Abstract
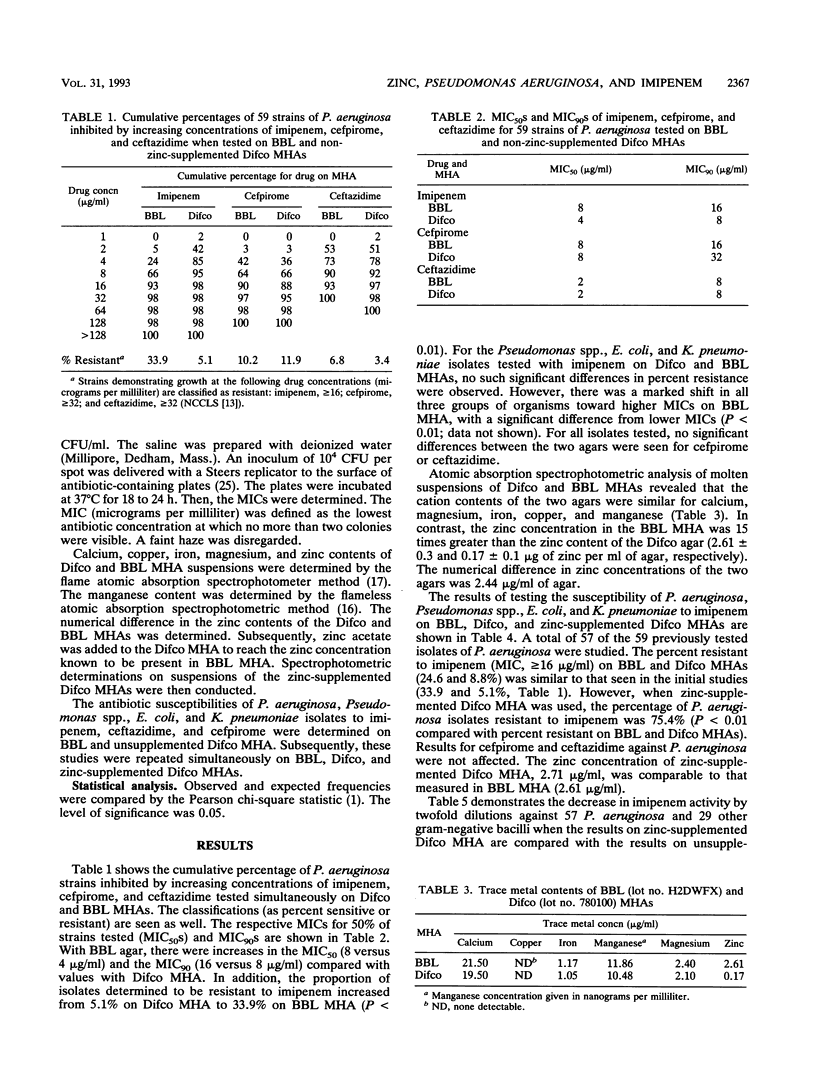
Serial dilution susceptibility testing of imipenem against 59 clinical isolates of Pseudomonas aeruginosa, conducted simultaneously on single lots of Difco and BBL Mueller-Hinton agar (MHA), resulted in MICs for 90% of strains tested of 8 and 16 micrograms/ml, respectively. MICs for Escherichia coli, Klebsiella pneumoniae, and Pseudomonas spp. were also higher on BBL MHA. Quantification of the cation content of the two MHAs by atomic absorption spectroscopy demonstrated that the zinc concentration in BBL MHA was 15 times greater than that measured in Difco MHA (2.61 and 0.17 micrograms/ml, respectively). Concentrations of calcium, magnesium, iron, manganese, and copper in the two agars were similar. Addition of zinc to Difco MHA resulted in increases in MICs of imipenem for P. aeruginosa but not in the MICs of ceftazidime or cefpirome for P. aeruginosa (P < 0.01). A lesser zinc effect was seen on the activity of imipenem against E. coli, K. pneumoniae, and Pseudomonas spp. The activities of ceftazidime and cefpirome were similar on both MHAs when tested against all gram-negative organisms in this study. Thus, the effect of zinc in MHA was clearly demonstrated by a significant increase in the MICs of imipenem for P. aeruginosa, and, to a lesser extent, for other gram-negative bacilli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casillas E., Kenny M. A., Minshew B. H., Schoenknecht F. D. Effect of ionized calcium and soluble magnesium on the predictability of the performance of Mueller-Hinton agar susceptibility testing of Pseudomonas aeruginosa with gentamicin. Antimicrob Agents Chemother. 1981 Jun;19(6):987–992. doi: 10.1128/aac.19.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Malamy M. H., Tally F. P. Beta-lactamase-mediated imipenem resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986 Nov;30(5):645–648. doi: 10.1128/aac.30.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne J., Vézina G., Levesque R. C. Cloning and expression of the imipenem-hydrolyzing beta-lactamase operon from Pseudomonas maltophilia in Escherichia coli. Antimicrob Agents Chemother. 1988 Jun;32(6):819–826. doi: 10.1128/aac.32.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H., Sfikakis P., Voutsinas D., Galanakis N., Daikos G. K. Evaluation of imipenem/cilastatin against nosocomial infections and multiresistant pathogens. J Antimicrob Chemother. 1986 Dec;18 (Suppl E):175–179. doi: 10.1093/jac/18.supplement_e.175. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E. Penetration of beta-lactams through Pseudomonas aeruginosa porin channels. Antimicrob Agents Chemother. 1987 Aug;31(8):1216–1221. doi: 10.1128/aac.31.8.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Nishino T. Decreases of the susceptibility to low molecular weight beta-lactam antibiotics in imipenem-resistant Pseudomonas aeruginosa mutants: role of outer membrane protein D2 in their diffusion. J Antimicrob Chemother. 1990 Feb;25(2):191–198. doi: 10.1093/jac/25.2.191. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Woodruff W. A. Roles of porin and beta-lactamase in beta-lactam resistance of Pseudomonas aeruginosa. Rev Infect Dis. 1988 Jul-Aug;10(4):770–775. doi: 10.1093/clinids/10.4.770. [DOI] [PubMed] [Google Scholar]
- Jones R. N. Review of the in vitro spectrum of activity of imipenem. Am J Med. 1985 Jun 7;78(6A):22–32. doi: 10.1016/0002-9343(85)90098-1. [DOI] [PubMed] [Google Scholar]
- Kenny M. A., Pollock H. M., Minshew B. H., Casillas E., Schoenknecht F. D. Cation components of Mueller-Hinton agar affecting testing of Pseudomonas aeruginosa susceptibility to gentamicin. Antimicrob Agents Chemother. 1980 Jan;17(1):55–62. doi: 10.1128/aac.17.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp H., Gerckens L., Sundelof J. G., Kahan F. M. Antibacterial activity of imipenem: the first thienamycin antibiotic. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S389–S410. doi: 10.1093/clinids/7.supplement_3.s389. [DOI] [PubMed] [Google Scholar]
- Mett H., Rosta S., Schacher B., Frei R. Outer membrane permeability and beta-lactamase content in Pseudomonas maltophilia clinical isolates and laboratory mutants. Rev Infect Dis. 1988 Jul-Aug;10(4):765–769. doi: 10.1093/clinids/10.4.765. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Carbapenems: special properties contributing to their activity. Am J Med. 1985 Jun 7;78(6A):33–40. doi: 10.1016/0002-9343(85)90099-3. [DOI] [PubMed] [Google Scholar]
- O'Rourke E. J., Lambert K. G., Parsonnet K. C., Macone A. B., Goldmann D. A. False resistance to imipenem with a microdilution susceptibility testing system. J Clin Microbiol. 1991 Apr;29(4):827–829. doi: 10.1128/jcm.29.4.827-829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock H. M., Minshew B. H., Kenny M. A., Schoenknecht F. D. Effect of different lots of Mueller-Hinton agar on the interpretation of the gentamicin susceptibility of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Sep;14(3):360–367. doi: 10.1128/aac.14.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Studemeister A. E., DiVincenzo C. A., Lerner S. A. Resistance to imipenem in Pseudomonas aeruginosa: clinical experience and biochemical mechanisms. Rev Infect Dis. 1988 Jul-Aug;10(4):892–898. doi: 10.1093/clinids/10.4.892. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Satake S., Yoshihara E., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes reconstituted from purified porins of the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 May;34(5):685–690. doi: 10.1128/aac.34.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silley P., Griffiths J. W., Monsey D., Harris A. M. Mode of action of GR69153, a novel catechol-substituted cephalosporin, and its interaction with the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1990 Sep;34(9):1806–1808. doi: 10.1128/aac.34.9.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Dufresne J., Levesque R. C., Nikaido H. Decreased outer membrane permeability in imipenem-resistant mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Aug;33(8):1202–1206. doi: 10.1128/aac.33.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuye A. In vitro activity and beta-lactamase stability of N-formimidoyl thienamycin compared to that of second and third generation cephalosporins. Chemotherapy. 1982;28(4):267–275. doi: 10.1159/000238089. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Iyobe S., Inoue M., Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Jan;35(1):147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Kays M. B., Friedrich L. V., Brown E. W., Koonce J. R. Pseudoresistance of Pseudomonas aeruginosa resulting from degradation of imipenem in an automated susceptibility testing system with predried panels. J Clin Microbiol. 1991 Feb;29(2):398–400. doi: 10.1128/jcm.29.2.398-400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Wu P. J., Livermore D. M. Biochemical characterization of a beta-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother. 1990 May;34(5):755–758. doi: 10.1128/aac.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]


