the cochlea. I estimation by tracer perfusion. Hear Res. 1991;56:29–36. doi: 10.1016/0378-5955(91)90150-8. [DOI] [PubMed] [Google Scholar]
- 42.Salt AN, Ohyama K, Thalmann R. Radial communication between the perilymphatic scalae of the cochlea. Ii estimation by bolus injection of tracer into the sealed cochlea. Hear Res. 1991;56:37–43. doi: 10.1016/0378-5955(91)90151-x. [DOI] [PubMed] [Google Scholar]
- 43.Salt AN, Plontke SKR. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10:1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juhn S, Hamaguchi Y, Goycoolea M. Review of round window membrane permeability. Acta Otolaryngol Suppl. 1989;457:43–48. doi: 10.3109/00016488809138883. [DOI] [PubMed] [Google Scholar]
- 45.Goycoolea M, Lundman L. Round window membrane, structure function and permeability: A review. Microsc Res Tech. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea pig cochlea. Hear Res. 1988;35:119–130. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 47.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291–1298. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 48.Henderson D, McFadden SL, Liu CC, Hight N, Zheng XY. The role of antioxidants in protection from impulse noise. Ann N Y Acad Sci. :368–380. doi: 10.1111/j.1749-6632.1999.tb08655.x. [DOI] [PubMed] [Google Scholar]
- 49.Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Sendowski I, Abaamrane L, Raffin F, Cros A, Clarencon D. Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2006;221:119–127. doi: 10.1016/j.heares.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Uemaetomari I, Tabuchi K, Hoshino T, Hara A. Protective effect of calcineurin inhibitors on acoustic injury of the cochlea. Hear Res. 2005;209:86–90. doi: 10.1016/j.heares.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Coleman JKM, Kopke RD, Liu J, Ge X, Harper EA, Jones GE, Cater TL, Jackson RL. Pharmacological rescue of noise induced hearing loss using n-acetylcysteine and acetyl-l-carnitine. Hear Res. 2007;226:104–113. doi: 10.1016/j.heares.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Campbell KCM, Meech RP, Klemens JJ, Gerberi MT, Drystad SSW, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise- and drug-induced hearing loss with d-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15 doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- 55.Duan M, Chen Z, Qiu J, Ulfendahl M, Laurell G, Borg E, Ruan R. Low-dose, long-term caroverine administration attenuates impulse noise-induced hearing loss in the rat. Acta Otolaryngol. 2006;126:1140–1147. doi: 10.1080/00016480500540519. [DOI] [PubMed] [Google Scholar]
- 56.Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim T-S, Tabata Y, Ito J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–533. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- 57.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–142. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 58.Coleman JKM, Littlesunday C, Jackson R, Meyer T. Am-111 protects against permanent hearing loss from impulse noise trauma. Hear Res. 2007;226:70–78. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Harris KC, Hu B, Hangauer D, Henderson D. Prevention of noise-induced hearing loss with src-ptk inhibitors. Hear Res. 2005;208:14–25. doi: 10.1016/j.heares.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Van de Water TR, Bonny C, de Ribaurpierre F, Puel JL, Zine A. A peptide inhibitor of c-jun n-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rybak LP, Whitworth CA. Ototoxicity: Therapeutic opportunitities. Drug Discov Today. 2005;10:1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 62.Wanamaker H. Perfusion of the inner ear: Basic science considerations. Curr Opin Otolaryngol Head Neck Surg. 2001;9:329–332. [Google Scholar]
- 63.O'Leary SJ, Klis FL, deGroot JCMJ, Hamers FPT, Smoorenburg GF. Perilymphatic application of cisplatin over several days in albino guinea pigs: Dose-dependency of electrophysiological and morphological effects. Hear Res. 2001;154:135–145. doi: 10.1016/s0378-5955(01)00232-5. [DOI] [PubMed] [Google Scholar]
- 64.Rybak L. Mechanisms of cisplatin ototoxicity and progress in ototprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15:364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- 65.Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 66.Lefebvre PP, Staecker H, Van de Water T, Moonen G, Malgrange B. Pharmacologic treatment of inner ear: From basic science to the patient. Acta Otorhinolaryngol Belg. 2002;56:45–49. [PubMed] [Google Scholar]
- 67.Sergi B, Fetoni AR, Ferraresi A, Troiani D, Azzena GB, Paludetti G, Maurizi M. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngolog Suppl. 2004;552:42–45. doi: 10.1080/03655230410017111. [DOI] [PubMed] [Google Scholar]
- 68.Li G, Frenz DA, Brahmblatt S, Feghali JG, Ruben RJ, Berggren D, Arezzo J, Van de Water TR. Round window membrane delivery of l-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotox. 2001;22:163–176. doi: 10.1016/s0161-813x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 69.Wimmer C, Mees K, Stumpf P, Welsch U, Reichel O, Suckfull M. Round window application of d-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced otototoxicity. Otol Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Ekborn A, Laurell G, Ehrsson H, Miller J. Intracochlear administration of thiourea protects against cisplatin-induced outer hair cell loss in the guinea pig. Hear Res. 2003;181:109–115. doi: 10.1016/s0378-5955(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 71.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicitiy and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Faulconbridge RV, Lloyd, Fetoni A, Guitton MJ, Pujol R, Puel JL. Local application of sodium thiosulfate precents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology. 2003;45:380–393. doi: 10.1016/s0028-3908(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 73.Stocks RMS, Gould HJ, Bush AJ, Dudney BW, Pousson M, Thompson JW. Ototoxic protection of sodium thiosulfate: Daily vs constant infusion. Otolaryngol Head Neck Surg. 2004;131:115–119. doi: 10.1016/j.otohns.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 74.Hochman J, Blakley BW, Wellman M, Blakley L. Prevention of aminoglycoside-induced sensorineural hearing loss. J Otolaryngol. 2006;35:153–156. [PubMed] [Google Scholar]
- 75.Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, Doi T, Kuriyama H, Miller JM, Yamashita T. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- 76.Bhandare N, Antonelli PJ, Morris C, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469–479. doi: 10.1016/j.ijrobp.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Jereczek-Fossa BA, Zarowski A, Milani F, Orecchia R. Radiotherapy-induced ear toxicity. Cancer Treat Rev. 2003;29:417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 78.Ye Q, Tilleing J, Hartmann R, Gstoettner W, Kiefer J. Application of a corticosteroid (triamcinolon) protects inner ear function after surgical intervention. Ear Hear. 2007;28:361–369. doi: 10.1097/01.aud.0000261655.30652.62. [DOI] [PubMed] [Google Scholar]
- 79.Light J, Silverstein H. Transtympanic perfusion: Indications and limitations. Curr Opin Otolaryngol Head Neck Surg. 2004;12:378–383. doi: 10.1097/01.moo.0000134438.91734.38. [DOI] [PubMed] [Google Scholar]
- 80.Mosnier I, Bouccara D, Sterkers O. Management of idiopathic sudden sensorineural hearing loss. Otorhinolaryngol Nova. 1999;9:217–223. [Google Scholar]
- 81.Hoffmann KK, Silverstein H. Inner ear perfusion: Indications and applications. Curr Opin Otolaryngol Head Neck Surg. 2003;11:334–339. doi: 10.1097/00020840-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 82.Jackson LE, Silverstein H. Chemical perfusion of the inner ear. Otolaryngol Clin North Am. 2002;35:639–653. doi: 10.1016/s0030-6665(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 83.Chandrasekhar SS. Intratympanic dexamethasone for sudden sensorineural hearing loss: Clinical and laboratory evaluation. Otol Neurotol. 2001;22:18–23. doi: 10.1097/00129492-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Plontke SK, Lowenheim H, Preyer S, Leins P, Dietz K, Koitschev A, Zimmermann R, Zenner H-P. Outcomes research analysis of continuous intratympanic glucocorticoid delivery in patients with acute severe to profound hearing loss:Basis for planning randomized controlled trials. Acta Otolaryngol. 2005;125:830–839. doi: 10.1080/00016480510037898. [DOI] [PubMed] [Google Scholar]
- 85.Kopke RD, Hoffer ME, Wester D, O'Leary MJ, Jackson RL. Targeted topical steroid therapy in sudden sensorineural hearing loss. Otol Neurotol. 2001;22:475–479. doi: 10.1097/00129492-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Plaza G, Herraiz C. Intratympanic steroids for treatment of sudden hearing loss after failure of intravenous therapy. Otolaryngol Head Neck Surg. 2007;137:74–78. doi: 10.1016/j.otohns.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 87.Gianoli GJ, Li JC. Transtympanic steroids for treatment of sudden hearing loss. Otolaryngol Head Neck Surg. 2001;125:142–146. doi: 10.1067/mhn.2001.117162. [DOI] [PubMed] [Google Scholar]
- 88.Dallan I, Bruschini L, Nacci A, Bruschini P, Traino C, Rognini F, Fattori B. Transtympanic steroids as a salvage therapy in sudden hearing loss: Preliminary results. ORL J Otorhinolaryngol Relat Spec. 2005;68:247–252. doi: 10.1159/000093093. [DOI] [PubMed] [Google Scholar]
- 89.Rauch S. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–1074. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Haynes DS, O'Malley M, Cohen S, Watford K, Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2007;117:3–15. doi: 10.1097/01.mlg.0000245058.11866.15. [DOI] [PubMed] [Google Scholar]
- 91.Battista R. Intratympanic dexamethasone for profound idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2005;132:902–905. doi: 10.1016/j.otohns.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 92.Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: Basic mechanisms and options for therapy. Acta Otolaryngol Suppl. 2002;548:38–43. doi: 10.1080/00016480260094965. [DOI] [PubMed] [Google Scholar]
- 93.Yang GSY, Song H, Keithley EM, Harris JP. Intratympanic immunosuppressives for prevention of immune-mediated sensorineural hearing loss. Am J Otol. 2000;21:499–504. [PubMed] [Google Scholar]
- 94.Matteson EL, Fabry DA, Strome SE, Driscoll CLW, Beatty CW, McDonald TJ. Autoimmune inner ear disease: Diagnostic and therapeutic approaches in a multidisciplinary setting. J Am Acad Audiol. 2003;14:225–230. [PubMed] [Google Scholar]
- 95.Richardson RT, Noushi F, O'Leary S. Inner ear therapy for neural preservation. Audiol Neurootol. 2006;11:343–356. doi: 10.1159/000095896. [DOI] [PubMed] [Google Scholar]
- 96.Beck DL. Hair cells: Review, regeneration and protection. Oticon Clinical Update. 2007;1:14. [Google Scholar]
- 97.Kuang R, Hever G, Zajic G, Yan O, Collins F, Louis JC, Keithley E, Magal E. Glial cell line-derived neurotrophic factor. Potential for otoprotection. Annals of the New York Academy of Sciences. 1999 doi: 10.1111/j.1749-6632.1999.tb08648.x. [DOI] [PubMed] [Google Scholar]
- 98.Lopez I, Honrubia V, Lee SC, Chung WH, Gang L, Beykirch K, Micevych P. The protective effect of brain-derived neurotrophic factor after gentamicin ototoxicity. The American Journal of Otology. 1999;20:317–324. [PubMed] [Google Scholar]
- 99.Oestreicher E, Knipper M, Arnold A, Zenner HP, Felix D. Neurotrophin 3 potentiates glutamatergic responses of ihc afferents in the cochlea in vivo. European Journal of Neuroscience. 2000;12:1584–1590. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- 100.Gillespie LN, Clark GM, Phillip M. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- 101.Gluekert R, Mario, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentiation-associated changes in afferent and effert processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. The Journal of Comparative Neurology. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- 102.Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowh of auditory nerve peripheral processes:Effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85:1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- 103.Maruyama J, Miller JM, Ulfendahl M. Glial cell-line derived neurotrophic factor and antioxidants preserve electrical responsicenes of the spiral ganglion neruons after experimentally induced deafness. Neurobiology of Disease. 2008;29:14–21. doi: 10.1016/j.nbd.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. Bdnf-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 105.Shepherd, Coco, Epp Neurotrophins and electrical stimulation for protection oand repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008 doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanazaki S, Stover T, Kawamoto K, Pirieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronicelectrical stimulation prevent viii cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 107.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuind auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pau H, Clarke RW. Advances in genetic manipulations in the treatment of hearing disorders. Clin Otolaryngol. 2004;29:574–576. doi: 10.1111/j.1365-2273.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 109.Scully JL, Rippberger C, Rehmann-Sutter C. Non-professionals'evaluations of gene therapy ethics. Soc Sci Med. 2004;58:1415–1425. doi: 10.1016/S0277-9536(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 110.Lalwani A, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. The Laryngoscope. 2002;112:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 111.Staeker H, Gabaizadeh R, Federoff H, Van de Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngology-Head and Neck Surgery. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- 112.Hakuba N, Watabe K, Ohashi T, Eto Y, Taniguchi M, Yang L, Tanaka J, Hata R, Gyo K. Adenovirus-mediated overexpression of a gene prevents hearing loss and progressive inner hair cell loss after transient cochlear ischemia in gerbils. Gene Therapy. 2003;10:426–433. doi: 10.1038/sj.gt.3301917. [DOI] [PubMed] [Google Scholar]
- 113.Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with tgf-b1 and gdnf. Molecular Therapy. 2003;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 114.Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attentuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Molecular Therapy. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- 115.Kawamoto K, Sha S-H, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Molecular Therapy. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 116.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 117.Kawamoto K, Ishimoto S-i, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. The Journal of Neuroscience. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown SD, Hardistry-Hughes RE, Mburu P. Quiet as a mouse: Dissecting the molecular and genetric basis of hearing. Genetics. 2008;9:277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- 119.Kesser BW, Hashisaki GT, Holt JR. Gene transfer in human vestibular epithelia and the prospects for inner ear gene therapy. Laryngoscope. 2008;118:821–831. doi: 10.1097/MLG.0b013e318164d0aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kudo T, Kure S, Ikeda K, Xia A-P, Katori Y, Suzuki M, Kojima K, Ichinohe A, Suzuki Y, Aoki Y, Kobayashi T, Matsubara Y. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of corti and non-syndromic deafness. Human Molecular Genetics. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- 121.Van Eyken E, Laer LV, Fransen E, Topsakal V, Hendrickx J-J, Demeester K, Van de Heyning P, Miaki-Torko E, Hannula S, Sorri M, Jensen M, Parving A, Bille M, Baur M, Pfister M, Bonaconsa A, Mazzoli M, Orzan E, Espeso A, Stephens D, Verbruggen K, Huyghe J, Dhooge i, Huygen P, Kremer H, Cremers C, Kunst S, Pawelczyk M, Sliwinska-Kowalska m, Steffens M, VWienker T, Van Camp, Guy The contribution of gjb2 (connexin 26) 35delg to age-related hearing impairment and noise-induced hearing loss. Otology & Neruotology. 2007;28:970–975. doi: 10.197/MAO.0b013e3180dca1b9. [DOI] [PubMed] [Google Scholar]
- 122.Cohen-Salmon M, Ott T, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Current Biology. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maeda Y, Fukushima K, Kawasaki A, Nisjizaki K, Smith RJH. Cochlear expression of a dominant negative gjb2r75w construct delivered through the round window membrane in mice. Neuroscience Research. 2007;58:250–254. doi: 10.1016/j.neures.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochar A, de Silva MG, Dahl H-HM, Rose SD, Behlke MA, Smith JH. Advances in molecular and cellular therapies for hearing loss. Molecular Gene Therapy. 2008;16:224–236. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- 125.Staecker H, Brough DE, Praetorius M, Baker K. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37:1091–1108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 126.Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel E, Hackney C, Zenner H-P. Gene disruption of p27kip1 allows cell proliferation in the postnatal and adult organ of corti. Proc Nat Acad Sci. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Z-y. Cell cycle, differentiation and regeneration. Cell Cycle. 2006;5:2609–2612. doi: 10.4161/cc.5.22.3503. [DOI] [PubMed] [Google Scholar]
- 128.Cheng AG, Cunningham LL, Rubel E. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- 129.Oliveira S, Storm G, Schiffelers Targeted delivery of sirna. J Biomed Biotechnol. 2006;2006:1–9. [Google Scholar]
- 130.Xie FY, Woodle MC, Lu PY. Harnessing in vivo sirna delivery for drug discovery and therapeutic development. Drug Discov Today. 2006;11:67–73. doi: 10.1016/S1359-6446(05)03668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gary DJ, Puri N, Won T-T. Polymer-based sirna delivery: Perspectives on teh fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release. 2007;121:64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 132.Maeda Y, Fukushima K, Nishizaki K, Smith RJH. In vitro and in vivo suppression of gjb2 expression by rna interference. Hum Mol Genet. 2005;14:1641–1650. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- 133.Sellick P, Layton MG, Rodger J, Robertson D. A method for introducing non-silencing sirna into the guinea pig cochlea in vivo. J Neurosci Methods. 2008;167:237–245. doi: 10.1016/j.jneumeth.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 134.Li H, Corrales E, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends in Molecular Medicine. 2004;10:309–315. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Nakagawa T, Ito J. Application of cell therapy to inner ear diseases. Acta Oto-Laryngologica. 2004;124:6–9. doi: 10.1080/03655230310016735. [DOI] [PubMed] [Google Scholar]
- 136.Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo bdnf gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14:77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- 138.Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hearing Research. 2007;232:29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coleman B, Hardman J, Coco A, Epp SB, de Silva MG, Crook JM, Shepherd RK. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hildebrand MS, Dahl H-HM, Hardman J, Coleman B, Shepherd RK, De Silva MG. Survival of partially differentiated mouse embryonic stem cells in the scala media of the buinea pig cochlea. Journal of the Association for Research in Otolaryngology. 2005;6:341–354. doi: 10.1007/s10162-005-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu Z, Ulfendaho M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Research. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 142.Sakamoto T, Nakagawa T, Endo T, Kim T-s, Iguchi F, Naito Y, Sasai Y, Ito J. Fates of mouse embryonic stem cells transplanted into the inner ears of adult mice and embryonic chickens. Acta Oto-Laryngologica. 2004;124:48–52. doi: 10.1080/03655230310016825. [DOI] [PubMed] [Google Scholar]
- 143.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. PNAS. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinez-Monedero R, Oshima K, Heller S, Edge A. The potential role of endogenous stem cells in regeneration fo the inner ear. Hearing Research. 2007;22:48–52. doi: 10.1016/j.heares.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oshima K, Grimm C, Corrales E, Senn P, Martinez-Mondero R, Geleoc G, Edge A, Holt J, Heller S. Differential distribution of stem cells in teh auditory and vestibular organs of the inner ear. Journal of the Association for Research in Otolaryngology. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matsuoka A, Kondo T, Miyamoto R, Hashino E. Enhanced survival of bone-marrow derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117:1629–1635. doi: 10.1097/MLG.0b013e31806bf282. [DOI] [PubMed] [Google Scholar]
- 147.Naito Y, Nakamura T, Nakagawa T, Iguchi F, Endo T, Fujino K, Kim T-S, Hiratsuka Y, Tamura T, Kanemaru S, Shimizu Y, Ito J. Transplantation of bone marrow stromal cells into the cochlea of chinchillas. Neuroreport. 2004;15:1–4. doi: 10.1097/00001756-200401190-00001. [DOI] [PubMed] [Google Scholar]
- 148.Plontke SK, Plinkert P, Plinkert B, Koitschev A, Zenner H, Lowenheim H. Transtympanic endoscopy for drug delivery to the inner ear using a new microendoscope. Adv Otorhinolaryngol. 2002;59:149–155. doi: 10.1159/000059253. [DOI] [PubMed] [Google Scholar]
- 149.Banerjee, Parnes The biology of intratympanic drug administration and pharmacodynamics of round window drug absorption. Otolaryngol Clin North Am. 2004;37:1035–1051. doi: 10.1016/j.otc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 150.Alzamil KS, Linthicum FHJ. Extraneous round window membranes and plugs: Possible effect on intratympanic therapy. Ann Otol Rhinol Laryngol. 2000;109:30–32. doi: 10.1177/000348940010900105. [DOI] [PubMed] [Google Scholar]
- 151.Goycoolea The round window membrane under normal and pathological conditions. Acta Otolaryngol Suppl. 1992;493:43–55. [PubMed] [Google Scholar]
- 152.Arnold, Senn, Henig, Michaelis, Deingruber, Scheler, Steinhoff, Riphagen, Lamm Novel slow- and fast-type drug release round-window microimplants for local drug application to the cochlea: An experimental study in guinea pigs. Audiol Neurootol. 2005;10:53–63. doi: 10.1159/000082575. [DOI] [PubMed] [Google Scholar]
- 153.Pasic T, EW R. Rapid changes in cochlear nucleus cell size following blockage of auditory nerve electrical activity in gerbils. J Comp Neurol. 1989;283:474–480. doi: 10.1002/cne.902830403. [DOI] [PubMed] [Google Scholar]
- 154.Noushi, Richardson, Hardman, Clark, O'Leary Delivery of neurotrophin-3 to the cochlea using alginate beads. Otology & Neurotology. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- 155.Albrecht J, Gaudet S, Jensen KF. Rapid free flow isoelectric focusing via novel electrode structures. 9th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS); Boston. 2005. [Google Scholar]
- 156.Ito, Endo, Nakagawa, Kita, Kim, Iguchi A new method for drug application to the inner ear. ORL J Otorhinolaryngol Relat Spec. 2005;67:272–275. doi: 10.1159/000089407. [DOI] [PubMed] [Google Scholar]
- 157.Endo T, Nakagawa T, Kita T, Iguchi F, Kim T-S, Tamura T, Iwai K, Tabata Y, Ito J. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–2020. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- 158.Tamura T, Kita T, Nakagawa T, Endo T, Kim T-S, Ishihara T, Mizushima Y, Higaki M, Ito J. Drug delivery to the cochlea using plga nanoparticles. Laryngoscope. 2005;115:2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 159.Praetorius M, Brunner C, Lehnert B, Klingmann C, Schmidt H, Staecker H, Schick B. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127:486–490. doi: 10.1080/00016480600895102. [DOI] [PubMed] [Google Scholar]
- 160.Ge, Jackson, Liu, Harper, Hoffer, Wassel, Dormer, Kopke, Balough Distribution of plga nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137:619–623. doi: 10.1016/j.otohns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 161.Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MHF, Hong K, Zheng WW, Lalwani AK. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 162.Schuknecht HF. Ablation therapy in the management of meniere's disease. Acta Otolaryngol Suppl. 1957;132:3–42. [PubMed] [Google Scholar]
- 163.Sakata E, Ito Y, Itoh A. Clinical experiences of steroid targeting therapy to inner ear for control of tinnitus. Int Tinnitus J. 1997;3:117–121. [PubMed] [Google Scholar]
- 164.Blakley BW. Update on intratympanic gentamicin for meniere's disease. Laryngoscope. 2000;110:236–240. doi: 10.1097/00005537-200002010-00009. [DOI] [PubMed] [Google Scholar]
- 165.Seidman MD, Van de Water TR. Pharmacologic manipulation of the labyrinth with novel and traditional agents delivered to the inner ear. Ear Nose Throat J. 2003;82:276–300. [PubMed] [Google Scholar]
- 166.Lange G. Transtympanic gentamycin in the treatment of ménière's disease. Rev Laryngol Otol Rhinol (Bord) 1995;116:151–152. [PubMed] [Google Scholar]
- 167.Hoffer M, Balough B, Henderson J, DeCicco M, Wester D, O'Leary M, Kopke R. Use of sustained release vehicles in the treatment of meniere's disease. Otolaryngol Clin North Am. 1997;30:1159–1166. [PubMed] [Google Scholar]
- 168.Alzamil KS, Linthicum FH., Jr Extraneous round window membranes and plugs: Possible effect on intratympanic therapy. Ann Otol Rhinol Laryngol. 2000;109:30–32. doi: 10.1177/000348940010900105. [DOI] [PubMed] [Google Scholar]
- 169.Salt AN, Hale SA. Longitudinal distribution of drugs in perilymph assessed by cochlear action potentials. 28th Mid Winter Research Meeting of the ARO.2005. [Google Scholar]
- 170.Salt AN, Hale SA, Plontke SK. Perilymph sampling from the cochlear apex: A reliable method to obtain higher purity perilymph samples from the scala tympani. J Neurosci Methods. 2005 doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 172.Hashimoto Y, Iwasaki S, Mizuta K, Arai M, Mineta H. Pattern of cochlear dmamge caused by short-term kanamycin application using the round window microcatheter method. Acta Otolaryngol. 2007;127:116–121. doi: 10.1080/00016480600794438. [DOI] [PubMed] [Google Scholar]
- 173.Plontke SKR, Wood AW, Salt AN. Analysis of gentamicin kinetics in fluids of the inner ear with round window adminstration. Otol Neurotol. 2002;23:967–974. doi: 10.1097/00129492-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 174.Seidman MD. Glutamate antagonists, steroids, and antioxidants as therapeutic options for hearing loss and tinnitus and the use of an inner ear drug delivery system. Int Tinnitus J. 1998;4:148–154. [PubMed] [Google Scholar]
- 175.Lefebvre PP, Staecker H. Steroid perfusion of the inner ear for sudden sensorineural hearing loss after failure of conventional therapy: A pilot study. Acta Otolaryngol. 2002;122:698–702. [PubMed] [Google Scholar]
- 176.Laurell G, Teixera M, Sterkers O, Bagger-Sjoback D, Eksbor S, Lidman O, Ferrary E. Local administration of antioxidants to the inner ear: Kinetics and distribution. Hear Res. 2002;173:198–209. doi: 10.1016/s0378-5955(02)00613-5. [DOI] [PubMed] [Google Scholar]
- 177.Silverstein H. Use of a new device, the microwick, to deliver medication to the inner ear. Ear Nose Throat J. 1999;78:595–600. [PubMed] [Google Scholar]
- 178.Hillman TM, Arriaga MA, Chen DA. Intratympanic steroids: Do they acutely improve hearing in cases of cochlear hydrops? Laryngoscope. 2003;113:1903–1907. doi: 10.1097/00005537-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 179.Killian P, Hoffer M. Microwick-delivered transtympanic gentamicin: Hearing effects. Otolaryngol Head Neck Surg. 2004;131:153–154. [Google Scholar]
- 180.Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 181.Theeuwes F, Yum SI. Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann Biomed Eng. 1976;4:343–353. doi: 10.1007/BF02584524. [DOI] [PubMed] [Google Scholar]
- 182.Lehner R, Brugger H, Maassen MM, Zenner H. A totally implantable drug delivery system for local therapy of the middle and inner ear. Ear Nose Throat J. 1997;76:567–570. [PubMed] [Google Scholar]
- 183.Praetorius M, Limberger A, Muller M, Lehner R, Schick B, Zenner H, Plinkert P, Knipper M. A novel microperfusion system for the long term local supply of drugs to the inner ear: Implantation and function in the rat model. Audiol Neurootol. 2001;6:250–258. doi: 10.1159/000046130. [DOI] [PubMed] [Google Scholar]
- 184.De Ceulaer G, Johnson S, Yperman M, Daemers K, Offeciers FE, O'Donoghue GM, Govaerts PJ. Long-term evaluation of the effect of intracochlear steroid deposition on electrode impedance in cochlear implant patients. Otol Neurotol. 2003;24:769–774. doi: 10.1097/00129492-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 185.Paasche G, Bockel F, Tasche C, Lesinski-Schiedat A, Lenarz T. Changes of postoperative impedances in cochlear implant patients: The short-term effects of modified electrode surfaces and intracochlear corticosteroids. Otol Neurotol. 2006;27:639–647. doi: 10.1097/01.mao.0000227662.88840.61. [DOI] [PubMed] [Google Scholar]
- 186.Praetorius M, Baker K, Brough DE, Plinkert P, Staecker H. Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hear Res. 2007;227:53–58. doi: 10.1016/j.heares.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 187.Oestreicher E, Arnold W, Ehrenberger K, Felix D. New approaches for inner ear therapy with glutamate receptors. Acta Otolaryngol. 1999;119:174–178. doi: 10.1080/00016489950181611. [DOI] [PubMed] [Google Scholar]
- 188.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: Round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 189.Eshraghi AA, Van de Water TR. Cochlear implantation trauma and noise-induced hearing loss: Apoptosis and therapeutic strategies. Anat Rec. 2006;228A:473–481. doi: 10.1002/ar.a.20305. [DOI] [PubMed] [Google Scholar]
- 190.Konishi T, Kelsey E. Effect of sodium deficiency on cochlear potentials. J Acoust Soc Am. 1968;43:462–470. doi: 10.1121/1.1910853. [DOI] [PubMed] [Google Scholar]
- 191.Chen Z, Mikulec AA, McKenna MJ, Sewell WF, Kujawa SG. A method for intracochlear drug delivery in the mouse. J Neurosci Methods. 2006;150:67–73. doi: 10.1016/j.jneumeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 192.Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK. Marker retention in the cochlea following injections through the round window membrane. Hear Res. 2007;232:78–86. doi: 10.1016/j.heares.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Richardson RT, Wise A, O'Leary S, Hardman J, Casley D, Clark G. Tracing neurotrophin-3 diffusion and uptake in the guinea pig cochlea. Hear Res. 2004;198:25–35. doi: 10.1016/j.heares.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 194.Prieskorn DM, Miller JM. Technical report: Chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140:212–215. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 195.Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- 196.Eshraghi AA, Adil E, He J, Graves R, Balkany TJ, Van de Water TR. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol Neurotol. 2007;28:842–849. doi: 10.1097/mao.0b013e31805778fc. [DOI] [PubMed] [Google Scholar]
- 197.Wang J, Ladrech S, Pujol R, Brabet P, Van de Water TR, Puel J-L. Caspase inhibitors, but not c-jun nh2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- 198.Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the adminstration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 199.Shinomori, Spack, Jones, Kimura Volumetric and dimensional analysis of the guinea pig inner ear. Ann Otol Rhinol Laryngol. 2001;110:91–98. doi: 10.1177/000348940111000117. [DOI] [PubMed] [Google Scholar]
- 200.Schindler RA, Gladstone HB, Scott N, Hradek GT, Williams H, Shah SB. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factor. Am J Otol. 1995;16:304–309. [PubMed] [Google Scholar]
- 201.Wang J, Pignol B, Chabrier P-E, Saido T, Lloyd R, Tang Y, Lenoir M, Puel J-L. A novel dual inhibitor of calpains and lipid peroxidation (bn82270) rescues the cochlea from sound trauma. Neuropharmacology. 2007;52:1426–1437. doi: 10.1016/j.neuropharm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 202.Pettingill LN, Richardson RT, Wise AK, O'Leary S, Shepherd RK. Neurotrophic factors and neural prostheses: Potential clinical applications based uon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1–5. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Garnham C, Reetz G, Jolly C, Miller J, Salt A, Beal F. Drug delivery to the cochlea after implantation: Consideration of the risk factors. Cochlear Implants Int. 2006;6:12–14. doi: 10.1179/cim.2005.6.Supplement-1.12. [DOI] [PubMed] [Google Scholar]
- 204.Duan ML, Ulfendahl M, Laurell G, Counter AS, Pyykko I, Borg E, Rosenhall U. Protection and treatment of sensorineural hearing disorders caused by exogeous factors: Experimental findings and potential clinical application. Hear Res. 2002;169:169–178. doi: 10.1016/s0378-5955(02)00484-7. [DOI] [PubMed] [Google Scholar]
- 205.Hochmair I, Nopp P, Jolly CN, Schmidt M, Schober H, Garnham C, Anderson I. Med-el cochlear implants: State fo the art and a glimpse into the future. Trends Amplif. 2006;10:201–220. doi: 10.1177/1084713806296720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Paasche G, Gibson P, Averbeck T, Becker H, Lenarz T, Stover T. Technical report: Modification of a cochlear implant electrode for drug delivery to the inner ear. Otol Neurotol. 2003;24:222–227. doi: 10.1097/00129492-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 207.Stover T, Paasche G, Lenarz T, Ripken T, Breitenfeld P, Lubatschowski H, Fabian T. Development of a drug delivery device: Using the femtosecond laser to modify cochlear implant electrodes. Cochlear Implants Int. 2007;8:38–52. doi: 10.1179/cim.2007.8.1.38. [DOI] [PubMed] [Google Scholar]
- 208.Paasche G, Bogel L, Leinung M, Lenarz T, Stover T. Substance distribution in a cochlea model using different pump rates for cochlear implant drug delivery electrode prototypes. Hear Res. 2006;212:74–82. doi: 10.1016/j.heares.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 209.Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 210.Kingma GG, Miller JM, Myers MW. Chronic drug infusion into the scala tympani if the guinea pig cochlea. J Neurosci Methods. 1992;45:127–134. doi: 10.1016/0165-0270(92)90050-n. [DOI] [PubMed] [Google Scholar]
- 211.Leary Swan EE, Fiering JO, Sewell WF, Kujawa SG, McKenna MJ, Borenstein JT, Mescher MJ. Microfabricated components for fully implantable microfluidic drug delivery to the inner ear. 34th Annual Meeting and Exhibition of the Controlled Release Society; Long Beach, CA. 2007. [Google Scholar]