One-sentence summary for table of contents: A new nairovirus isolated from ticks collected from cattle hides was characterized.
Keywords: Nairovirus, Kupe virus, Dugbe virus, arbovirus, tick-borne virus, Kenya, research
Abstract
We have previously described isolation and preliminary identification of a virus related to Dugbe virus (DUGV), family Bunyaviridae, genus Nairovirus. Six isolates of the virus were obtained from pools of Amblyomma gemma and Rhipicephalus pulchellus ticks collected from hides of cattle in Nairobi, Kenya, in October 1999. We report results of further characterization of this virus, including growth kinetics in cell culture and full-length genome sequencing and genetic characterization, which show it to be distinct from DUGV. We suggest that this is a new virus in the family Bunyaviridae, genus Nairovirus, and we propose that it be designated Kupe virus.
The genus Nairovirus in the family Bunyaviridae comprises 7 species groups containing primarily tick-borne viruses, some of which have been identified as human or animal pathogens. The genome of the nairoviruses consists of 3 segments of negative-sense, single-stranded RNA, small (S), medium (M), and large (L), which encode the nucleocapsid protein, glycoproteins (Gn and Gc), and viral polymerase, respectively. Additionally, an M segment–encoded nonstructural protein, NSM, was recently identified in the nairovirus Crimean-Congo hemorrhagic fever virus (CCHFV) (1). In recent years, nucleotide and amino acid sequence information has become available so that additional characterization of these viruses is possible, including further analysis of relationships among members of the genus. Full-length sequence data are now available for CCHFV, Hazara virus (HAZV) and Dugbe virus (DUGV), and partial sequences are available for many other members of the genus. CCHFV, which ranges from sub-Saharan Africa to western People’s Republic of China, is currently the most well characterized member of the genus. DUGV, also well characterized, is commonly isolated in surveillance studies conducted in Africa and appears to be endemic in most of the drier parts of this continent. DUGV is transmitted by ticks to vertebrates, including humans, and causes a mild febrile illness and thrombocytopenia (2).
In a recent survey of ticks infesting market livestock in Nairobi, Kenya, we identified 26 isolates of DUGV and additionally obtained several isolates of a virus that was identified as a nairovirus related most closely to DUGV (3). We report further characterization of the K611 isolate of this virus, including the full-length genome. Our findings suggest that this is a new virus in the genus Nairovirus, and we propose that it be designated Kupe virus (Kupe is the Kiswahili word for tick).
Materials and Methods
Isolates of viruses were obtained from pools of ticks collected at abattoirs in Nairobi, Kenya, as described (3). The K611 isolate used in this study was obtained from a pool of Amblyomma gemma ticks in October 1999.
Characterization of Viruses in Cell Culture and Mice
Growth of Kupe virus and DUGV was tested in Vero (African green monkey kidney), LLC-MK2 (rhesus monkey kidney), BHK (baby hamster kidney), SW-13 (human adrenal cortex carcinoma), HeLa (human cervical adenocarcinoma), HUH-7 (human hepatocarcinoma), and C6/36 (Aedes albopictus mosquito) cells in culture. Growth kinetics of the 2 viruses were compared in a 13-day growth curve in which cells were infected at a multiplicity of infection of 0.01 and aliquots removed daily. Virus titers were assayed on Vero cell monolayers in 6-well plates by using a published double-overlay method (4). Second overlays containing neutral red were added at 6-days postinfection.
Nucleic Acid Sequencing
Viruses to be sequenced were amplified in Vero cells, and viral RNA was extracted from cell culture supernatant by using the QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA, USA). Reverse transcription–PCR was conducted by using the Titan One Tube Reverse Transcription–PCR system (Roche, Indianapolis, IN, USA). Amplified products were purified by agarose gel electrophoresis, and DNA fragments were extracted by using the MinElute Gel Extraction Kit (QIAGEN). Purified DNA fragments were sequenced by using the BigDye 3.1 kit (PE Applied Biosystems, Foster City, CA, USA) and analyzed by using a model 3130 automated sequencer (PE Applied Biosystems). Both strands of the DNA were sequenced.
The full-length genome of Kupe virus isolate K611 was sequenced, beginning with fragments amplified by Nairobi sheep disease virus (NSDV)–specific primers or DUGV-specific primers from each segment. Full-length sequence was obtained by using a previously described method of primer walking and the 5′/3′ Rapid Amplification of cDNA Ends (RACE) Kit (Roche), which was used to determine the sequence of the segment ends (5). Fragments of the S (nt 413–916), M (nt 408–2372), and L (nt 6656–8185) segments from other Kupe virus isolates were also sequenced for comparison (3). Additionally, fragments of the S, M, and L segments from isolates of DUGV collected in 1999 from the Nairobi abattoirs were sequenced by using primers designed from the published sequence of DUGV (3).
Genome Characterization and Comparison with Other Viruses
The nucleotide sequence of each segment of the Kupe virus genome was analyzed for open reading frames (ORFs) by using the EditSeq module of Lasergene (DNASTAR, Inc., Madison, WI, USA) and translated into deduced amino acid sequence. Identification of protein motifs and potential sites for glycosylation was accomplished by using Prosite (http://ca.expasy.org/prosite), psi-BLAST and CDS-BLAST (www.ncbi.nlm.nih.gov/BLAST), NetOGlyc 3.1, and MOTIFS in the Wisconsin Package version 11.1.2 (6,7). Nucleotide and amino acid sequences were compared with DUGV, CCHFV, NSDV, and HAZV sequences. GenBank accession numbers for sequences used in this study are listed in Table 1 or in the text below. Sequence alignments were performed by using the PILEUP and GAP programs in the Wisconsin Package. Sequence identities were calculated by using the GAP program (Wisconsin Package) or MegAlign (Lasergene; DNASTAR, Inc.). Phylogenetic analysis of alignments was conducted by using the maximum parsimony method with 500 bootstrap replicates in MEGA, version 3.1 (www.megasoftware.net).
Table 1. Virus sequences used in phylogenetic comparisons*.
| Genome segment | Virus | Strain | GenBank nucleotide accession no. | GenBank amino acid accession no. |
|---|---|---|---|---|
| Small | Dugbe | ArD44313 | AF434161 | AAL73396 |
| Dugbe | KT281/75 | AF434165 | AAL73400 | |
| Dugbe | IbAr1792 | AF434164 | AAL73399 | |
| Dugbe | IbH11480 | AF434163 | AAL73398 | |
| Dugbe | ArD16095 | AF434162 | AAL73397 | |
| Nairobi sheep disease | RV082 | AF504294 | AAM33324 | |
| Hazara | JC280 | M86624 | AAA43842 | |
| Crimean-Congo hemorrhagic fever | IbAr10200 | U88410 | AAB48501 | |
|
|
Kupe |
K611 |
EU257626 |
NA |
| Medium | Dugbe | ArD44313 | M94133 | AAA42974 |
| Hazara | JC280 | DQ813514 | ABH07417 | |
| Crimean-Congo hemorrhagic fever | IbAr10200 | AF467768 | AAM48106 | |
|
|
Kupe |
K611 |
EU257627 |
NA |
| Large | Dugbe | ArD44313 | U15018 | AAB18834 |
| Hazara | JC280 | DQ076419 | AAZ38668 | |
| Crimean-Congo hemorrhagic fever | IbAr10200 | AY389508 | AAQ90157 | |
| Kupe | K611 | EU257628 | NA |
*NA, not available.
Results
Viruses were isolated from pools of ticks collected from livestock driven to market at 2 abattoirs in Nairobi, Kenya, as described (3). Several isolates made from pools of A. gemma and Rhipicephalus pulchellus ticks collected on 4 days during the fall of 1999 were identified as similar to DUGV on the basis of nucleotide sequence of a fragment of the S segment genomic RNA. This virus has been designated Kupe virus.
Growth kinetics of Kupe virus and DUGV were compared in 7 cell types (Figure 1). Neither virus replicated in C6/36 mosquito cells. Kupe virus and DUGV replicated in all mammalian cell types tested, and maximum titers were observed 1–2 or 2–4 days postinfection, respectively. The Kupe virus titer increased more rapidly than the DUGV titers and achieved peak titers 1–2 days earlier. The subsequent decrease in titer was also more rapid (Figure 1). In all mammalian cell types except BHK cells, we observed earlier appearance of cytopathic effects (CPE) in Kupe virus–infected cells; CPE progressed more rapidly in DUGV-infected BHK cells. However, in all but LLC-MK2 cells, Kupe virus caused greater overall destruction of the cell monolayer by the end of the growth curve experiment. In Vero cell plaque assays, DUGV plaques were slower to form than those caused by Kupe virus, although plaque morphology of the 2 viruses was similar (2–4 mm in diameter).
Figure 1.
Growth of Dugbe (black lines) and Kupe (red lines) viruses in A) Vero (African green monkey kidney), B) LLC-MK2 (rhesus monkey kidney), C) BHK (baby hamster kidney), D) SW-13 (human adrenal cortex carcinoma), E) HeLa (human cervical adenocarcinoma), and F) HUH-7 (human hepatocarcinoma) cells in culture.
Genome Analysis
The 3 genomic RNA segments of Kupe virus, isolate K611, were completely sequenced, ORFs were identified, and deduced amino acid sequences were determined. Similar to other viruses in this family, the ends of each RNA segment contain a conserved sequence, the terminal 9 nt of which are identical to those found in all segments of DUGV, CCHFV, and HAZV and in the S segment of NSDV (sequence of other NSDV segments not available). The S segment of Kupe virus has 1,694 nt, an ORF of 483 aa, and 5′ and 3′ noncoding regions (NCRs) of 49 nt and 193 nt, respectively. The DUGV S segment has 1,716 nt, 5′ and 3′ NCRs of 51 nt and 213 nt, and an ORF of 483 aa (8,9).
The Kupe virus M segment RNA has 4,818 nt and contains 1 ORF flanked by 5′ and 3′ NCRs of 47 nt and 121 nt, respectively. The DUGV M segment has 4,888 nt and its 5′ and 3′ NCRs are 47 nt and 185 nt, respectively (9). As observed in other nairoviruses, the Kupe virus M ORF, which has 1,549 aa, is longer than those of other members of Bunyaviridae (9,10). The Kupe virus M ORF contains 8 potential sites for N-linked glycosylation (N-gly); the DUGV M ORF contains 10 potential sites (Table 2). Kupe virus contains a unique potential N-gly site in the Gn and Gc glycoprotein regions (aa 612 and aa 1514) and was missing potential sites found at aa 30, 80, 848, and 1258 in DUGV. Further analysis is necessary to determine which of the potential N-gly sites are used in DUGV and Kupe virus proteins. DUGV and Kupe virus M segment ORFs contain a highly variable, mucin-like region near the amino terminus, as described for the genome of CCHFV (9,11). This ≈100-aa region in DUGV and Kupe virus is shorter than the 243–248-aa region identified in CCHFV, but this region in both viruses contains similarly high amino acid sequence variability, increased frequency of serine, threonine, and proline residues, and more highly predicted O-linked glycosylation than for the rest of the ORF.
Table 2. Potential N-linked glycosylation sites in the medium segment of Dugbe and Kupe viruses.
| Amino acid location* |
Region† | |
|---|---|---|
| Dugbe virus | Kupe virus | |
| 25 | 16 | Mucin-like, variable |
| 30 | – | Mucin-like, variable |
| 80 | – | Mucin-like, variable |
| 142 | 140 | Upstream of Gn |
| 413 | 414 | Gn |
| – | 612 | Gn |
| 827 | 829 | Unknown |
| 848 | – | Gc precursor |
| 1201 | 1203 | Gc |
| 1258 | – | Gc |
| 1420 | 1421 | Gc |
| –- | 1514 | Gc |
*Amino acid location in the translated open reading frame. †Gn and Gc are glycoproteins.
Previous studies of CCHFV and DUGV suggest that precursors of Gn and Gc glycoproteins are produced and then post-translationally cleaved to form mature glycoproteins. Potential tetrapeptide cleavage sites for SKI-1/S1P protease or a related protease have been identified immediately upstream of the N-termini of the CCHFV (RRLL519– Gn, RKPL1040– Gc) and DUGV (RKLL374– Gn, RKLL896– Gc[predicted]) glycoproteins; similar peptides are found in the Kupe virus ORF (RRIL375 and RRLL898) (11–13). Additionally, a furin-like cleavage recognition motif (RSKR247) has been identified in CCHFV upstream of the amino terminus of Gn that has been shown to produce an additional glycoprotein; however, DUGV and Kupe virus do not share this motif (14). They contain an additional SKI-1/S1P-like cleavage motif in this region (DUGV–RRII204; Kupe virus–RRIL202).
As reported for DUGV and CCHFV, the length of the L segment RNA (12,330 nt) and ORF (4,050 aa) of Kupe virus is almost twice that of other bunyaviruses (15,16). The L RNA contains a 5′ NCR of 40 nt and a 3′ NCR of 137 nt; the 5′ and 3′ NCRs of DUGV are 40 and 104 nt, respectively. The Kupe virus ORF aa sequence shows a high degree of homology to that of DUGV, with the exception of a highly variable region (Kupe virus aa 755–896) that shows low homology (24.8%) and in which the DUGV sequence is 14 aa shorter than Kupe virus (42 nt deletion in DUGV relative to Kupe virus). In this same region, a 92-nt deletion has been shown in CCHFV relative to DUGV, and a similar deletion is observed in HAZV (17). All conserved motifs in the RNA-dependent RNA polymerase (RDRP) module (region 3), as well as other conserved domains upstream and downstream of the polymerase module (regions 1, 2, and 4), were conserved in the Kupe virus ORF, as shown in DUGV and CCHFV (16). Kupe virus L segment ORF also contains several protein motifs previously identified in DUGV and CCHFV, including an ovarian tumor–like cysteine protease domain, a DNA topoisomerase–like domain (aa 76–94), and a C2H2-type zinc finger motif (aa 608–631) (17,18). However, Kupe virus ORF did not contain the leucine zipper motif identified in CCHFV and DUGV.
Phylogenetic Analysis
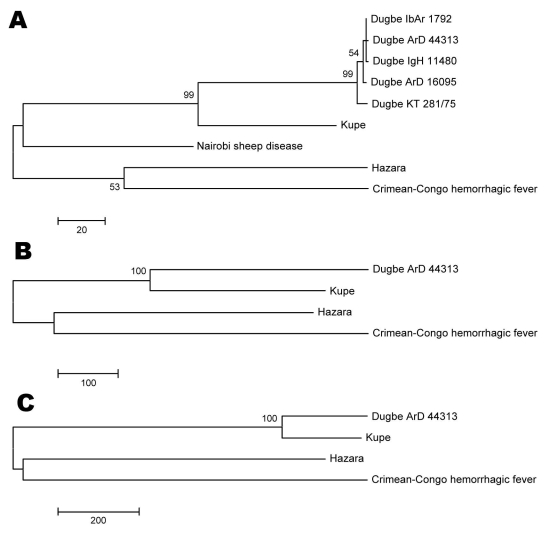
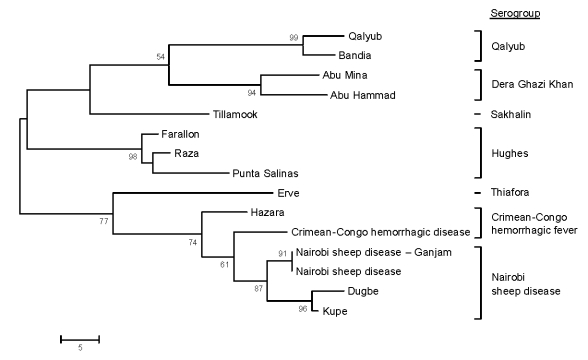
Nucleotide and deduced amino acid sequences of Kupe virus segments were compared with sequences from other nairoviruses available in GenBank and with partial sequences of DUGV isolates obtained in the 1999 Kenya survey in which Kupe virus was isolated (Tables 3–6) (3). Comparison of full-length S segment sequences showed 68.8%–69.4% nt and 74.9%–75.5% aa sequence identity between Kupe virus and 5 strains of DUGV. Identities among the 5 DUGV strain sequences were nt 90.9%–99.4% and aa 98.1%–99.8%. Pairwise, full-length S segment nucleotide and amino acid identities among DUGV, CCHFV, NSDV, and HAZV ranged from 59.0%–64.1% and 55.3%–63.2%, respectively (see Table 3 for specific pairwise identities). A 428-nt fragment of the S segment, corresponding to Kupe S nt 44–471, was also sequenced from 26 DUGV isolates obtained during the 1999 abattoir survey (GenBank accession nos. FJ422213–FJ422238) and compared with available DUGV sequences from GenBank (Table 1) and Kupe virus. Results of these comparisons are shown in Table 6. Nucleotide and amino acid sequence identities among 5 Kupe virus isolates for a 504-nt fragment (nt 413–916) of the S segment were 95.0%–98.4% and 98.8%–100.0%, respectively (GenBank accession nos. EU257626, EU816906–EU816909). Results of phylogenetic analysis of the full-length S segment amino acid sequence alignment is shown in Figure 2, panel A. Kupe virus is shown as most closely related to DUGV, although it is distinct from the clade containing the 5 DUGV strains.
Table 3. Pairwise comparison of full-length nucleotide and amino acid sequences of the small segment of Kupe virus with other nairoviruses*.
| Virus | Kupe | Dugbe ArD44313 | Dugbe ArD16095 | Dugbe KT281/75 | Dugbe IbH11480 | Dugbe IbAr1792 | NSDV | HAZV | CCHFV |
|---|---|---|---|---|---|---|---|---|---|
| Kupe | 69.3 | 69.4 | 69.4 | 68.8 | 69.1 | 65.1 | 60.4 | 61.2 | |
| Dugbe ArD44313 | 75.2 | 99.3 | 91.1 | 98.9 | 99.1 | 63.6 | 60.0 | 59.6 | |
| Dugbe ArD16095 | 75.2 | 99.4 | 91.7 | 99.2 | 99.2 | 64.1 | 59.9 | 59.6 | |
| Dugbe KT281/75 | 74.9 | 98.1 | 98.3 | 91.0 | 90.9 | 63.3 | 59.1 | 59.0 | |
| Dugbe IbH11480 | 74.9 | 99.6 | 99.4 | 98.6 | 99.4 | 63.7 | 59.6 | 60.3 | |
| Dugbe IbAr1792 | 75.5 | 99.8 | 99.6 | 98.3 | 99.8 | 63.8 | 59.6 | 60.1 | |
| NSDV | 64.0 | 59.9 | 60.1 | 59.5 | 59.9 | 60.1 | 63.5 | 63.1 | |
| HAZV | 57.6 | 55.7 | 55.7 | 55.3 | 55.7 | 55.9 | 63.2 | 60.4 | |
| CCHFV | 57.5 | 56.4 | 56.2 | 56.4 | 56.0 | 56.2 | 62.7 | 59.5 |
*Nucleotide identity (%) is shown above the diagonal, and amino acid identity (%) is shown below the diagonal. NSDV, Nairobi sheep disease virus; HAZV, Hazara virus; CCHFV, Crimean-Congo hemorrhagic fever virus.
Table 6. Nucleotide and amino acid sequence comparisons between fragments of Kupe and Dugbe viruses*.
| Segment and virus | Virus | ||
|---|---|---|---|
| Small (428 nt) | Kupe |
Dugbe, Kenya, 1999 |
Other Dugbe† |
| Kupe | 68.8–70.9 | 69.2–70.9 | |
| Dugbe, 1999, Kenya | 67.6–69.7 | 89.3–97.9 | |
| Other Dugbe† |
69.0 |
95.8–100.0 |
|
| Medium (308 nt) | Kupe |
Dugbe, Kenya, 1999 |
Dugbe ArD44313 |
| Kupe | 63.9–65.2 | 65.8 | |
| Dugbe, 1999, Kenya | 61.8–64.7 | 86.8–92.3 | |
| Dugbe ArD44313 |
63.7 |
93.1–98.0 |
|
| Large (603 nt) | Kupe |
Dugbe, Kenya, 1999 |
Dugbe ArD44313 |
| Kupe | 81.8–82.4 | 81.3 | |
| Dugbe, 1999, Kenya | 94.5–96.0 | 91.0–92.0 | |
| Dugbe ArD44313 | 94.5 | 96.0–98.0 | |
*Nucleotide identity (%) is shown above the diagonal, and amino acid identity (%) is shown below the diagonal. †Dugbe viruses listed in Table 1.
Figure 2.
Phylogenetic trees produced by using maximum-parsimony analysis with 500 bootstrap replicates on alignments of full-length amino acid sequences of the A) small segment, B) medium segment, and C) large segment of Kupe virus with other available full-length nairovirus sequences. Scale bars indicate branch length and bootstrap values >50% are shown above branches.
Full-length M segment sequences are available for only 3 of the known nairoviruses: DUGV (strain ArD 44313), HAZV, and CCHFV. Comparison of these viruses with Kupe virus M segment sequence showed 61.9%, 54.7%, and 52.1% nt identity and 57.0%, 47.7%, and 43.0% aa identity, respectively (Table 4). Additionally, a 308-nt fragment (Kupe M segment, nt 2181–2488) was sequenced from 25 DUGV isolates obtained in Kenya in 1999 (GenBank accession nos. FJ422239–FJ422263) and compared with DUGV ArD44313 and Kupe virus. Results of these comparisons are shown in Table 6. Sequence identities between 5 Kupe virus isolates for a 1,965-nt fragment of the M segment (nt 408–2372) were 90.9%–98.8% for nt and 96.0%–99.4% for aa (GenBank accession nos. EU257627, EU816902–EU816905). Phylogenetic analysis of full-length M segment amino acid sequences resulted in a tree with topology similar to that of the S segment tree (Figure 2, panel B).
Table 4. Pairwise comparison of full-length nucleotide and amino acid sequences of the medium segment of Kupe virus with other nairoviruses*.
| Virus | Kupe | Dugbe ArD44313 | HAZV | CCHFV |
|---|---|---|---|---|
| Kupe | 61.9 | 54.7 | 52.1 | |
| Dugbe ArD44313 | 57.0 | 53.7 | 52.5 | |
| HAZV | 47.7 | 44.4 | 50.8 | |
| CCHFV | 43.0 | 38.3 | 41.4 |
*Nucleotide identity (%) is shown above the diagonal, and amino acid identity (%) is shown below the diagonal. HAZV, Hazara virus; CCHFV, Crimean-Congo hemorrhagic fever virus.
Full-length L segment sequences are available only for DUGV (strain ArD 44313), HAZV, and CCHFV. Comparison of these sequences with Kupe virus sequence showed 77.4%, 62.8%, and 61.5% nt identity and 89.0%, 66.3%, and 63.7% aa identity, respectively (Table 5). As expected from this data, phylogenetic analysis of full-length L segment aa sequence resulted in a tree showing Kupe virus more closely related to Dugbe virus than in the S or M segment trees (Figure 2, panel C).
Table 5. Pairwise comparison of full-length nucleotide and amino acid sequences of the large segment of Kupe virus with other nairoviruses*.
| Virus | Kupe | Dugbe ArD44313 | HAZV | CCHFV |
|---|---|---|---|---|
| Kupe | 77.4 | 62.8 | 61.5 | |
| Dugbe ArD44313 | 89.0 | 63.4 | 62.1 | |
| HAZV | 66.3 | 66.1 | 62.3 | |
| CCHFV | 63.7 | 63.4 | 64.0 |
*Nucleotide identity (%) is shown above the diagonal, and amino acid identity (%) is shown below the diagonal. HAZV, Hazara virus; CCHFV, Crimean-Congo hemorrhagic fever virus.
Nucleotide and amino acid sequence comparisons of a 441-nt fragment of the highly conserved L segment RDRP catalytic core domain (Kupe virus nt 6986–7426) were also made between Kupe virus and sequences of 14 other viruses representing 7 groups of the Nairovirus genus published by Honig et al. (19). A phylogenetic tree derived from the amino acid alignment of these sequences shows Kupe virus most closely related to DUGV (82.8% nt identity/95.9% aa identity), NSDV (74.9%/92.5%), CCHFV (71.9%/88.4%), and HAZV (71.7%/87.8%) (Figure 3). An additional 603-nt L fragment alignment overlapping the RDRP core domain (Kupe virus nt 7292–7894) included sequences from 26 DUGV isolates obtained in Kenya in 1999 (GenBank accession nos. EU359010–EU359035), DUGV ArD 44313, and Kupe virus. Results of these comparisons are shown in Table 6. Sequence identities among 5 Kupe virus isolates for this fragment were nt 91.2%–100.0% and aa 98.5%–100.0% (GenBank accession nos. EU257628, EU816898–EU816901).
Figure 3.
Phylogenetic tree produced by using maximum parsimony analysis with 500 bootstrap replicates on amino acid alignment of nairovirus large segment fragment (147 aa sequence translated from 441 nt sequence). Scale bar indicates branch length and bootstrap values >50% are shown above branches.
Discussion
Although little genetic information is available for most viruses in the genus Nairovirus, current classification of the diverse group of viruses in the genus is in relative agreement with available genetic analyses (19,20). Genetic information is useful in identifying emerging viruses and in analysis of relationships between viruses, especially given the segmented nature of the nairovirus genome, which can lead to generation of new viruses by segment reassortment (21). Within the genus, however, limited species and strain comparisons are available, making the definition of a genetic classification criteria difficult, and the segmented nature of the genome confounds the analysis. These findings are shown by a recent in-depth genetic analysis of CCHFV strains that demonstrated a high degree of genomic plasticity and RNA segment reassortment among virus strains studied (22).
Detailed study of the complete genome of 13 geographically and temporally diverse strains of CCHFV demonstrated nt/aa sequence identities of 80%/92%, 69%/73%, and 78%/90% for the S, M and L segments, respectively (22). Similarly, comparison of published full-length S segment sequences from 5 strains of DUGV isolated in Senegal, Nigeria, and Kenya between 1964 (IbAr1792) and 1985 (ArD443143) demonstrated sequence identities >90% at the nucleotide and amino acid levels (Table 3). Likewise, >89% identities were observed when a fragment of S segment sequence from these 5 strains was compared with 26 DUGV isolates from the 1999 Kenya abattoir survey (Table 3). S segment sequence identity between Kupe virus and DUGV falls well below identities observed among strains of either DUGV (Tables 3, 6) or CCHFV and is closer to that observed in S segment sequence comparisons among DUGV, CCHFV, NSDV, and HAZV (Table 3) (22).
Although comparison of full-length M segment sequence among multiple DUGV strains is not possible because of lack of available sequence information, sequence identities for comparison of a fragment of the M segment of DUGV ArD44313 and the 26 isolates obtained in Kenya in 1999 were >86% for nt and >93% for aa. In contrast, identities observed between Kupe virus and the DUGV sequences were considerably lower and, similar to the S segment sequence, were closer to identities observed among DUGV, CCHFV, and HAZV. In addition, differences in the number and positions of potential N-gly sites in the M segment ORF between DUGV and Kupe virus suggest substantial differences between these viruses.
Comparison of Kupe virus L segment sequences was inconclusive in determining its relationship to DUGV. Again, because of lack of available sequence information, comparison of multiple full-length DUGV strains is not possible at this time; comparison of a fragment of the L segment between the Kenya DUGV isolates and DUGV ArD44313 showed identities >91%. The relatively high full-length L segment nt/aa sequence identities of 77.4%/89.0% observed between Kupe virus and DUGV strain ArD 44313 are similar to identity levels reported among full-length L segment comparisons of CCHFV strains. This finding suggests that the L segment of Kupe virus may have been acquired from DUGV by reassortment. However, identities observed for the highly conserved 603-nt L segment fragment between Kupe virus and DUGV ArD44313 were somewhat lower compared with identities between DUGV ArD44313 and other Kenya DUGV isolates. These lower identities, combined with differences observed in the L segment variable region (Kupe virus aa 755–896), suggest otherwise.
Little is known about the ecology of Kupe virus other than its isolation from ticks infesting cattle. DUGV has been reportedly isolated from several tick species, including A. gemma and R. pulchellus, the species from which Kupe virus was isolated (19,23,24). In the 1999 Kenya abattoir survey, DUGV was isolated from 4 species of ticks, A. variegatum, A. gemma, A. lepidum, and R. pulchellus (3). Although ≈1,000 specimens each of A. variegatum and A. lepidum were collected and tested in that study, no isolates of Kupe virus were found in those species, which suggested that vector hosts for DUGV and Kupe virus may differ (3). Specific vector competence studies will be needed to resolve this point. The pathogenesis, if any, of Kupe virus in mammals is unknown.
Kupe virus and DUGV were observed to replicate and cause CPE in a variety of cultured mammalian cell types. Kupe virus was observed to have a more rapid increase and subsequent decrease in viral titer, an earlier onset of visible CPE, and greater destruction of the cell monolayer in most of the mammalian cells tested. These findings show that this virus is more virulent than DUGV in the mammalian cells tested.
Taxonomic classification of viruses is an evolving discipline that in early years was based primarily on morphologic characters. More recently, better classification has been obtained by using antigenic relationships and information gained from genetic characteristics. The International Committee on Taxonomy of Viruses has defined a virus species as “a polythetic class of viruses that constitute a replicating lineage and occupy a particular ecological niche” (25). This definition and its use in virus classification has been the subject of much discussion in the literature, and its application to newly described viruses is often difficult because of incomplete descriptive information about the new virus and other viruses in the group to which it is related (26,27).
For Kupe virus, nucleotide and amino acid sequence variation between the S and M segments of Kupe virus and DUGV, or any other genetically characterized nairovirus, was greater than expected between strains of a single virus in the genus Nairovirus. We also noted differences in other genetic characteristics between Kupe virus and DUGV, including M segment N-gly sites, L segment variable region, and NCR length variations. This evidence, combined with increased virulence of Kupe virus in cultured mammalian cells and potential differences in vector hosts, shows that Kupe virus is substantially different from, although closely related to, DUGV and is a new virus in the genus Nairovirus. However, further studies are necessary to determine the hosts, vectors, and geographic range of Kupe virus along with its virulence as a human or animal pathogen. Such information will aid in appropriate classification of this new virus.
Biography
Ms Crabtree is a researcher at the Centers for Disease Control and Prevention in Fort Collins, Colorado. Her research interest is the molecular biology of arboviruses.
Footnotes
Suggested citation for this article: Crabtree MB, Sang R, Miller BR. Kupe virus, a new virus in the family Bunyaviridae, genus Nairovirus, Kenya. Emerg Infect Dis [serial on the Internet]. 2009 Feb [date cited]. Available from http://www.cdc.gov/EID/content/15/2/147.htm
References
- 1.Altamura LA, Bertolotti-Ciarlet A, Teigler J, Paragas J, Schmaljohn CS, Doms RW. Identification of a novel C-terminal cleavage of Crimean-Congo hemorrhagic fever virus PreGN that leads to generation of an NSM protein. J Virol. 2007;81:6632–42. 10.1128/JVI.02730-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sang R, Onyango C, Gachoya J, Mabinda E, Konongoi S, Ofula V, et al. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis. 2006;12:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BR, Mitchell CJ, Ballinger ME. Replication, tissue tropisms and transmission of yellow fever virus in Aedes albopictus. Trans R Soc Trop Med Hyg. 1989;83:252–5. 10.1016/0035-9203(89)90667-6 [DOI] [PubMed] [Google Scholar]
- 5.Crabtree MB, Sang RC, Stollar V, Dunster LM, Miller BR. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch Virol. 2003;148:1095–118. 10.1007/s00705-003-0019-7 [DOI] [PubMed] [Google Scholar]
- 6.Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj J. 1998;15:115–30. 10.1023/A:1006960004440 [DOI] [PubMed] [Google Scholar]
- 7.Devereux J. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–95. 10.1093/nar/12.1Part1.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridgen A, Dalrymple DA, Elliott RM. Dugbe nairovirus S segment: correction of published sequence and comparison of five isolates. Virology. 2002;294:364–71. 10.1006/viro.2001.1324 [DOI] [PubMed] [Google Scholar]
- 9.Marriott AC, el-Ghorr AA, Nuttall PA. Dugbe nairovirus M RNA: nucleotide sequence and coding strategy. Virology. 1992;190:606–15. 10.1016/0042-6822(92)90898-Y [DOI] [PubMed] [Google Scholar]
- 10.Elliott RM. Molecular biology of the Bunyaviridae. J Gen Virol. 1990;71:501–22. 10.1099/0022-1317-71-3-501 [DOI] [PubMed] [Google Scholar]
- 11.Sanchez AJ, Vincent MJ, Nichol ST. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J Virol. 2002;76:7263–75. 10.1128/JVI.76.14.7263-7275.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent MJ, Sanchez AJ, Erickson BR, Basak A, Chretien M, Seidah NG, et al. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J Virol. 2003;77:8640–9. 10.1128/JVI.77.16.8640-8649.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergeron E, Vincent MJ, Nichol ST. Crimean-Congo hemorrhagic fever virus glycoprotein processing by the endoprotease SKI-1/S1P is critical for virus infectivity. J Virol. 2007;81:13271–6. 10.1128/JVI.01647-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez AJ, Vincent MJ, Erickson BR, Nichol ST. Crimean-Congo hemorrhagic fever virus glycoprotein precursor is cleaved by Furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J Virol. 2006;80:514–25. 10.1128/JVI.80.1.514-525.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marriott AC, Nuttall PA. Large RNA segment of Dugbe nairovirus encodes the putative RNA polymerase. J Gen Virol. 1996;77:1775–80. 10.1099/0022-1317-77-8-1775 [DOI] [PubMed] [Google Scholar]
- 16.Kinsella E, Martin SG, Grolla A, Czub M, Feldmann H, Flick R. Sequence determination of the Crimean-Congo hemorrhagic fever virus L segment. [erratum in 2004;330:361]. Virology. 2004;321:23–8. 10.1016/j.virol.2003.09.046 [DOI] [PubMed] [Google Scholar]
- 17.Honig JE, Osborne JC, Nichol ST. Crimean-Congo hemorrhagic fever virus genome L RNA segment and encoded protein. Virology. 2004;321:29–35. 10.1016/j.virol.2003.09.042 [DOI] [PubMed] [Google Scholar]
- 18.Makarova KS, Aravind L, Koonin EV. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem Sci. 2000;25:50–2. 10.1016/S0968-0004(99)01530-3 [DOI] [PubMed] [Google Scholar]
- 19.Honig JE, Osborne JC, Nichol ST. The high genetic variation of viruses of the genus Nairovirus reflects the diversity of their predominant tick hosts. Virology. 2004;318:10–6. 10.1016/j.virol.2003.09.021 [DOI] [PubMed] [Google Scholar]
- 20.Ward VK, Marriott AC, Polyzoni T, el-Ghorr AA, Antoniadis A, Nuttall PA. Expression of the nucleocapsid protein of Dugbe virus and antigenic cross-reactions with other nairoviruses. Virus Res. 1992;24:223–9. 10.1016/0168-1702(92)90009-X [DOI] [PubMed] [Google Scholar]
- 21.Hewson R, Gmyl A, Gmyl L, Smirnova SE, Karganova G, Jamil B, et al. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J Gen Virol. 2004;85:3059–70. 10.1099/vir.0.80121-0 [DOI] [PubMed] [Google Scholar]
- 22.Deyde VM, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Crimean-Congo hemorrhagic fever virus genomics and global diversity. J Virol. 2006;80:8834–42. 10.1128/JVI.00752-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David-West TS, Porterfield JS. Dugbe virus: a tick-borne arbovirus from Nigeria. J Gen Virol. 1974;23:297–307. 10.1099/0022-1317-23-3-297 [DOI] [PubMed] [Google Scholar]
- 24.Karabatsos N, ed. International catalog of arboviruses, 3rd ed. San Antonio (TX): American Society of Tropical Medicine and Hygiene; 1985. [Google Scholar]
- 25.Van Regenmortel MH. Applying the species concept to plant viruses. Arch Virol. 1989;104:1–17. 10.1007/BF01313804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs AJ, Gibbs MJ. A broader definition of ‘the virus species.’. Arch Virol. 2006;151:1419–22. 10.1007/s00705-006-0775-2 [DOI] [PubMed] [Google Scholar]
- 27.van Regenmortel MH, Mahy BW. Emerging issues in virus taxonomy. Emerg Infect Dis. 2004;10:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]