Abstract
The authors conduct a systematic review of the literature to identify interventions designed to enhance breast cancer screening, diagnosis, and treatment among minority women. Most trials in this area have focused on breast cancer screening, while relatively few have addressed diagnostic testing or breast cancer treatment. Among patient-targeted screening interventions, those that are culturally tailored or addressed financial or logistical barriers are generally more effective than reminder-based interventions, especially among women with fewer financial resources and those without previous mammography. Chart-based reminders increase physician adherence to mammography guidelines but are less effective at increasing clinical breast examination. Several trials demonstrate that case management is an effective strategy for expediting diagnostic testing after screening abnormalities have been found. Additional support for these and other proven health care organization-based interventions appears justified and may be necessary to eliminate racial and ethnic breast cancer disparities.
Keywords: breast cancer, screening, diagnosis, treatment, race, ethnicity, intervention
Breast cancer is the most common noncutaneous malignancy and the second most common cause of cancer death among U.S. women (Ries et al. 2005). With over 200,000 cases diagnosed each year, the lifetime risk of breast cancer among U.S. women is 1 in 8 (American Cancer Society 2005). Although breast cancer mortality declined by 2.3% per year between 1990 and 2002, racial and ethnic disparities increased during that time, primarily due to a greater decline in breast cancer mortality among white women compared to minority women (Jemal et al. 2004; Ries et al. 2005). Five-year female breast cancer survival is currently 87.5% among whites, 75.0% among blacks, 83.0% among Hispanics/Latinos, 89.4% among Asians/Pacific Islanders, and 79.6% among American Indians/Alaska Natives (Jemal et al. 2004).
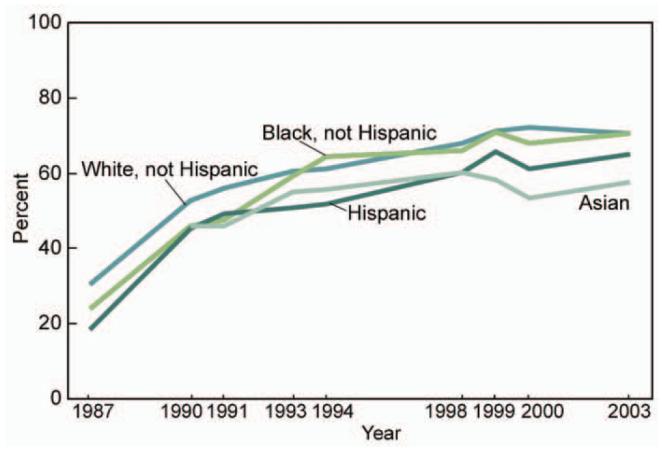
Disparities in breast cancer survival may be related to racial and ethnic differences at each stage of detection and management, including screening, timeliness of diagnostic testing after abnormal screening, quality of care during breast cancer treatment, and follow-up upon completion of breast cancer therapy (Aziz and Rowland 2002; McWhorter and Mayer 1987). No single element of care explains all of the mortality disparities. For example, breast cancer mortality remains higher among black women compared to white women despite evidence that screening mammography rates have been similar in these two groups since about 1993 (Figure 1). In addition, compared to white women, Hispanic/Latino women have lower mammography rates and lower 5-year breast cancer survival, while Asian/Pacific Islander women have lower mammography rates and higher 5-year breast cancer survival (National Center for Health Statistics 2005; Jemal et al. 2004). Although debate exists regarding the accuracy of self-reported mammography screening (McPhee et al. 2002), these results suggest that screening mammography is only one of several factors important to racial/ethnic differences in breast cancer mortality. Another factor is quality of mammography screening, which may be inferior in institutions that serve primarily minority women (Hirschman, Whitman, and Ansell 2007). For example, the cancer detection rate for screening mammography in the U.S. population is 0.0042-0.0063 (May et al. 2000), while the detection rate in institutions that serve primarily low-income women was 0.001 in one series (Moss and Steinhauer 2002) and 0.0024 in another (Hirschman, Whitman, and Ansell 2007). Lower quality screening and missed abnormalities may therefore contribute to delayed diagnosis and worse outcomes in minority women.
Figure 1.
Use of Mammography within the Past 2 Years for Women 40 Years of Age and over by Race and Hispanic Origin
A factor that may also contribute to racial/ethnic mortality disparities is delay in follow-up of abnormal screening mammography results. Patients with lower educational attainment, including many minority women, are more likely to experience delay in follow-up testing compared to patients with higher educational attainment (Yabroff et al. 2004). Regarding quality of breast cancer treatment, research indicates black, Mexican American, and Puerto Rican women with early stage (I or II) breast cancer are 20 to 50% more likely to receive inappropriate treatment compared to white women with similar stage disease (Li 2005). In contrast, Japanese-, Chinese-, Korean-, and Vietnamese-American women are 20 to 40% less likely to receive inappropriate treatment compared to white women (Li 2005). Differences in care following treatment (survivorship care) may also contribute to disparities in breast cancer mortality. Research suggests that the quality of surveillance and medical care that women receive following breast cancer treatment is not as high among racial/ethnic minorities compared to whites (Ashing-Giwa et al. 2004).
New Contribution
The goal of this article is to systematically review the literature regarding health care organization-based interventions to improve breast cancer screening, diagnosis, and treatment among racial and ethnic minority women. Previous reviews have mainly focused on health care organization- and community-based interventions to increase breast cancer screening (Yabroff and Mandelblatt 1999; Legler et al. 2002; Mandelblatt and Yabroff 1999) but none has reviewed health care organization-based interventions to improve other important aspects of breast cancer care, including diagnostic testing and treatment. Recent efforts to enhance breast cancer diagnosis and treatment among uninsured and underinsured women have been bolstered by the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) and the national Breast and Cervical Cancer Prevention and Treatment Act of 2000 (BCCPTA). These programs are administered through health centers throughout the country, and many have recently developed and tested novel strategies for enhancing breast cancer services. Reviewing these strategies can help identify those that are most effective and highlight areas that require further study. Greater appreciation of successful screening, diagnostic, and treatment strategies will also increase the likelihood that providers and patients will make the best use of valuable NBCCEDP and BCCPTA resources.
Method
We conducted a systematic review of the literature to determine the effectiveness of health care organization-based interventions to enhance breast cancer screening, diagnosis, and treatment among racial and ethnic minorities. Initially, we used Medical Subject Headings (MeSH) to search MEDLINE via Ovid. Our search strategy was an intersection of MeSH terms referring to breast cancer (e.g., breast neoplasms), trial study design (e.g., intervention studies, randomized controlled trials, controlled clinical trials, evaluation studies, program evaluation, and peer review), and racial/ethnic minorities (e.g., minority groups, ethnic groups, Hispanic Americans, Mexican Americans, Asian Continental Ancestry, Asian Americans or American Native Continental Ancestry or Indians, North American, African American, African Continental Ancestry Group, or Oceanic Ancestry Group). Similar combinations of keywords were used to search the Cochrane CENTRAL Register of Controlled Trials and the Cumulative Index of Nursing and Allied Health Literature (CINAHL). Abstracts identified through this process were reviewed to determine eligibility for study inclusion.
Criteria for inclusion were as follows: published from 1986 through December 2005, in English, included human data, represented a published report, specific to minority health (overall patient population more than 50% minority or if less than 50% minority, included analysis by race or ethnicity), conducted in the United States, included an intervention that was health care organization-based, and used a randomized or concurrent controlled trial design. Full reports were printed and reviewed when the abstract met the inclusion criteria. A manual search was used to identify key references cited in full reports and review articles.
Trials that included community-based interventions were considered if the primary interventions were health care organization-based. A standardized review form was used for each full report to confirm eligibility, assess the study and participant characteristics, and abstract the data needed to address the study questions (Beach et al. 2006). A primary and secondary reviewer evaluated each article. The primary reviewer completed the review form, and the secondary reviewer checked each item on the form for completeness and accuracy. When disagreements arose, issues were discussed among all authors until consensus was reached.
Included studies were evaluated for quality and given a summary score (0 to 27) based upon reporting features, external validity, bias, and confounding (Downs and Black 1998). Consistency of scoring across authors was enhanced by using a scoring form and by discussing the quality score of five studies (12% of the total), which all authors reviewed. Once consensus was reached on the quality score of these studies, each author reviewed an equal proportion of the remaining studies.
Conceptual Model
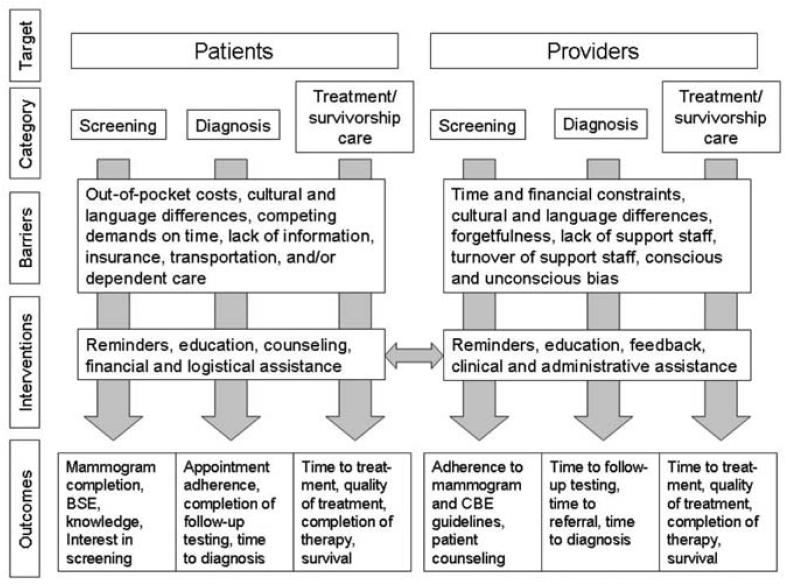
Our conceptual model builds upon the general model presented in the introductory paper of this supplement (Chin et al. 2007) and highlights the main patient and provider barriers to screening, diagnosis, and treatment/survivorship care, as well as the types of interventions that can be used to overcome these barriers (Figure 2). Patient barriers include out-of-pocket costs, lack of health insurance, cultural and language differences, lack of information, and logistical challenges such as lack of transportation or child care. For providers, barriers include time and financial constraints, lack of staff support, staff turnover, language and cultural differences, forgetfulness, and bias. Health care interventions can focus on a single barrier (such as lack of patient or provider knowledge) or can be multifaceted and address several barriers at once. Examples of focused interventions are reminder letters for patients who are due for mammography or chart reminders that inform providers about the screening status of their patients. Case management is an example of a patient intervention that simultaneously addresses multiple financial and logistical barriers to care. An example of a multifaceted provider intervention is one that provides written guidelines that are reviewed in a didactic setting, a chart reminder regarding screening tests, and administrative assistance in completing radiology request forms. In the conceptual model, a double arrow connects patient and provider interventions because both groups can be targeted simultaneously in health care organization-based trials. Outcomes in the conceptual model reflect the intervention category (screening, diagnosis, treatment) and include both process and outcome measures.
Figure 2.
Conceptual Model of Health Care Organization-Based Breast Cancer Interventions
Results
The electronic and manual searches yielded 215 unique citations, of which 154 were eligible for full review. After applying the inclusion criteria, 42 studies were retained for this report. Thirty-six trials focused on breast cancer screening, five focused on follow-up testing, two addressed breast cancer treatment, and one of the treatment trials addressed an element of survivorship care. The most common reasons for exclusion were that the study was not a concurrent or randomized controlled trial, was not health care organization-based, or had fewer than 50% minority patients and did not include subgroup analysis by patient race/ethnicity. Of the studies used for this report, 7 were published between 1986 and 1990, 27 were published between 1991 and 2000, and 8 were published between 2001 and 2005.
Study quality varied both within and between categories of intervention. For the 36 breast cancer screening trials, the range of quality scores was 12 to 25 and the mean was 18.8 out of 27. For the five diagnostic testing interventions, the range of quality scores was 13 to 19, and the mean was 16.4 out of 27. The average quality score for the breast cancer treatment trials was 19.5 out of 27. Most studies scored well on reporting, which reflects the inclusion of study aims, participant characteristics, interventions, confounders, and findings. Scores were also generally high for external validity, a measure of how well the study population represented the population from which participants were drawn. Given the overt nature of the interventions, it was not surprising that most studies did not score as well on participant or researcher blinding. Confounding was also a problem in several studies that did not adequately adjust for important patient characteristics.
Following the conceptual model, results are presented based upon intervention category (screening, diagnosis, treatment) and target (patient only, patient and provider, provider only). Because most of the trials focused on breast cancer screening, these are described first. This is followed by a description of the five diagnostic testing trials, which are categorized by the number and type of interventions used. Breast cancer treatment trials are described last. To assist with interpretation of results, information regarding the health insurance status of study participants is provided for each trial.
Interventions to Enhance Breast Cancer Screening
The 36 breast cancer screening trials were diverse with respect to target population, intervention type, and study design. Fourteen studies targeted only patients, 18 studies targeted both patients and providers, and 4 studies targeted only providers. Black women represented 30% or more of the study population in 23 studies. In half of these studies, 30% or more of the study participants were white. Hispanic women represented 30% or more of the patient population in five studies. One study population consisted entirely of Asian American women. Among the most common patient-targeted interventions were reminder letters and telephone calls, written educational materials, in-person counseling, mammography vouchers, and classroom education. Common provider interventions were chart reminders, chart flow sheets, written educational materials, and chart audits with feedback. Patient outcomes included rates of mammography completion, breast self-examination, and intention to ask a provider about mammography. Provider outcomes included adherence to mammography and clinical breast examination (CBE) guidelines and advice regarding breast self-examination. None of the studies examined breast cancer diagnosis as an outcome. A randomized controlled trial design was used in 31 studies, while a concurrent controlled trial design was used in 5 studies.
Trials that Included Only Patient Interventions (Table 1)
Table 1.
Trials that Included Only Patient Interventions to Increase Breast Cancer Screening
| Reference | Design | Intervention(s) | Control or Limited Intervention(s) | Intervention (control n), Duration, Race/Ethnicity | Health Insurance, Income | Setting | Results (intervention vs. control, results as odds ratios, relative risk, or actual percentage) | Quality Score (out of 27) |
|---|---|---|---|---|---|---|---|---|
| Kiefe et al. 1994 | RCT | Voucher for free screening mammography at local facility. | Counseling, mammography pamphlet, referral, map, telephone number to low-cost mammogram facility. | 61 (58), 2 months, 77% black, 12% Hispanic, 13% white, 7% Other. | 100% Medicare, 92% with household income less than $10,000. | General medicine clinic at a public hospital. | Mammography; intervention (44%), control (10%), (p < .001). | 21 |
| Skinner et al. 1994 | RCT | Baseline telephone interview plus tailored letter addressing perceived barriers to mammography, breast cancer risk factors, and screening status. | Baseline telephone interview plus standard breast cancer screening letter. | ~217 (~218), 8 months, 84% white, 16% black. | Insurance not reported, 43% with income below $26,000. | 2 family practice groups in North Carolina | Mammography; intervention (44%), control (31%) p = .16) | 20 |
| Skaer et al. 1996 | RCT | Mammogram recommendation, instructions, and a free mammogram voucher. | Mammogram recommendation and instructions. | 40 (40), 2 months, 100% foreign-born Hispanic. | 77.5% to 82.5% uninsured. | Two migrant health clinics | Mammography; intervention (87.5%), control (17.5%), OR 47.03 (CI 9.28, 238.37). | 20 |
| Janz et al. 1997 | RCT | Mammography reminder letter from primary care physician, grocery coupon upon completion of mammogram, telephone counseling by community peer. | Usual care. | 223 (237), 12 months, 74% white, 23% black. | All Medicare eligible, income not reported. | Community-based primary care practices. | Mammography; intervention (38%), control (16%), (p < .001). | 20 |
| Weber and Reilly 1997 | RCT | Personalized reminder letter from primary care physician (PCP) plus standardized case management protocol using community health educators (CHE); a second reminder letter from CHE 2 wks after PCP letter, a structured outreach protocol involving patient education, reminders, identification/removal of barriers to care, and systems navigation. | Personalized PCP reminder letter followed by usual care. | 186(190), 13 months, 42% white, 36% black, 7% Hispanic, 4% Asian, 1% Other. | 5% uninsured, 21% Medicaid, 60% of household incomes less than $15,000. | 6 primary care practices affiliated with an urban community teaching hospital. | Mammography; intervention (25%), control group (9.8%), (p < .001). | 25 |
| Davis et al. 1998 | RCT | Small group interactive session with culturally relevant, learner-developed educational video +/- National Cancer Institute (NCI), mammography brochure. | In-person mammogram recommendation from study investigator. | 298 (147), 24 months, 69% black, 30% white. | Insurance not reported, 80-84% low income. | University affiliated outpatient clinics at a public hospital. | Mammography; small group interactive (29%), in-person recommendation (21%), recommendation plus NCI brochure (18%), (P = -05, χ2)- | 20 |
| Margolis et al. 1998 | RCT | Lay health advisor (LHA) mammography instructions, CBE with culturally tailored NP, and same-day mammography. | Baseline questionnaire and breast examination. | 857 (801), 25 months, ~62% white, ~18% black, ~13% Native American, ~6% Other. | 69% Medicare or Medicaid, 24% private insurance, average income $1064 per month. | Non-primary care clinics at county medical center (surgery, orthopedics, ophtho, dental, psychiatry) | Mammography; intervention (60%), control (50%), (p = .006). No difference among those up-to-date at baseline. | 22 |
| Mishra et al. 1998 | RCT | Four 2-hr breast health educational sessions. | Usual care. | 51 (37) 8 weeks, 100% Latina. | 51% uninsured, 50% with household income < $10,000. | Community-based primary care clinics and social service organizations. | Increase in proportion who conduct BSE once a month; intervention (24% to 67%) control (22% to 35%), p = .01) | 21 |
| Dolan et al. 1999 | RCT | Same-day appointment for screening mammography following PCP appointment. | Physician recommendation for screening mammography. | 408 (512), 12 months, 43% white, 39% black, 18% Other. | 14% Medicaid, 4% no insurance, income not reported. | Urban academic general internal medicine practice. | Mammography; intervention (66%), control (56%), (p = .003). | 21 |
| Mayer et al. 2000 | RCT | Reminder letters (from physicians or mammogram facility) w/ contact information for mammogram facility. | No reminder letters (during the study period only). | 1039 (523), 23 months, 83% white, 7.5% Latina, 3% black, 4% Other. | 5% uninsured, 93% other-insurance, 55% with annual family income < $40,000. | Women were patients of primary care providers who referred to 1 of 6 mammogram centers. | Mammography; physician letter (47.7%), facility letter (46.6%), control (28.3%), (p <.001). (No significant difference between interventions groups). | 20 |
| Champion et al. 2002 | RCT | Tailored telephone counseling and/or tailored primary care physician letter, information regarding breast cancer risk, instructions regarding mammography appointment logistics. | Baseline telephone interview. | 707 (683), 2 years, 21-83% black, 15-77% white. | Insurance not reported, 77% low income at high minority site, 24% low income at low minority sites. | University-based general medicine clinic and two HMO clinics. | Mammography; telephone counseling OR=1.66 (CI 1.12,2.46), tailored mailing OR=1.72(CI 1.18, 2.52), telephone counseling plus mailing OR = 2.16 (CI 1.46, 3.19). | 18 |
| Valdez et al. 2002 | RCT | Five 1-hour educational modules regarding breast cancer and screening. | Usual care. | 614 (583), 12 months, 100% Latinas. | 62% uninsured, 61% with household income less than $18,200. | Three community health centers, two HMO sites and a community-based organization. | Intention to ask physician about mammography; intervention (85%) control (74%), p < .0001, χ2). | 18 |
| Fitzgibbon et al. 2004 | RCT | Culturally tailored classroom instruction on breast health and diet (low fat/high fiber). | Mailed written materials covering same health curriculum as the classroom instruction. | 127 (129), 8 months, 100% Latinas. | Insurance not reported, income not reported. | Urban community health center. | Monthly breast self exam; intervention (45.7%), control (22.3%), (p = .003). | 21 |
| Smith-West et al. 2004 | RCT | Stage 1: Personalized reminder letter. Stage 2: If no mammogram 6 months later, woman randomized to receive either tailored breast cancer risk letter or tailored telephone call. All given information on mammography scheduling and no-cost mammography program. | Usual care. | 159 (161), 12 months, 91% black. | Insurance not reported, participants chosen from a low-income sample. | Family Health Care of Alabama, a federally qualified health center, serving mostly low-income, rural black patients. | Stage 1: Mammography following personalized letter: intervention (14%), control (14%) Stage 2: Mammography following tailored letter (13%) vs. tailored telephone call (15%), difference not significant. | 19 |
In the 14 trials that included only patient interventions, we identified 3 primary intervention types: reminder-based, culturally tailored, and multifaceted. The most common outcome assessed in these trials was screening mammography, but breast self-examination, intention to ask a provider about mammography, and breast cancer knowledge were also assessed.
Reminder-based Interventions
Results from five studies suggest that the success of reminder-based interventions, such as letters and telephone calls, is related to the demographic and socioeconomic characteristics of the study population. For example, one study assessed the effect of tailored letters and telephone counseling on screening mammography in two groups (Champion et al. 2002). In the first group, 77% of participants were white, 50% were employed, and 24% earned less than $15,000 per year. In the second group, 83% were black, 10% were employed, and 77% earned less than $15,000 per year. Health insurance status was not assessed. Tailored letters alone increased mammography in the first group but only the combination of the tailored letters and telephone counseling was effective in the second group. Results from other studies mirrored these findings. For example, personalized reminder letters did not increase screening mammography rates in a population that consisted primarily of low-income black women who had never had a mammogram before (insurance status not reported) (Smith-West et al. 2004). In another study, a combination of reminder letters and telephone calls was also less effective among black women compared to their white counterparts (Janz et al. 1997). Insurance status was not reported in that study but all women were Medicare eligible. In contrast, a study among primarily white (83%), insured (93%), and educated (>80% high school graduates) women showed both generic and tailored reminder letters resulted in increased likelihood of mammography over the study period (Mayer et al. 2000). Factors associated with increased likelihood of mammography were previous mammography and age 65 years and older (i.e., Medicare eligible) versus age 50 to 64 years. Black women were less likely to obtain mammography, but this effect was not significant. There was also a nonsignificant positive association between education and mammogram likelihood. Results from these studies suggest that women with lower income and/or lower educational attainment face barriers to mammography that are not effectively addressed by low-intensity interventions such as reminder letters and telephone counseling.
Culturally Tailored Interventions
Recent immigrants to the United States face a multitude of cultural barriers to breast cancer screening (Swan et al. 2003), and several of the trials we identified used a culturally tailored approach to increase screening (Fisher et al. 2007). For example, Margolis et al. found that after controlling for insurance type, women who received CBEs by nurse practitioners who had received cultural sensitivity training were more likely to subsequently obtain screening mammography. This effect was greatest among Native American and Southeast Asian women (Margolis et al. 1998). Valdez et al. used culturally tailored waiting room videos to address knowledge deficits, fear, and misconceptions regarding screening mammography among Latina women attending a primary care clinic. Intervention and control groups had similar rates of health insurance. Bivariate analysis revealed that viewing Spanish language videos was associated with increased breast cancer knowledge and intention to ask physicians about mammography (Valdez et al. 2002). Similarly, culturally tailored classroom instruction on breast health improved perceived self-efficacy, proficiency in the BSE, and monthly BSE rates among Latina women compared to controls who had similar rates of health insurance (Mishra et al. 1998) or had similar immigration and marital status (Fitzgibbon, Gapstur, and Knight 2004). Davis et al. (1998) used feedback from focus groups consisting primarily of black women to develop a breast health educational video that used patient preferences regarding presentation style and format. Use of this video in combination with culturally tailored small-group education increased mammography 29% compared to 18% in the control group. Insurance status was not reported in this study, but the groups did not differ based upon age, race, income, education, or literacy level. In each of these studies, the authors cited use of culturally tailored educational materials as essential to the intervention's success.
Multifaceted Interventions
Prior research has shown that financial and logistical barriers, such as out-of-pocket costs, inconvenience, and lack of transportation contribute to lower mammography rates among minority women (Caplan et al. 1996). We identified eight interventions that addressed financial and logistical concerns among low-income women, and this approach appeared to be more effective than reminder-based interventions at increasing screening mammography. For example, in a study of primarily low-income Medicare recipients, 44% obtained a mammogram within 60 days of receiving a free mammography voucher compared to only 10% of women in the control group (Kiefe et al. 1994). Similarly, the combination of a mammography voucher and provider advice was associated with a higher mammography rate at 30 days compared to provider advice alone among primarily uninsured, foreign-born Hispanic women after adjusting for multiple covariates, including health insurance status (Skaer et al. 1996). In another study, combining provider recommendation for mammography with same-day mammography appointments resulted in increased mammography completion after controlling for health insurance status (Dolan et al. 1999). A similar trial led to increased mammography when transportation was combined with appointment scheduling and dependent care, although insurance status in the intervention group differed from that of the control group (Weber and Reilly 1997) These studies suggest that multifaceted strategies can be used to help minority women overcome financial and logistical barriers to screening mammography.
Trials that Included Patient and Provider Interventions (Table 2)
Table 2.
Trials that Included Both Patient and Provider Interventions to Increase Breast Cancer Screening
| Reference | Design | Intervention(s) | Control or Limited Intervention(s) | Intervention (control n), Duration, Race/Ethnicity | Health Insurance, Income | Setting | Results | Quality Score (out of 27) |
|---|---|---|---|---|---|---|---|---|
| Becker et al. 1989 | RCT | For patients; preventive care services reminder letter. For providers; chart reminder regarding preventive care services. |
For patients; telephone interview. | 366(188), 7 months, 50-59.5% black, 40.5-50% white. | 36-45% no insurance, income not reported. | University internal medicine clinic. | Mammography: physician reminder only (30.3%), physician and patient reminder (31.1%), control (10.6%), (p = .020, χ2). | 22 |
| McPhee et al. 1989 | RCT | For patients; half received letters recommending CBE/mammograms and reminder postcards. For providers; chart reminders or audit-based feedback via monthly didactic sessions. | For patients; half received letters recommending CBE/mammo-grams and reminder postcards. No provider intervention. | 41 physicians (20 physicians), 9 months, Patients: 41% white, 25% black, 17% Hispanic, 14% Asian, 3% Other. | 37% with Medicaid, 15% uninsured, income not reported. | General internal medicine practice at an academic hospital | Percent increase in clinical breast exam: audit with feedback (14.1%, p = .006), reminders (18.5%, p <.001), patient education (1.7%, p = .666). Percent increase in mammography: audit with feedback (10%, p = .050), reminders (11%, p = .031), patient education (11.8%, p = .006). | 20 |
| Nattinger et al. 1989 | CCT | For patients; educational mammography handout. For providers; ACS questionnaire plus either: monthly feedback with peer comparisons (feedback arm) or pre-completed mammogram orders for eligible patients (visit-based arm). | For providers: questionnaire about knowledge of and agreement with ACS cancer screening guidelines. | 281 (227), 6 months, 60% black, 32% white, 8% Hispanic, 2% Other. | Insurance not reported, income not reported. | General internal medicine clinic at an academic hospital. | Mammography: educational handout (54%), resident physician feedback (62%), control (36%) (p < .001 when each intervention is compared to control) | 19 |
| Ornstein et al. 1991 | RCT | For patients: PCP signed reminder letters. For providers: all of the elements in the control group plus cancer screening chart reminders. | For providers: health promotion educational sessions, audit with feedback. | Patients: 5821 (1576) Providers: 39 (10), 1 year, Patients: 61% black 38% white 2% Other. | 18% Medicare or Medicaid, 40% uninsured, Income not reported. | Academic family practice clinic. | Percent increase in mammography: chart reminders alone (10.7%, p < .0001), chart reminders plus patient letters (15.7%, p < .0001), patient letters alone (2.8%, p = .35) control (15.7% (p < .0001). | 18 |
| Dickey and Pettiti 1992 | CCT | For patients: health information sheet, provider-completed patient health diary, waiting room posters. For providers; health diary attached to chart. | For providers; verbal and written information about the full intervention and copies of patient health diary forms. | 200 (100), 18 months, 49-55% Spanish speaking. | Insurance not reported, income not reported. | University-affiliated community-based family practice clinic. | Percent increase in mammography; intervention (40.9-61.8%), (p < .005). control (37.3-40.9%), (not significant). | 19 |
| Landis et al. 1992 | RCT | For patients; mammography reminder letter, mammography prescription. For providers: mammography reminder on chart. | For patients; placebo mailing comprised of patient satisfaction survey. | 79 (43), eight months, 83% white, 17% black. | 13-40% no insurance, income not reported. | University-affiliated family practice clinic. | Mammography; intervention (25%), control (5%), (p = .07). | 19 |
| Burack et al. 1994 | RCT | For patients: postcard appointment reminders, dedicated scheduling system for women who missed mammography appointment, and reduction or elimination of out-of-pocket mammogram costs. For providers; chart reminder regarding mammogram. | For patients; dedicated telephone appointment line, telephone reminders, and reduction or elimination of out-of-pocket mammogram costs. For providers and staff: breast cancer awareness program. | 1363 (1322) 14 months, approximately 96% black (inferred from Burack et al. 1996). | 43% Medicaid, 26% no insurance, income not reported. | Health department primary care practices, HMO clinics, outpatient practice sites of a private hospital. | Mammography appointments; intervention (rate varied by site from 38% to 65%) control (rate varied by site from 11% to 37%) intervention rate exceeded control rate by 13% (CI 6 to 20) to 29% (CI 21 to 38) depending upon site. Mammography completion: intervention (rate varied by site from 43% to 64%) control (rate varied by site from 25% to 46%) intervention rate exceeded control rate by 12% (CI 5 to 19) to 25% (CI 16 to 34) depending upon site. | 19 |
| Herman et al. 1995 | RCT | For patients: mammography educational materials provided in-person and reviewed by nurses (patient education group). For providers; completed health maintenance flow sheet on chart, partially completed mammography order form on chart (prevention team). | For providers: breast cancer screening monograph plus preventive services lectures (control). | 348 (192), 6 months, 41-58% white, 34-53% black, 9-13% Hispanic. | Medicare-eligible population, income not reported. | Internal medicine clinics at a university-affiliated public hospital. | Clinical breast exam offered: prevention team (31.5%), (p = .003 compared to prevention team), patient education (21.9%), p = .04 compared to prevention team), control (17.8%) Mammography; prevention team (36.4%),(p = .001 compared to control) patient education (31.4%), (p = .005 compared to control) control (18%). | 19 |
| Burack et al. 1996 | RCT | For patients: personalized mammography reminder letter signed by HMO medical director and elimination of out-of-pocket mammogram costs. For providers: chart reminder regarding mammogram. | For patients: elimination of out-of-pocket mammogram costs. | 1772 (596), 12 months, 96% black (among those for whom information was available). | All patients insured, 49-61% Medicaid, income not reported. | HMO clinics. | Mammography at site 1: intervention (~30%) control (~30%), (p = .524) Mammography at site 2 (fewer Medicaid patients): intervention (36%), control (22%), (p = .002). | 21 |
| Burack and Gimotty 1997 | RCT | For patients: postcard appointment reminders, dedicated scheduling system for women who missed mammogram appointments, and reduction or elimination of out-of-pocket mammogram costs. For providers: chart reminder regarding mammogram. | For patients; dedicated telephone appointment line, telephone reminders, and reduction or elimination of out-of-pocket mammogram costs. Breast cancer awareness program for providers and staff. | 1413 (1413), 26 months, approximately 96% black (inferred from Burack et al. 1996). | 55% from health department clinics not insured, 100% insured at HMO clinic, income not reported. | Health department primary care practices and an HMO clinic. | Mammography at health department clinics: full intervention (44%), limited intervention (28%), (OR = 1.84 CI 1.4, 2.4). Mammography at HMO clinic: full intervention (45%), limited intervention (46%), (OR = 1.06 CI .8, 1.42). | 19 |
| Dietrich et al. 1998 | RCT | For patients: health education materials and patient-held health diary. For providers: preventive care flow sheet in patient charts, workshop and ongoing education for key clinic personnel. | Usual care. | 1381 (1499), 24 months, 23-31% black, 22% Hispanic, 22-26% white, 2.3-4.9% Other, 21-27% unknown. | 25-28% Medicaid, 26-33% no insurance, income not reported. | Community-based migrant health centers. | Percent increase in breast self-exam advice from providers: intervention (8.6%, p < .001) control (5.5%, p = .06). No intervention effect on mammography or clinical breast exam. | 20 |
| Manfredi et al. 1998 | RCT | For patients: mailed health maintenance cards. For providers: chart flow sheets, chart reminders, protocols, continuing medical education, provider feedback, on-site staff training, use of cancei-screening guidelines. | For providers: chart flow sheets and an HMO letter recommending adoption of NCI guidelines. | 24 intervention centers (23 control centers), 2 years, 39 of 47 practices were located in census tracts that were 75 to 99% black and/or Hispanic | 43% private insurance, 44% Medicare or Medicaid, 6% uninsured, income not reported. | Primary care HMO-affiliated practices located in low-income, urban minority communities | HMO patients: Mammography rate change: intervention (-13.9%), control (-1.0%) (not significant). CBE rate change: intervention (7.7%) and control (9%) (not significant) Non-HMO pts: Mammography rate change: intervention (-7.9%), control (-17.3%) (not significant). CBE rate change: intervention (9.9%), control (-5.4%), (p ≤ .05). | 18 |
| Paskett et al. 1999 | CCT | Intervention City: For patients: educational sessions, health literature, community events, advertisements, church program, birthday cards, personalized follow-up letters for abnormal tests. For providers; chart reminders, exam room prompts, in-service meetings, alerts for abnormal test results, follow-up protocol. | Usual care in the comparison city. | 908 (1021) 36 months, 66% black at baseline, 64% black at follow-up. | Insurance not reported, Participants had “low incomes.” | Housing communities in two cities. | Increase in mammography: in intervention city (from 31% to 56%) (p = .049) comparison city (from 33% to 40%) (not significant) | 16 |
| Rimer et al. 1999 | RCT | For patients: tailored print communications (TPC) or tailored print communications plus tailored telephone counseling (TPC + TTC). | For providers: tailored chart prompt intervention (PI). | 384 total, 24 months, 81% black, 19% white. | 21%-34% uninsured, 77% with income less than $20,000. | Community health centers providing care for low-income patients. | Mammography; PI (86%) TPC (82%) TPC + TTC (85%) (group differences not significant). | 20 |
| Taylor et al. 1999 | RCT | For patients: motivational video, informational pamphlet, bus passes, telephone and postcard reminder. For providers: one-on-one mammography education and computer-generated chart prompts for physicians and nurses. | Usual care. | 232 (82), 15 months, 39% black, 42% white, 18% Other. | 10% uninsured, income not reported. | Adult medicine clinic of a county-owned hospital serving inner-city residents. | Mammography: intervention (49%) control (22%) p <.001). | 18 |
| Nguyen et al. 2000 | RCT | For patients: Vietnamese-language educational materials. For providers: manual and computerized reminders, continuing medical education, newsletters, and enrollment in the NCI Physician's Data Query oncology information program. | Usual care. | 9 physicians and their patients (11 physicians and their patients), 30 months, Vietnamese American physicians and their Vietnamese American patients. | Insurance not reported, income not reported. | Private practice physician offices throughout California | Increase in clinical breast examination; intervention (81.3% to 85.9%) p = .081)control (72.9% to 81.8%) (p = .003) Increase in mammography: intervention (68.1% to 79.2%) p = .O81) control (61.4% to 79.5%) (p = .003) | 12 |
| Roetzheim et al. 2004 | RCT | For patients: completion of screening checklist For providers: chart stickers indicating status of screening tests, patient-completed cancer screening checklists in chart, 45 min training session, training manual, feedback on intervention compliance and ongoing support from research staff. | Usual care. | 600 (596), 12 months, 24-34% black, 45-51% white, 21-24% Hispanic. | 100% insured, 13.9-16.8% Medicaid, income not reported. | 8 primary care clinics participating in a county-funded health insurance plan. | Mammography at 12 months; intervention (75.7%), control (71.1%), (p = .023). | 21 |
| Roetzheim et al. 2005 | RCT | As above for Roetzheim et al 2004 except no ongoing support from research staff. | Usual care. | 600 (596) 24 months, 24-34% black, 45-51% white, 21-24% Hispanic. | 100% insured, 13.9-16.8% Medicaid income not reported. | 8 primary care clinics participating in a county-funded health insurance nlan. | Mammography at 24 months; intervention (67%), control (64.5%), (p = .023). | 20 |
Of the 18 studies that included both patient and provider interventions, 7 compared the effectiveness of patient interventions to provider interventions and 11 compared a combination of patient and provider interventions to provider interventions alone or to usual care. The outcomes assessed in these trials included screening mammography, clinical breast examination, and advice regarding breast self-examination.
Screening Mammography
Provider interventions were more effective than patient interventions at increasing screening mammography rates in six of the seven trials that compared the two intervention targets. For example, chart-based reminders regarding preventive care services were associated with a higher rate of mammography completion (30%) in an ethnically diverse, underinsured population compared to a patient reminder letter alone (11%), while adding a patient reminder letter to the chart reminder intervention yielded no increase in screening mammography (Becker et al. 1989). Ornstein et al. (1991) found that physician chart reminders, but not patient reminder letters, increased mammography in a primarily black and underinsured population. This effect was stronger among those who had Medicare, Medicaid, or no insurance compared to those who had an HMO, PPO, or third-party insurance. In another study, physicians received either chart reminders for cancer screening or feedback regarding their compliance with screening guidelines (McPhee et al. 1989). After adjusting for multiple patient characteristics including receipt of Medicaid, these interventions were each more successful at increasing mammography compared to patient reminder letters alone.
In a series of studies conducted in health department and HMO clinics in the Detroit area, Burack and colleagues found physician-targeted chart reminders were more effective than patient reminder letters at increasing screening mammography rates even when out-of-pocket mammography costs were eliminated (Burack et al. 1994; Burack et al. 1996; Burack and Gimotty 1997). At the two HMO clinics in this study, the chart reminder approach was more successful in the clinic that had the higher proportion of commercially insured HMO patients versus Medicaid-subsidized HMO patients (Burack et al. 1996), suggesting that the effectiveness of the physician-targeted approach was related to patient socioeconomic status. In contrast to the above studies, Rimer et al. (1999) did not find that physician chart reminders led to higher screening mammography compared to either tailored patient letters or a combination of tailored letters and tailored telephone counseling. However, a ceiling effect may explain this result, as a majority of participants had health insurance and 84% were up-to-date with mammography at baseline.
One study compared the effectiveness of a combined provider and patient intervention to a provider intervention alone. In that trial, a combination of provider education, chart reminders, partially completed mammography requisitions, and patient education was associated with a higher mammography completion rate compared to provider education alone in an older, Medicare-eligible population (Herman, Speroff, and Cebul 1995). Provider education consisted of a lecture on preventive services and a monograph that included background articles and guidelines related to preventive care.
In the screening studies that compared a combination of provider and patient interventions to usual care, six found a positive intervention effect. In one study, completion of screening test checklists by patients, with the assistance of their providers, resulted in a greater increase in screening mammography compared to the control group over an 18-month period, although analysis did not adjust for insurance status (Dickey and Petitti 1992). In another study, patient-completed screening checklists were placed in the medical charts along with stickers reminding the providers of each patient's screening test status. This strategy led to increased screening mammography compared to usual care at the end of the intervention (12 months) and also 1 year after completion of the intervention among those who received county-sponsored health insurance (Roetzheim et al. 2004; Roetzheim et al. 2005). Similarly, Nattinger, Panzer, and Janus (1989) found that a combination of chart reminders, partially completed mammography requisitions, and patient educational materials was effective at increasing screening mammography, as was physician feedback regarding the proportion of patients who were up-to-date with screening mammography. Both interventions were superior to usual care in this study, although health insurance status was not included in the analysis. In two other studies, multifaceted interventions, including motivational videos, informational pamphlets, bus passes, telephone/postcard reminders, and chart prompts, led to increases in screening mammography among minority women with (Taylor et al. 1999) and without (Paskett et al. 1999) adjusting for health insurance status. Unfortunately, the design of these studies made it impossible to determine which of the interventions or combination of interventions led to this effect.
Three of the trials that used combined patient and provider interventions were not effective at increasing screening mammography in racially and ethnically diverse patient populations. For example, health educational material and screening test checklists for patients, combined with preventive care flow sheets and in-service training for providers did not lead to increased mammography in community-based migrant health centers that served a racially and ethnically diverse patient population (Dietrich et al. 1998). The proportions of patients with no insurance or public insurance were similar at the intervention and control sites. Using subgroup analysis, the authors noted that the intervention was more successful in clinics with stable medical director leadership compared to those that had experienced turnover at that level. Another study attempted to increase mammography rates through a combination of patient-oriented health maintenance cards and physician-oriented chart reminders, in-service training, and feedback (Manfredi et al. 1998). This approach did not succeed, and the authors hypothesized that physician turnover may also have reduced its effectiveness, although this hypothesis was not formally tested. In a study among Vietnamese-American patients, a combination of Vietnamese-language educational materials for patients and chart reminders, in-service training, and newsletters for physicians also did not improve adherence to mammography screening guidelines (Nguyen et al. 2000). An emphasis on urgent care, as opposed to preventive care, in clinic scheduling was cited as a potential reason for the lack of effectiveness of this intervention.
Clinical Breast Examination
Four of the above studies also examined the effect of combined patient and provider interventions on provider adherence to CBE guidelines. Two of these trials were successful, while two were not. In one study, the combination of patient education, provider education, chart reminders, and assistance completing mammography requisitions was associated with a higher rate of CBE compared to provider education alone (Herman, Speroff, and Cebul 1995). Another study also demonstrated increased CBE rates compared to controls when providers received chart reminders regarding overdue screening tests and patients received letters and postcards regarding CBEs (McPhee et al. 1989). In contrast, CBE rates did not increase with a combination of patient and provider interventions in racially and ethnically diverse populations (Manfredi et al. 1998; Dietrich et al. 1998).
Advice Regarding Breast Self Examination
In the Dietrich et al. (1998) study, the combination of patient and provider interventions was associated with a greater likelihood of physicians providing advice regarding breast self examination. Study participants received health education materials and a health diary, while providers received chart-based sticker reminders and preventive care flow sheets as well as periodic in-service training.
Trials that Included Only Provider Interventions (Table 3)
Table 3.
Trials that Included Only Provider Interventions to Increase Breast Cancer Screening
| Reference | Design | Intervention(s) | Control or Limited Intervention(s) | Intervention (control n), Duration, Race/Ethnicity | Health Insurance, Income | Setting | Results | Quality Score (out of 27) |
|---|---|---|---|---|---|---|---|---|
| Schreiner et al. 1988 | RCT | Chart reminder indicating which screening procedures were due. | Usual care. | 20 physicians (22 physicians), 5 months, 35-39% white, 49-54% black, 11-12% Hispanic. | Insurance not reported, income not reported. | Two university-and hospital-affiliated general medicine clinics. | Clinical breast exam rate 6 months after completion of intervention: intervention (42%), control (27%), (p < .02). | 18 |
| Chambers et al. 1989 | RCT | Date of last mammogram printed on encounter form. | Usual care. | 639 (623) 6 months, 69-72% non-white. | 28% Medicaid, 16% no insurance, income not reported. | University family practice clinic. | Mammography: intervention (26.6%), control (20.6%), (p = .011). | 19 |
| Litzelman et al. 1993 | RCT | Chart reminders with required responses. | Chart reminders without required responses. | Providers: 92 (86), Patients: 2827 (2580), 6 months, 62% black. | Insurance not reported, income not reported. | Academic primary care general internal medicine practice | Mammography: intervention (54%), control (47%), (p = .036). | 18 |
| McCarthy et al. 1997 | CCT | MA/LPNs to check for eligibility, offer screening mammograms, prepare MD order and schedule mammogram after physician's signature obtained. | Usual care. | 1250 (4684), 15 months, 73-82% black, 15-24% white, 2.6-3.5% Other. | 31% Medicaid or Medicare, 68% private insurance, income not reported. | 3 general internal medicine clinics at a large, urban teaching hospital | Percent increase in mammography: intervention (9%), control (1-2%), (p = .004). | 18 |
Of the four screening trials that used only provider interventions, three were designed to increase physician adherence to screening mammography guidelines and one was designed to increase provider adherence to clinical breast examination guidelines.
Screening Mammography
Increased provider adherence to mammography screening guidelines was achieved in three provider-targeted interventions. One trial used chart reminders and controlled for insurance status (Chambers et al. 1989), one used a chart flow sheet that required physician response but did not control for insurance status (Litzelman et al. 1993), and one provided administrative assistance in completing radiology requisition forms for patients who were due for screening mammography (McCarthy et al. 1997). In the latter study, the patient insurance profiles were similar at the intervention and control clinics. In all of the studies, the authors highlighted the time pressures faced by health care providers and the potential benefits of assisting providers with clerical tasks.
Clinical Breast Examination
As with two of the combined patient and provider interventions to increase CBE, the provider-only intervention to increase CBE achieved limited success. Conducted in two university-affiliated general medicine clinics serving a diverse patient population, this trial's chart reminder strategy did not lead to a higher CBE rate by the end of the intervention period (Schreiner et al. 1988). Six months after the intervention period, the CBE rate was higher in the intervention clinic compared to the control clinic. However, patient load was significantly lower in the intervention clinic, and the analysis did not control for health insurance status.
Interventions to Expedite Diagnostic Testing
Women with abnormal screening mammograms or clinical breast examinations comprised the target population in all five studies of diagnostic testing interventions. None of the interventions targeted health care providers. Black women comprised the majority of participants in four of the studies, while Hispanic women comprised the majority in one study. Outcomes assessed included time to follow-up appointments and time to breast biopsy. One study examined stage at diagnosis in the intervention group compared to the control group. None of the studies evaluated long-term patient survival.
Of the five interventions we identified, all achieved success using some form of case management to monitor follow-up and diagnostic activities. Case management is defined as a collaborative process that assesses, plans, implements, coordinates, and evaluates options and services to meet an individual's health needs through communication and available resources (Case Management Society of America 2002). Case management is considered “minimal” when it involves basic services such as outreach, client assessment/case planning, and referral to service providers (Korr and Cloninger 1991). Two of the interventions used a minimal model of case management. A more extensive “coordination” model of case management is characterized by the basic case management services plus advocacy on behalf of clients, direct casework with clients, and reassessment (Korr and Cloninger 1991). Of the studies we identified, one used a coordination model of case management. A “sociomedical” model of case management was used in the other two studies. In this model, issues related to housing, work, food security, substance use/abuse, domestic violence, and mental health are assessed and addressed in addition to immediate health care and advocacy needs (Lantz et al. 2004).
Patient Interventions (Table 4)
Table 4.
Trials to Expedite Follow-up Testing
| Reference | Design | Intervention(s) | Control or Limited Intervention(s) | Intervention n (control n), Duration, Race/Ethnicity | Health Insurance, Income | Setting | Results | Quality Score (out of 27) |
|---|---|---|---|---|---|---|---|---|
| Manfredi et al. 1990 | CCT | Nurse instructions on making referral appointments; stamped, pre-addressed form patients returned upon referral completion; mailed reminder notes; telephone reminders. | Usual care. | 69 (95), 16 months, 69.5% black. | 48.2% uninsured in control group, 61.3% uninsured in intervention group, income not reported. | Four urban Department of Health neighborhood health centers. | Referral completion: Intervention (84.1%), control (54.7%), (CI 14.8, 44.0). | 13 |
| Bressler et al. 1993 | CCT | Breast cancer screening and breast self-exam education, clinical breast exam and referral for mammogram when indicated, tracking of those referred for biopsy. | Usual care. | 164 (335), 4 years, 83-86% black, 8% white, 4-5% Hispanic, 2-4% Other. | Insurance not stated, patients had “low income”. | Outpatient general medicine clinic at large county hospital. | Proportion diagnosed with early stage breast cancer: intervention (25%), control (6%), (p = .001). | 17 |
| Lacey et al. 1993 | CCT | Nurse and outreach worker directed education, screening, referral, and tracking/follow-up. | Usual care. | 100 (270) 18 months 99% black. | Patients were “primarily uninsured,” income not reported, 75% unemployed. | Urban community-based health center with a breast screening clinic. | Diagnostic appointment rates: intervention (92%), control (72%), (p < .001). | 16 |
| Freeman, Muth, and Kemer 1995 | CCT | Navigator assistance with follow-up appointments, obtaining financial and medical clearance for surgical procedures, retrieving lost test results, language translation when necessary. | Usual care. | 131 (493), 27 months, 64% black, 26% Hispanic, 10% white. | ~20% Medicaid, ~40% no insurance, patients had “low income.” | Primary care clinic in a major community hospital. | Biopsy completion: intervention (85.7%), control (56.5%), (not significant). Biopsy completion within 4 weeks: intervention (71.4%), control (38.5%), (p = .047). | 17 |
| Ell et al. 2002 | CCT | Stepped care appointment reminders, reinforcing telephone calls, assistance with logistical barriers, systems navigation, social work assessment +/- counseling +/- referral to psychosocial oncology +/- mental health services. | Usual care. | 605 (476), 12 months, 71% Hispanic, 18% black, 11% Other. | 17% without insurance at one location, 78% without insurance at other location, income not reported. | Large public and private urban screening, diagnostic, and treatment-referral centers in Los Angeles and New York. | Follow-up appointment adherence in Los Angeles; intervention (93%), control (72%), (p = .001). Follow-up appointment adherence in New York: intervention (90% ), control (69%), (p = .001) Diagnostic resolution within 240 days for American College of Radiology Score of 3: interventions vs. control (89% vs. 67%, p = .008) in Los Angeles and (75% vs. 49%, p = .001) in New York | 19 |
Minimal Model of Case Management
In the first study, clinic nurses provided case management, tracking, and telephone/letter follow-up to women referred for diagnostic evaluation for a suspected malignancy (Manfredi, Lacey, and Warnecke 1990). Adherence to follow-up subspecialty appointments was over 90% in both the intervention and control groups if the appointments occurred within 15 days of the referral. When all time periods were assessed, the appointment adherence rate was 88% in the intervention group and 66% in the control group, regardless of insurance status. In this multi-ethnic population, the follow-up appointment adherence rate did not differ for black females compared to the group as a whole. Rates of diagnostic testing (i.e., follow-up breast ultrasound or biopsy) were not measured in this study.
A minimal model of case management was also used in the Breast Cancer Screening Program (BCSP) at Chicago's Stroger (formerly Cook County) Hospital, which serves primarily low-income, uninsured, or underinsured minority patients. In this trial, patients were educated regarding abnormal test results, referred for further evaluation and biopsy, and tracked for completion of recommended diagnostic testing (Bressler et al. 1993). Among women in the program, 85% of those referred for consultation kept their appointment, and 90% of women referred for biopsy underwent the procedure. Although appointment adherence rates were not reported for the control group, a higher proportion of women in the BCSP (25%) had in situ or Stage I breast cancer compared to those in the control group (6%).
Coordination Model of Case Management
Lacey et al. (1993) used clinic nurses to manage a computerized tracking system, to conduct telephone follow-up and appointment scheduling, and to attend the breast oncology clinic on days when referred patients were seen (Lacey et al. 1993). Among women with abnormal screening test results, a higher proportion of women who received coordination services (92%) kept their follow-up appointments with the breast oncology clinic compared to those who did not receive coordination services (72%). Nearly all patients in this study were black (99%), unemployed, and uninsured. The authors attributed the success of this intervention to the coordination services provided by the project staff.
Sociomedical Medical Model of Case Management
Both of the studies that used a sociomedical model of case management reported benefits with respect to completion of diagnostic studies. Freeman et al. (1995) used a screening form that allowed a patient navigator to identify barriers to follow-up testing and assess each patient's financial, communication, social service, emotional, and psychological needs (Freeman, Muth, and Kerner 1995). The navigators provided coordination services to women with abnormal screening tests and referred patients for social and psychological services as indicated. Of those who received case management services, 88% completed the recommended breast biopsy. In comparison, only 57% of those in the control group, which had a higher rate of health insurance, underwent a breast biopsy. Among those who completed a breast biopsy, 71% of intervention patients did so within four weeks compared to 39% in the control group. This patient navigator program served primarily black and Hispanic women, 91% of whom reported being “highly satisfied” with the assistance they received. In the Screening Adherence Follow-up (SAFe) program, social workers screened for psychosocial barriers to follow-up testing and provided counseling or a referral to psychosocial oncology when indicated (Ell et al. 2002). These services augmented appointment reminder calls and systems navigation services, which were provided by social workers and peer counselors. Implemented in health centers in New York and Los Angeles, this intervention was associated with a 90% adherence rate with follow-up subspecialty appointments. Adherence among the control population was less than 75%. In addition, enrollees had higher rates of timely follow-up for abnormal mammograms that were “probably benign” (recommend follow-up within 240 days), as well as those that were “suspicious for cancer” (recommended follow-up within 60 days). However, these results did not account for health insurance status and the control group consisted of a random sample of women who either refused study participation or could not be located to invite participation.
Interventions to Improve Treatment
Most health care organization-based interventions to enhance breast cancer treatment among minority women focus on expediting treatment initiation rather than improving quality of breast cancer care or survivorship care. Of the interventions we initially identified, one described a controlled trial but did not report results (Hiatt et al. 2001), one described an uncontrolled patient navigator intervention (Burhansstipanov et al. 1998), and two reported results from controlled trials (Ell et al. 2002; Goodwin et al. 2003). Only the controlled trials met all of the inclusion criteria for this review. As described above, Ell and colleagues evaluated the Screening Adherence Follow-Up (SAFe) program, a case management-based intervention that combined health education, counseling, and systems navigation. The treatment-related outcome in this study was time to initiation of cancer treatment, defined as the time interval from the date of the breast cancer diagnosis to the date of the first confirmed treatment appointment. In this study, a greater proportion of enrollees (62%) received their first breast cancer treatment within 30 days after receiving a diagnosis compared to controls (40%). In addition, the median time to treatment initiation was 24 days for enrollees and 29 days for controls. However, due to the small number of patients diagnosed with breast cancer, neither of these differences was statistically significant.
Goodwin et al. (2003) evaluated the effect of nurse case management on breast cancer treatment in a racially diverse population of female Medicare recipients. Interactions between nurse case managers and patients occurred in the patients' homes, via telephone, in outpatient clinics, and in the hospital setting. Case manager services included education, counseling, advocacy, and coordination of care. Compared to those in the control group, women in the intervention group were more likely to receive breast-conserving surgery (29% vs. 19%) and radiation therapy (36% vs. 19%). Among women who received breast-conserving surgery, a higher proportion of those in the intervention group received adjuvant radiation (78% vs. 45%) and axillary dissection (71% vs. 45%). Being aged 75 and older, being unmarried, living alone, and being a racial/ethnic minority were all associated with lower rates of stage-appropriate treatment in the control group but not in the intervention group, suggesting that women with indicators of poor social support were more likely to benefit from nurse case management. As for survivorship care, there was a trend toward increased breast reconstruction surgery in the intervention group compared to the control group (9% vs. 3%) but this effect was not significant. In addition, 2 months after surgery, a significantly higher proportion of women in the intervention group reported normal or near-normal arm function compared to the control group (93% vs. 84%).
Discussion
Of the studies that met our inclusion criteria, most focused on breast cancer screening rather than diagnostic testing or breast cancer treatment. The screening interventions were generally effective but were more likely to be effective if they were conducted among white, educated populations. Interventions to expedite diagnostic testing used varying levels of case management, and all succeeded in facilitating at least one step in the diagnostic process. None of the diagnostic testing trials compared different models of case management so we could not determine whether a less comprehensive (and therefore less costly) model is as effective as the most comprehensive approach. Only two studies examined interventions to enhance breast cancer treatment. None of the studies in this review examined long-term patient outcomes, such as tumor recurrence or 5-year survival. In addition, none of the trials addressed potential provider bias in breast cancer screening, diagnosis, or treatment.
Screening
A recent population-based study of women 40 years and older revealed that 70% of whites, 70% of blacks, 65% of Hispanics or Latinos, and 63% of Asians reported obtaining a mammogram in the previous 2 years (National Center for Health Statistics 2005). Several of the studies in this review documented much lower baseline mammography rates among both intervention and control groups. This discrepancy likely reflects both the shorter time frame of evaluation (6 to 12 months) and the higher rates of uninsurance and underinsurance among the trial populations. As many as 50 to 60% of trial participants were uninsured, while nationally, rates of uninsurance are not as high: 20% among blacks, 33% among Hispanics and Latinos, 19% among Asians, and 15% among whites (DeNavas-Walt, Proctor, and Hill-Lee 2005).
We found that patient-targeted screening trials employed a variety of strategies, including reminder letters and telephone calls, culturally tailored classroom instruction and videos, and assistance with financial and logistical needs. While reminder-based patient interventions increased mammography among women with higher educational attainment or previous mammography, this approach was less successful among those with lower educational attainment or no history of mammography. This suggests that barriers other than knowledge deficits prevent many women from obtaining appropriate screening tests. Foreign language represents an important barrier among recent immigrants (Swan et al. 2003), and studies among low acculturated Latinas demonstrated a positive effect of culturally tailored interventions (such as native language educational material and classroom instruction) on outcomes important to breast cancer screening, including breast cancer knowledge, BSE proficiency and practice, and intention to ask providers about mammography. Cultural barriers to screening are also important for black women as suggested by the effectiveness of culturally tailored videos and classroom instruction on screening mammography rates. Previous research has shown that patient involvement in developing health education materials ensures that the content of the materials is relevant to the patients' situation and is presented from their point of view (Rudd and Comings 1994).
Other important screening barriers include lack of health insurance, low income, and reduced access to transportation. In contrast to reminder-based interventions, interventions that addressed financial and logistical concerns increased mammography in patient populations that were diverse with respect to race, ethnicity, and insurance status. The dramatic increases in screening associated with mammogram vouchers suggest that financial barriers are paramount among low-income and minority populations. This is supported by an extensive literature on financial incentives and self-care behavior, including the Rand Health Insurance Experiment, which showed that out-of-pocket costs reduce adherence to medical recommendations for asymptomatic conditions in low-income populations (Shapiro, Ware, and Sherbourne 1986).
In studies among low-income and underinsured populations, provider-targeted interventions led to greater increases in screening mammography compared to patient-targeted interventions. This is not surprising given that physicians who receive mammography prompts typically interact with the patients about whom they are prompted whereas not all patients who receive mammography reminders interact with their physicians. Burack et al. (1994) found that a physician intervention led to increases in screening mammography even after removal of out-of-pocket mammography costs for low-income patients. This suggests that physician advice can be effective across a spectrum of patient income and insurance levels, and that more should be done to ensure that physicians make appropriate screening recommendations. In the studies we reviewed, chart reminders, audit with feedback, peer comparison, and preventive care flow sheets all increased physician adherence to mammography screening guidelines.
While these strategies worked for mammography, they were less successful at increasing CBE. This may be related to the greater amount of time needed for performing a CBE compared to ordering a mammogram. Clinics that primarily serve uninsured and underinsured patients tend to have fewer financial resources and more time constraints compared to those with higher proportions of insured patients (McAlearney 2002). In addition, patients who visit public or community health centers tend to have higher rates of comorbidity and other barriers to efficient care, including language differences (Lemon et al. 2006). Physicians with time pressures due to patient care needs may be less inclined to conduct clinical screening examinations, especially if support staff are not available to provide assistance. In addition, clinicians may be less inclined to conduct a CBE if they believe that CBE plus mammography does not reduce breast cancer mortality compared to mammography alone (Humphrey et al. 2002). Therefore, while the less time-intensive practice of mammography ordering was amenable to change in most clinical settings, CBE practice was more difficult to change. Further research is needed regarding CBE as a screening strategy, especially among women with limited access to mammography.
Diagnosis
Previous research has shown that several barriers contribute to delay in diagnostic testing following abnormal screening mammography or CBE. These include fear of finding cancer, not understanding the recommended exam, worrying about the exam or treatment, cost, transportation and child care issues, and language or cultural differences between patients and providers (Ell et al. 2002; Lantz et al. 2004). Because of the number and complexity of barriers that many women face, all of the trials we identified used some form of case management. These trials differed both in the intensity of case management and in the outcomes studied. Those that used either the minimal or coordination case management model demonstrated increased adherence with follow-up appointments, while those that used the more comprehensive sociomedical model demonstrated increased breast biopsy rates and earlier diagnostic resolution. None of the studies used a randomized controlled trial design, and selection bias must be considered before results can be generalized. In addition, because comparisons between models of case management were not made in any of the trials, we cannot quantify the benefits associated with higher levels of case management. Nevertheless, case management appears to be an effective strategy for overcoming many of the social, economic, and logistical barriers to follow-up testing that minority women face.
Treatment
Only two studies met our criteria for health care organization-based studies to improve breast cancer treatment (Ell et al. 2002; Goodwin et al. 2003). In the Ell et al. study, a sociomedical model of case management increased the number of patients who received breast cancer treatment within 30 days of diagnosis. While this effect was not statistically significant, it suggests that comprehensive case management, including assistance with scheduling and child or elder care, as well as evaluation and treatment for depression and anxiety, can facilitate treatment initiation among minority women. The Goodwin et al. study also used case management to provide a range of services, including assessment of social support and emotional and cognitive status, and referral for services as indicated. This intervention resulted in significant increases in breast-conserving surgery, adjuvant radiation, and axillary dissection. While women in the control group with indicators of poor social support were less likely to receive appropriate treatment, this was not the case in the intervention group, suggesting that a sociomedical model of case management is especially helpful to those facing multiple barriers to care. There was some evidence of improved survivorship care (e.g., increased breast reconstruction and increased postsurgical arm function) in the intervention group but many elements of survivorship care were not assessed by either Goodwin et al. or Ell et al. These elements include follow-up office visits, follow-up mammography, diagnosis and management of disease recurrence, and diagnosis and management of treatment sequelae. In addition, neither study evaluated the impact of case management on long-term survival. Future studies in this area should assess both quality of survivorship care and long-term outcomes.
Bigby et al. (2003) recently described a provider-targeted program aimed at improving breast cancer care for black women and reducing racial and ethnic disparities in breast cancer mortality. This intervention includes primary care physician training regarding cultural competency and breast cancer treatment options. The training program is a train-the-trainer model in which a core group of providers from six demonstration sites train providers and staff at other clinical sites. When published, results from this trial may shed light on the effects of health care organization-based interventions on the quality of breast cancer treatment and survivorship care.
Conclusions and Future Directions
We found that significant financial, logistical, and time barriers to breast cancer screening and follow-up testing exist for many minority patients and their providers. Despite these obstacles, several health care organization-based interventions achieved success in increasing screening behaviors and diagnostic testing. Trials that focused on barriers other than knowledge deficits, such as out-of-pocket costs for mammography or lack of transportation to specialist appointments, were successful even in uninsured and underinsured populations. We conclude that an important first step toward reducing breast cancer mortality among low-income and minority women is to identify financial and logistical barriers to screening and follow-up testing and provide services that overcome these barriers.
Recognition of the importance of financial barriers to breast cancer screening and treatment has led to the development of two federal breast cancer initiatives, the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) and the Breast and Cervical Cancer Prevention and Treatment Act (BCCPTA). The NBCCEDP was created in 1990 to assist uninsured and underinsured women obtain breast and cervical cancer screening and follow-up diagnostic testing. It is administered by the Centers for Disease Control and Prevention (CDC) through cooperative agreements with all 50 states, 4 U.S. territories, the District of Columbia, and 13 American Indian/Alaska Native tribes or organizations. Between 1991 and 2002, the NBCCEDP budget increased from $30 million to $192 million and the number of annual mammograms provided through this program increased from 38,869 to 292,601. During this time, 9,956 cases of breast cancer were diagnosed among women who may not have ever obtained screening mammography (Ryerson and Major 2005). Women diagnosed with breast cancer through the NBCCEDP can receive treatment for their disease through the BCCPTA, which extends Medicaid eligibility to uninsured women regardless of their income or assets (Lantz, Weisman, and Itani 2003). Since its inception in 2000, the BCCPTA has helped many women obtain breast cancer care with minimal delay (Kenney et al. 2004).
Despite the success of these programs, there is evidence that the NBCCEDP and the BCCPTA serve only a fraction of women who are eligible (Smith-Bindman et al. 2003; Kenney et al. 2004). For example, in a 1994 national survey, 34% of uninsured women aged 40 to 64 years reported having had a mammogram within the past 2 years. By 2003, this proportion had only increased to 42% (National Center for Health Statistics 2005). With an estimated 6.7 million uninsured women between the ages of 40 and 64 years in the United States (DeNavas-Walt, Proctor, and Hill-Lee 2005), these data suggest that approximately 3.9 million uninsured women do not obtain regular mammography. Lack of screening mammography and timely diagnosis significantly contribute to increased breast cancer mortality among low-income and minority women (McWhorter and Mayer 1987). To meet the unmet need for screening mammography, federal and state funding for the NBCCEDP should be increased to enhance the access of uninsured women to breast cancer screening. Increased efforts should also be made to raise patient awareness about the importance and availability of breast cancer screening through the NBCCEDP, and to ensure that all uninsured individuals have a medical home (Satcher et al. 2005).
In addition to overcoming patient-related barriers to screening, screening behaviors among providers must also be improved. We found that provider adherence to mammography guidelines can be improved with relatively simple chart reminders and flow sheets. Consequently, a portion of NBCCEDP funds should be used to develop a standardized chart-based reminder system, which could be implemented in all NBCCEDP-affiliated clinics. Improving provider compliance with CBE guidelines presents a greater challenge, especially in clinics that serve primarily minority patients. Progress in this area may require strategies that address the staffing and financial constraints that many community health centers face. The Institute of Medicine found that the ability of community health centers to provide high quality care declined in the late 1990s due to the growing number of uninsured patients, the proliferation of Medicaid managed care, and the erosion of the subsidies used to cover the cost of charity care (McAlearney 2002).
A potentially important area that was not evaluated in any of the studies in our review is provider bias. A growing body of research indicates that physicians' diagnostic decisions can be negatively influenced by patient race/ethnicity (van Ryn and Burke 2000; Finucane and Carrese 1990; Bogart et al. 2000; Schulman et al. 1999). The 2004 position paper of the American College of Physicians on racial/ethnic disparities in health care notes that physicians must “recognize that inherent biases can lead to disparities in health care among racial and ethnic minorities” and the recent Agency for Healthcare Research and Quality (AHRQ) report, “Strategies for Improving Minority Healthcare Quality,” called for more research into interventions aimed specifically at improving quality of care for racial/ethnic minorities, such as addressing provider bias (Beach et al. 2006; Groman and Ginsburg 2004). Future research that explores and addresses potential areas of provider bias that contribute to racial disparities in breast cancer mortality will be important.
It is somewhat encouraging that mammography screening rates have been similar among white and black women since about 1993 (National Center for Health Statistics 2005) despite the fact that uninsurance is more prevalent among blacks (20%) compared to whites (15%) (DeNavas-Walt, Proctor, and Hill-Lee 2005). However, debate exists as to the accuracy of self-reported screening mammography (McPhee et al. 2002) and continued efforts are needed to increase screening mammography among the subpopulation of black and white women who do not obtain regular screening, especially among those who lack health insurance. In addition, lower rates of screening mammography among Hispanic/Latino and Asian women continue to represent a challenge. Among Hispanic/Latino women, this phenomenon may reflect both a high rate of uninsurance (33%) (DeNavas-Walt, Proctor, and Hill-Lee 2005) and a language barrier (Swan et al. 2003). Language or cultural barriers may also account for the lower rate of mammography among Asian Americans whose level of uninsurance (19%) is comparable to that among African Americans (20%) (DeNavas-Walt, Proctor, and Hill-Lee 2005). Eliminating barriers to screening mammography and bringing all racial/ethnic groups closer to compliance with screening guidelines will require increased state and federal support for health centers that provide care to uninsured and underinsured women. While such an investment will be significant, a recent cost-effectiveness analysis found that the incremental cost of screening mammography per quality adjusted life year (QALY) is $37,000, which is below the commonly accepted benchmark for cost-effectiveness of $50,000 per QALY (Stout et al. 2006).
Equally important to breast cancer screening is timely follow-up of abnormal screening results. The NBCCEDP provides funding for the diagnostic evaluation of abnormal mammograms and clinical breast examinations. A recent survey of NBCCEDP-funded health centers found that most centers use some form of case management to assist women with diagnostic testing and treatment (Lantz et al. 2004). However, very little has been published regarding the cost-effectiveness of various case management models. While the comprehensive or sociomedical model may be the most effective, the cost of widespread implementation may be prohibitive. Therefore, we encourage efforts to find the most cost-effective model of case management. As breast cancer screening continues to increase, increased funding will be needed for case management to ensure that low income and minority women do not experience diagnostic delays due to lack of insurance, transportation, child care, or coordination of appointments.
Only two studies met our review criteria for interventions to reduce disparities in breast cancer treatment (Ell et al. 2002; Goodwin et al. 2003). The paucity of literature in this area indicates more research is needed to define what represents high-quality breast cancer care and to identify effective strategies for improving treatment and survivorship care among minority women. Fortunately, several initiatives are underway in this area. For example, the state-level experience in implementing the BCCPTA is being monitored (French et al. 2004) and a long-term assessment of the impact of the BCCPTA on breast cancer mortality has been described (Maloy et al. 2004). In addition, several programs are in place to monitor the quality of cancer care among all U.S. patients (Malin et al. 2006). Identifying and correcting racial and ethnic differences in breast cancer care will help ensure that advances made in screening and diagnostic testing are followed by state-of-the-art care. This will increase the likelihood that breast cancer survival among minority women will more closely match that of white women.
In a recent essay on cancer disparities research, Krieger (2005) emphasized the importance of monitoring patient survival, morbidity, and mortality. While the trials we reviewed examined important process outcomes, none addressed long-term health outcomes among study participants. It is possible that racial and ethnic disparities in breast cancer mortality will persist even after all disparities in screening, diagnosis, and treatment are identified and corrected. This could be due to racial or ethnic differences in tumor biology (Carey et al. 2006) or differences in response to therapy that have a biological or psychosocial basis (Antoni et al. 2006). Therefore, it is important that breast cancer survival, morbidity, and mortality be monitored not only in clinical trials but also, where possible, in trials designed to enhance breast cancer screening and diagnosis. As with clinical trials, identifying the impact of screening and diagnostic test interventions on health outcomes requires significant numbers of participants followed over months to years. National health initiatives such as the NBCCEDP and the BCCPTA are in a better position than individual health centers to conduct this type of research. In addition, the infrastructure of these programs permits communication among partnering agencies and health centers throughout the country. This infrastructure should continue to be used as a way to disseminate findings, such as those in this review.
In the absence of a national health program, which by nature ensures greater equity in health care services across racial, ethnic, and socioeconomic groups, the elimination of disparities in breast cancer mortality will require continued recognition of barriers to screening, diagnosis, treatment, and survivorship care, and increased support for interventions that reduce those barriers.
Table 5.
Trials to Enhance Breast Cancer Treatment
| Reference | Design | Intervention(s) | Control or Limited Intervention(s) | Intervention n (Control n), Duration, Race/Ethnicity | Health Insurance, Income | Setting | Results | Quality Score (out of 27) |
|---|---|---|---|---|---|---|---|---|
| Ell et al. 2002 | CCT | Stepped care appointment reminders, reinforcing telephone calls, assistance with logistical barriers, systems navigation, social work assessment +/- counseling +/- referral to psychosocial oncology +/- mental health services. | Usual care. | 605 (476), 12 months, 71% Hispanic, 18% black, 11% Other. | 17% without insurance at one location, 78% without insurance at other location, income not reported. |
Large public and private urban screening, diagnostic, and treatment-referral centers in Los Angeles and New York. | Initiated breast cancer treatment within 30 days after diagnosis: intervention (62%), control (40%), (not significant). Median length of time from diagnosis to treatment: intervention (24 days), control (29 days), (not significant). |
19 |
| Goodwin et al. 2003 | RCT | Nurse case management: baseline assessment, education, counseling, advocacy, care coordination. Goals were to ensure patients informed of treatment options and physicians aware of relevant issues. Specific treatments were not advocated by nurse case managers. | Usual care. | 169 (166), 12 months, 70% white, 21% black, 6.6% Hispanic, 2.4% Other. | All with Medicare, 59% with supplemental insurance, 10% with Medicaid, among those with information on household income, 53% with income <$15,000/year | 13 community and 2 public hospitals in southeastern Texas | Breast conserving surgery: intervention (28.6%), control (18.7%), (p < .031), radiation therapy: intervention (36%), control (19%), (p < .003), breast reconstruction: intervention (9.3%), control (2.6%), (p < .054), normal arm function two months post surgery: intervention (93%), control (84%), (p < .037). |
20 |
Acknowledgments
Authors' Note: This project was supported by the Robert Wood Johnson Foundation (RWJF) Finding Answers: Disparities Research for Change Program and the Department of Medicine at the University of Chicago. Dr. Masi is supported in part by a Centers for Population Health and Health Disparities Grant (P50 ES-12382) from the National Institute of Environmental Health Sciences and the National Cancer Institute. Dr. Peek is supported by an RWJF Harold Amos Medical Faculty Development award (53056) and an NIH career development award (K23 DK075006-01).
References
- American Cancer Society . Breast Cancer Facts & Figures 2005-2006. American Cancer Society, Inc.; Atlanta, GA: 2005. [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, Green-McDonald P, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumor biology: Pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashing-Giwa KT, Padilla G, Tejero J, Kraemer J, Wright K, Coscarelli A, Clayton S, Williams I, Hills D. Understanding the breast cancer experience of women: A qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psycho-Oncology. 2004;13:408–28. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NM, Rowland JH. Cancer survivorship research among ethnic minority and medically underserved groups. Oncology Nursing Forum. 2002;29(5):789–801. doi: 10.1188/02.ONF.789-801. [DOI] [PubMed] [Google Scholar]
- Beach MC, Gary TL, Price EG, Robinson K, Gozu A, Palacio A, Smarth C, Jenckes M, Feuerstein C, Bass EB, Powe NR, Cooper LA. Improving health care quality for racial/ethnic minorities: A systematic review of the best evidence regarding provider and organization interventions. BMC Public Health. 2006;6:104. doi: 10.1186/1471-2458-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DM, Gomez EB, Kaiser DL, Yoshihasi A, Hodge RH. Improving preventing care at a medical clinic: How can the patient help? American Journal of Preventive Medicine. 1989;5(6):353–59. [PubMed] [Google Scholar]
- Bigby J, Ko LK, Johnson N, David MM, Ferrer B. A community approach to addressing excess breast and cervical cancer mortality among women of African descent in Boston. Public Health Reports. 2003;118(4):338–47. doi: 10.1093/phr/118.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LM, Kelly JA, Catz SL, Sosman JM. Impact of medical and nonmedical factors on physician decision making for HIV/AIDS antiretroviral treatment. Journal of Acquired Immune Deficiency Syndrome. 2000;23(5):396–404. doi: 10.1097/00126334-200004150-00006. [DOI] [PubMed] [Google Scholar]
- Bressler J, Ansell D, Parker J, Dillard J, Whitman S. Breast cancer screening in an urban public hospital. Cancer. 1993;1993:3636–40. doi: 10.1002/1097-0142(19931215)72:12<3636::aid-cncr2820721214>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burack RC, Gimotty PA. Promoting screening mammography in inner-city settings: The sustained effectiveness of computerized reminders in a randomized controlled trial. Medical Care. 1997;35(9):921–31. doi: 10.1097/00005650-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Burack RC, Gimotty PA, George J, Simon MS, Dews P, Moncrease A. The effect of patient and physician reminders on use of screening mammography in a health maintenance organization. Results of a randomized controlled trial. Cancer. 1996;78(8):1708–21. [PubMed] [Google Scholar]
- Burack RC, Gimotty PA, George J, Stengle W, Warbasse L, Moncrease A. Promoting screening mammography in inner-city settings: A randomized controlled trial of computerized reminders as a component of a program to facilitate mammography. Medical Care. 1994;32(6):609–24. doi: 10.1097/00005650-199406000-00006. [DOI] [PubMed] [Google Scholar]
- Burhansstipanov L, Bad-Wound D, Capelouto N, Goldfarb F, Harjo L, Hatathlie L, Vigil G, White M. Culturally relevant “navigator” patient support: the Native Sisters. Cancer Practice. 1998;6(3):191–94. doi: 10.1046/j.1523-5394.1998.006003191.x. [DOI] [PubMed] [Google Scholar]
- Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for delay in breast cancer diagnosis. Preventive Medicine. 1996;25:218–24. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowans D, Conway K, Karaca G, Troester MA, Kit-Tse C, Edminston S, Deming SL, Ceradts J, Cheang MCU, Nielson TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Case Management Society of America . Standards of practice for case management. Case Management Society of America; Little Rock, Arkansas: 2002. [Google Scholar]
- Chambers CV, Balaban DJ, Carlson BL, Ungemack JA, Grasberger DM. Microcomputer-generated reminders: Improving the compliance of primary care physicians with mammography screening guidelines. Journal of Family Practice. 1989;29(3):273–80. [PubMed] [Google Scholar]
- Champion VL, Skinner CS, Menon U, Seshadri R, Anzalone DC, Rawl SM. Comparisons of tailored mammography interventions at two months postintervention. Annals of Behavioral Medicine. 2002;24(3):211–28. doi: 10.1207/S15324796ABM2403_06. [DOI] [PubMed] [Google Scholar]
- Chin MH, Walters AE, Cook SC, Huang E. Interventions to reduce racial and ethnic disparities in health care. Medical Care Research and Review. 2007;64(5 Suppl):7S–28S. doi: 10.1177/1077558707305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TC, Berkel HJ, Arnold CL, Nandy I, Jackson RH, Murphy PW. Intervention to increase mammography utilization in a public hospital. Journal of General Internal Medicine. 1998;13:230–33. doi: 10.1046/j.1525-1497.1998.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD, Hill-Lee C. Income, poverty, and health insurance coverage in the United States: 2004. Government Printing Office; Washington, DC: 2005. [Google Scholar]
- Dickey LL, Petitti D. A patient-held minirecord to promote adult preventive care. Journal of Family Practice. 1992;34:457–63. [PubMed] [Google Scholar]
- Dietrich AJ, Tobin JN, Hill-Sox C, Cassels AN, Negron F, Younge RG, Demby NA, Tosteson TD. Cancer early-detection services in community health centers for the underserved. Archives of Family Medicine. 1998;7:320–27. doi: 10.1001/archfami.7.4.320. [DOI] [PubMed] [Google Scholar]
- Dolan NC, McDermott MM, Morrow M, Venta L, Martin GL. Impact of same-day screening mammography availability: Results of a controlled clinical trial. Archives of Internal Medicine. 1999;159(4):393–98. doi: 10.1001/archinte.159.4.393. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell K, Padgett D, Vourlekis B, Nissly J, Pineda D, Sarabia O, Walther V, Blumenfield S, Lee PJ. Abnormal mammogram follow-up: a pilot study in women with low income. Cancer Practice. 2002;10(3):130–38. doi: 10.1046/j.1523-5394.2002.103009.x. [DOI] [PubMed] [Google Scholar]
- Finucane TE, Carrese JA. Racial bias in presentation of cases. Journal of General Internal Medicine. 1990;5(2):120–21. doi: 10.1007/BF02600511. [DOI] [PubMed] [Google Scholar]
- Fisher TL, Burnet DL, Huang ES, Chin MH, Cagney KA. Cultural leverage: Interventions utilizing culture to narrow racial disparities in health care. Medical Care Research and Review. 2007;64(5 Suppl):243S–282S. doi: 10.1177/1077558707305414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon ML, Gapstur SM, Knight SJ. Results of Mujeres Felices por ser Saludables: A dietary/breast health randomized clinical trial for Latino Women. Annals of Behavioral Medicine. 2004;28(2):95–104. doi: 10.1207/s15324796abm2802_4. [DOI] [PubMed] [Google Scholar]
- Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice. 1995;3:19–30. [PubMed] [Google Scholar]
- French C, True S, McIntyre R, Sciulli M, Maloy KA. State implementation of the Breast and Cervical Cancer Prevention and Treatment Act of 2000: A collaborative effort among government agencies. Public Health Reports. 2004;119(3):279–85. doi: 10.1016/j.phr.2004.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Satish S, Anderson ET, Nattinger AB, Freeman JL. Effect of nurse case management on the treatment of older women with breast cancer. Journal of the American Geriatric Society. 2003;51:1252–59. doi: 10.1046/j.1532-5415.2003.51409.x. [DOI] [PubMed] [Google Scholar]
- Groman R, Ginsburg J. Racial and ethnic disparities in health care: A position paper of the American College of Physicians. Annals of Internal Medicine. 2004;141(3):226–32. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- Herman CJ, Speroff T, Cebul R. Improving compliance with breast cancer screening in older women: Results of a randomized controlled trial. Archives of Internal Medicine. 1995;155(7):717–22. [PubMed] [Google Scholar]
- Hiatt RA, Pasick RJ, Stewart S, Bloom J, Davis P, Gardiner P, Johnston M, Luce J, Schorr K, Brunner W, Stroud F. Community-based cancer screening for underserved women: Design and baseline findings from the Breast and Cervical Cancer Intervention Study. Preventive Medicine. 2001;33(3):190–203. doi: 10.1006/pmed.2001.0871. [DOI] [PubMed] [Google Scholar]
- Hirschman J, Whitman S, Ansell D. The black:white disparity in breast cancer mortality: The example in Chicago. Cancer Causes & Control. 2007;18:323–33. doi: 10.1007/s10552-006-0102-y. [DOI] [PubMed] [Google Scholar]
- Humphrey LL, Chan BKS, Detlefsen S, Helfand M. Screening for breast cancer: systematic evidence review No. 15. Agency for Healthcare Research and Quality; Rockville, MD: 2002. [PubMed] [Google Scholar]
- Janz NK, Schottenfeld D, Doerr KM, Selig SM, Dunn RL, Strawderman M, Levine PA. A two-step intervention of increase mammography among women aged 65 and older. American Journal of Public Health. 1997;87(10):1683–86. doi: 10.2105/ajph.87.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- Kenney KA, Blake SC, Maloy K, Ranji UR, Salganicoff A. hearing their voices: lessons from the Breast and Cervical Cancer Prevention and Treatment Act 2004. 2004 [cited May 16 2006]. Available from http://www.kaiserfamilyfoundation.org/womenshealth/upload/Hearing-Their-Voices-Lessons-from-the-Breast-and-Cervical-Cancer-Prevention-and-Treatment-Act-Focus-Group-Findings-from-California-Report.pdf.
- Kiefe CI, McKay SV, Halevy A, Brody BA. Is cost a barrier to screening mammography for low-income women receiving Medicare benefits? A randomized trial. Archives of Internal Medicine. 1994;154(11):1217–24. [PubMed] [Google Scholar]
- Korr WS, Cloninger L. Assessing models of case management: An empirical approach. Journal of Social Services Research. 1991;14:153–207. [Google Scholar]
- Krieger N. Defining and investigating social disparities in cancer: Critical issues. Cancer Causes and Control. 2005;16:5–14. doi: 10.1007/s10552-004-1251-5. [DOI] [PubMed] [Google Scholar]
- Lacey L, Whitfield J, DeWhite W, Ansell D, Whitman S, Chen E, Phillips C. Referral adherence in an inner city breast and cervical cancer screening program. Cancer. 1993;72(3):950–55. doi: 10.1002/1097-0142(19930801)72:3<950::aid-cncr2820720347>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Landis SE, Hulkower SD, Pierson S. Enhancing adherence with mammography through patient letters and physician prompts. A pilot study. North Carolina Medical Journal. 1992;53(11):575–78. [PubMed] [Google Scholar]
- Lantz PM, Keeton K, Romano L, DeGroff A. Case management in public health screening programs: The experience of the National Breast and Cervical Cancer Early Detection Program. Journal of Public Health Management Practice. 2004;10(6):545–55. doi: 10.1097/00124784-200411000-00012. [DOI] [PubMed] [Google Scholar]
- Lantz PM, Weisman CS, Itani Z. A disease-specific Medicaid expansion for women: The Breast and Cervical Cancer Prevention and Treatment Act of 2000. Women's Health Issues. 2003;13:79–92. doi: 10.1016/s1049-3867(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Legler J, Meissner HI, Coyne C, Breen N, Chollette V, Rimer BK. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:59–71. [PubMed] [Google Scholar]
- Lemon SC, Zapka JG, Estabrook B, Benjamin E. Challenges to research in urban community health centers. American Journal of Public Health. 2006;96(4):626–28. doi: 10.2105/AJPH.2004.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethnicity & Disease. 2005;15(2 Suppl 2):S5–S9. [PubMed] [Google Scholar]
- Litzelman DK, Dittus RS, Miller ME, Tierney WM. Requiring physicians to respond to computerized reminders improved their compliance with preventive care protocols. Journal of General Internal Medicine. 1993;8:311–17. doi: 10.1007/BF02600144. [DOI] [PubMed] [Google Scholar]
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: How can we improve the quality of cancer care in the United States? Journal of Clinical Oncology. 2006;24(4):626–34. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- Maloy KA, Blake SC, Merrill C, Cyprien S. Evaluation of the Breast and Cervical Cancer Prevention and Treatment Act (BCCPTA) of 2000. 2004 [cited August 1, 2006]. Available from http://www .academyhealth.org/2004/ppt/merrill2.ppt. [Google Scholar]
- Mandelblatt JS, Yabroff KR. Effectiveness of interventions designed to increase mammography use: A meta-analysis of provider-targeted strategies. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:759–67. [PubMed] [Google Scholar]
- Manfredi C, Czaja R, Freels S, Trubitt M, Warnecke R, Lacey L. Prescribe for Health: Improving cancer screening in physician practices serving low-income and minority populations. Archives of Family Medicine. 1998;7:329–37. doi: 10.1001/archfami.7.4.329. [DOI] [PubMed] [Google Scholar]
- Manfredi C, Lacey L, Warnecke R. Results of an intervention to improve compliance with referrals for evaluation of suspected malignancies at neighborhood public health centers. American Journal of Public Health. 1990;80(1):85–87. doi: 10.2105/ajph.80.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Lurie N, McGovern PG, Tyrrell M, Slater JS. Increasing breast and cervical cancer screening in low-income women. Journal of General Internal Medicine. 1998;13(8):515–21. doi: 10.1046/j.1525-1497.1998.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May DS, Lee NC, Richardson LC, Giustozzi AG, Bobo JK. Mammography and breast cancer detection by race and Hispanic ethnicity: Results from a national program (United States) Cancer Causes & Control. 2000;11:697–705. doi: 10.1023/a:1008900220924. [DOI] [PubMed] [Google Scholar]
- Mayer JA, Lewis EC, Slymen DJ, Dullum J, Kurata H, Holbrook A, Elder JP, Williams SJ. Patient reminder letters to promote annual mammograms: A randomized controlled trial. Preventive Medicine. 2000;31(4):315–22. doi: 10.1006/pmed.2000.0718. [DOI] [PubMed] [Google Scholar]
- McAlearney JS. The financial performance of community health centers, 1996-1999. Health Affairs. 2002;21(2):219–25. doi: 10.1377/hlthaff.21.2.219. [DOI] [PubMed] [Google Scholar]
- McCarthy BD, Ulcickas-Yood M, Bolton MB, Boohaker EA, MacWilliam CH, Young MJ. Redesigning primary care processes to improve the offering of mammography. Journal of General Internal Medicine. 1997;12(6):357–63. doi: 10.1046/j.1525-1497.1997.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee SJ, Adair-Bird J, Jenkins CNH, Fordham D. Promoting cancer screening: A Randomized, controlled trial of three interventions. Archives of Internal Medicine. 1989;149:1866–78. doi: 10.1001/archinte.149.8.1866. [DOI] [PubMed] [Google Scholar]
- McPhee SJ, Nguyen TT, Shema SJ, Nguyen B, Somkin C, Vo P, Pasick R. Validation of recall of breast and cervical cancer screening by women in an ethnically diverse population. Preventive Medicine. 2002;35(5):463–73. doi: 10.1006/pmed.2002.1096. [DOI] [PubMed] [Google Scholar]
- McWhorter WP, Mayer WJ. Black/White differences in type of initial breast cancer treatment and implications for survival. American Journal of Public Health. 1987;77:1515–17. doi: 10.2105/ajph.77.12.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SI, Chavez LR, Magana JR, Nava P, Buciaga-Valdez R, Hubbell FA. Improving breast cancer control among Latinas: Evaluation of a theory-based educational program. Health Education & Behavior. 1998;25(5):653–70. doi: 10.1177/109019819802500511. [DOI] [PubMed] [Google Scholar]
- Moss M, Steinhauer J. Mammogram clinic's flaws highlight gaps in U.S. rules. The New York Times. 2002:24. [Google Scholar]
- National Center for Health Statistics . Health, United States, 2005 with chartbook on trends in the health of Americans. Government Printing Office; Washington, DC: 2005. [PubMed] [Google Scholar]
- Nattinger AB, Panzer RJ, Janus J. Improving the utilization of screening mammography in primary care practices. Archives of Internal Medicine. 1989;149:2087–92. [PubMed] [Google Scholar]
- Nguyen BH, Nguyen KP, McPhee SJ, Nguyen AT, Tran DQ, Jenkins CNH. Promoting cancer prevention activities among Vietnamese physicians in California. Journal of Cancer Education. 2000;15:82–85. doi: 10.1080/08858190009528662. [DOI] [PubMed] [Google Scholar]
- Ornstein SM, Garr DR, Jenkins RG, Rust PF, Arnon A. Computer-generated physician and patient reminders: Tools to improve population adherence to selected preventive services. Journal of Family Practice. 1991;32(1):82–90. [PubMed] [Google Scholar]
- Paskett ED, Tatum CM, D'Agostino R, Rushing J, Velez R, Michielutte R, Dignan M. Community-based interventions to improve breast and cervical cancer screening: results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:453–59. [PubMed] [Google Scholar]
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK. SEER cancer statistics review, 1975-2002. National Cancer Institute; Bethesda, MD: 2005. [Google Scholar]
- Rimer BK, Conaway M, Lyna P, Glassman B, Yarnall KSH, Lipkus I, Barber LT. The impact of tailored interventions on a community health center population. Patient Education and Counseling. 1999;37:125–40. doi: 10.1016/s0738-3991(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Roetzheim RG, Christian LK, Jacobsen PB, Cantor AB, Schroeder J, Abdulla R, Hunter S, Chirikos TN, Krischer JP. A randomized controlled trial to increase cancer screening among attendees of community health centers. Annals of Family Medicine. 2004;2:294–300. doi: 10.1370/afm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetzheim RG, Christman LK, Jacobsen PB, Schroeder J, Abdulla R, Hunter S. Long-term results from a randomized controlled trial to increase cancer screening among attendees of community health centers. Annals of Family Medicine. 2005;3:109–14. doi: 10.1370/afm.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RE, Comings JP. Learner developed materials: An empowering product. Health Education Quarterly. 1994;21:313–27. doi: 10.1177/109019819402100304. [DOI] [PubMed] [Google Scholar]
- Ryerson AB, Major AC. National Breast and Cervical Cancer Early Detection Program: 1991-2002 national report. National Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- Satcher D, Fryer GE, McCann J, Troutman A, Woolf SH, Rust G. What if we were equal? A comparison of the Black-White mortality gap in 1960 and 2000. Health Affairs. 2005;24(2):459–64. doi: 10.1377/hlthaff.24.2.459. [DOI] [PubMed] [Google Scholar]
- Schreiner DT, Petrusa ER, Rettie CS, Kluge RM. Improving compliance with preventive medicine procedures in a house staff training program. Preventive Medicine Procedures. 1988;81(12):1553–57. doi: 10.1097/00007611-198812000-00021. [DOI] [PubMed] [Google Scholar]
- Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ. The effect of race and sex on physicians' recommendations for cardiac catheterization. New England Journal of Medicine. 1999;340(8):618–26. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- Shapiro MF, Ware JE, Sherbourne CD. Effects of cost sharing on seeking care for serious and minor symptoms. Annals of Internal Medicine. 1986;104:246–51. doi: 10.7326/0003-4819-104-2-246. [DOI] [PubMed] [Google Scholar]
- Skaer TL, Robison LM, Sclar DA, Harding GH. Financial incentive and the use of mammography among Hispanic migrants to the United States. Health Care for Women International. 1996;17(4):281–91. doi: 10.1080/07399339609516245. [DOI] [PubMed] [Google Scholar]
- Skinner CS, Strecher VJ, Hospers H. Physicians' recommendations for mammography: do tailored messages make a difference? American Journal of Public Health. 1994;84(1):43–49. doi: 10.2105/ajph.84.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bindman R, Chu PW, Miglioretti DL, Sickles EA, Blanks R, Ballard-Barbash R, Bobo JK, Lee NC, Wallis MG, Patnick J, Kerlikowske K. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290(16):2129–37. doi: 10.1001/jama.290.16.2129. [DOI] [PubMed] [Google Scholar]
- Smith-West D, Greene P, Pulley L, Kratt P, Gore S, Weiss H, Siegfried N. Stepped-care, community clinic interventions to promote mammography use among low-income rural African American women. Health Education & Behavior. 2004;31(4 Suppl):29S–44S. doi: 10.1177/1090198104266033. [DOI] [PubMed] [Google Scholar]
- Stout NK, Rosenberg MA, Trenthan-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. Journal of the National Cancer Institute. 2006;98(11):774–82. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: Results from the 2000 National Health Interview Survey. Cancer. 2003;97(6):1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- Taylor V, Thompson B, Lessler D, Yasui Y, Montano D, Johnson KM, Mahloch J, Mullen M, Li S, Bassett G, Goldberg HI. A clinic-based mammography intervention targeting inner-city women. Journal of General Internal Medicine. 1999;14(2):104–11. doi: 10.1046/j.1525-1497.1999.00295.x. [DOI] [PubMed] [Google Scholar]
- Valdez A, Banerjee K, Ackerson L, Fernandez M. A multimedia breast cancer education intervention for low-income Latinas. Journal of Community Health. 2002;27(1):33–51. doi: 10.1023/a:1013880210074. [DOI] [PubMed] [Google Scholar]
- van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians' perceptions of patients. Social Science & Medicine. 2000;50(6):813–28. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- Weber BE, Reilly BM. Enhancing mammography use in the inner city. A randomized trial of intensive case management. Archives of Internal Medicine. 1997;157(10):2345–49. [PubMed] [Google Scholar]
- Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:749–57. [PubMed] [Google Scholar]
- Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. national survey. Cancer Epidemiology, Biomarkers & Prevention. 2004;13(5):723–32. [PubMed] [Google Scholar]