SUMMARY
Gonorrhea is the second most commonly reported infectious disease in the United States, and incidence has been increasing in recent years. Antibiotic resistance among clinical isolates has reached a critical point at which the CDC currently recommends only a single class of antibiotic for treatment. These developments have hastened the search for a vaccine to protect against gonococcal infections. Vaccine efforts have been thwarted by the ability of the gonococcus to antigenically vary most surface structures. The transferrin-iron transport system is not subject to high frequency phase or antigenic variation and is expressed by all pathogenic Neisseria. Vaccine formulations comprised of epitopes of the transferrin-binding proteins complexed with inactivated cholera toxin generated antibodies with potentially protective characteristics. These antigens, and others predicted from genome sequence data, could be developed into a vaccine that protects against neisserial infections.
Keywords: Transferrin receptor, vaccine, antigens, adjuvants, Gram-negative bacteria, outer membrane, iron transporters
Gonorrhea infections and complications
Gonorrhea, caused by Neisseria gonorrhoeae, is the second most commonly reported infectious disease in the United States. In 2006, there were 358,366 reported cases according to the CDC [1], which represents an increase of 5.5% from the previous year. Rates of disease have been increasing at an alarming rate in the Western and Southeastern United States; in these regions, incidence increased 2.9 and 12.3%, respectively, between 2005 and 2006. Gonorrhea is a world-wide problem, impacting both industrialized and developing countries. In 1999, the WHO estimated the global prevalence of gonorrhea at 62.4 million cases [2]. Even this extraordinary number likely represents an underestimate as the disease is under-reported and often asymptomatic.
Localized gonococcal infections impact both males and females [3]. In males, the infection is usually symptomatic, with hallmark manifestations including a purulent urethral discharge and dysuria. If untreated, the infection can ascend resulting in epididymitis. In females, up to 50% of infections may be asymptomatic [4–6], thus prolonging the time to diagnosis and treatment. This population also serves as an important reservoir for the disease. If symptoms are present, the most common is a purulent cervical discharge; however this is often minimal and/or goes unnoticed. As a result, ascending infection, and the consequent morbidity, is disproportionately suffered by the female population; these infections include pelvic inflammatory disease, salpingitis and ectopic pregnancy. Ultimately, untreated infections in both women and men can lead to infertility. Disseminated gonococcal infections can also be a downstream consequence of asymptomatic infection; as one might expect, this manifestation is most commonly experienced by females. Symptoms include fever, rash and septic arthritis. Finally, perinatal infections also occur, which can result in conjunctivitis in newborns. If untreated this can lead to blindness; however this rarely occurs in developed countries due to the mandatory administration of ophthalmic antibiotics following a vaginal childbirth.
Increasing urgency for development of preventative approaches
While gonococcal infections are treatable with antibiotics, a dramatic increase in the incidence of antibiotic resistance has occurred in the recent past [7, 8]. Penicillin resistance emerged in the 1970’s and resistance to tetracycline emerged approximately 10 years later. This led to the abandonment of these antibiotics as recommended treatment regimens. While a decrease in the incidence of these resistances has occurred in the absence of selection, in 2004 16% of strains tested were resistant to one or both of these antimicrobial agents. Until recently, the fluoroquinolones were recommended therapy for treatment of gonococcal infections. However, in 2006 resistance to this class of antibiotic had risen to 13.8%, compared to 9.4% the year before. This increase led the CDC to announce in 2007 that fluoroquinolones were no longer the recommended therapy [7]. This development is of great concern as it restricts the treatment of gonococcal infections to a single class of antimicrobial agents: the third generation cephalosporins. Clearly, new approaches are urgently needed to treat this common sexually transmitted infection (STI), which has profound health consequences. One promising new development in the treatment of gonococcal infections is the identification of novel topical agents with microbicidal activity against N. gonorrhoeae [9].
In addition to emerging antibiotic resistance, another concern is the role that STIs play in the transmissibility of HIV. HIV genomic RNA levels are higher in the seminal plasma and vaginal secretions of men and women with concurrent gonococcal infections [10, 11]. When the bacterial STI is treated with antibiotics, HIV RNA and virus titers decrease. Therefore, gonococcal infection coincident with HIV infection exposes contacts to higher viral doses, increasing the risk of acquiring the infection. This observation, along with the emergence of antibiotic resistances, has hastened the search for an effective, cross-protective and long-lasting gonococcal vaccine.
Challenges in the development of a gonococcal vaccine
Gonococcal infections do not elicit protective immunity and there is no vaccine to prevent the disease. Many challenges have been identified in the decades-long search for a protective gonococcal vaccine. First, the gonococcal cell surface is extremely variable, being composed of protein and polysaccharide antigens that rapidly change in antigenic character. The list of antigens that are subject to high-frequency phase and antigenic variation includes pilin, opacity proteins (Opa), lipooligosaccharides (LOS), and several outer membrane iron transporters. Two basic mechanisms are employed by the Neisseriae to generate antigenic diversity. The pilin protein, which polymerizes to form the macromolecular pilus structure critical for adherence, is antigenically variable as a result of homologous recombination between an expression locus and any one of several storage or silent pil loci. Variant pilin proteins arise at a frequency of approximately 1/1000 per cell per generation, demonstrating the degree of pilin heterogeneity found within gonococcal populations [12]. The second basic mechanism employed by the Neisseriae to generate antigenic diversity is slipped-strand mispairing, which occurs at the site of polymeric sequence tracts [13]. Increases or decreases in the number of repeats result in changes in expression. In most loci, the repeats are located in the structural gene, in which case alteration in the number of repeats can lead to frame-shift mutations. For example, the N. gonorrhoeae genome contains up to 11 different Opa loci, each of which consists of a promoter and a set of repeats within the coding region. At any one time, 0–11 of these Opa genes may be expressed into full-length protein; however, in vitro the tendency is to express fewer and in vivo up to 5 Opa proteins are expressed simultaneously [14]. The slipped-strand mispairing mechanism is also responsible for variable expression of some iron transport proteins (Fig. 1). The loci encoding LbpAB [15], HpuAB [16], and FetA [17] all contain polymeric repeat regions, which result in rapid, on-off switching of these gene products [13].
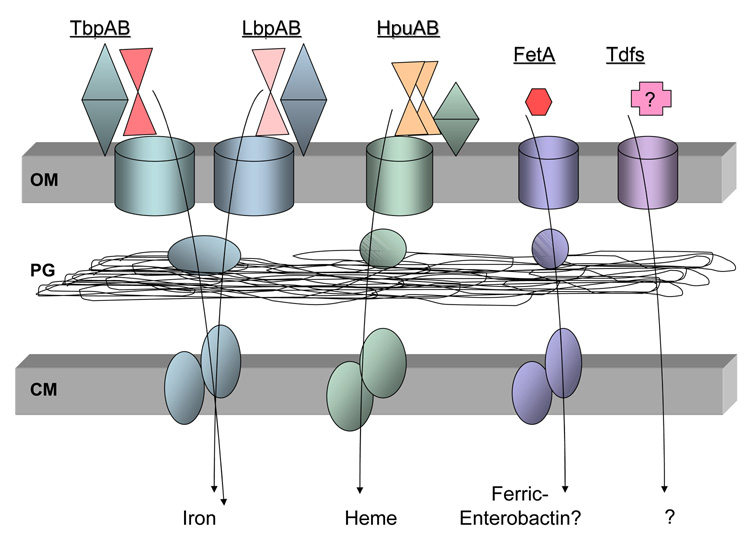
Figure 1. Iron transport systems expressed by N. gonorrhoeae.
The gonococcal cell envelope is represented by two membranes (OM: outer membrane; CM: cytoplasmic membrane) shaded in gray. Between these two membranes lies the peptidoglycan (PG), which is shown in the periplasmic space. The barrel shapes in the outer membrane depict the integral, TonB-dependent transporters for each iron uptake system. The lipoprotein members of each system (blue, teal or green) are depicted as entirely surface exposed and consisting of two lobes. Each outer membrane receptor or system binds to a different iron source: TbpAB, human transferrin (red); LbpAB, human lactoferrin (pink); HpuAB, hemoglobin (orange); FetA, ferric-enterobactin. The incompletely characterized Tdfs (TdfF, TdfG, TdfH and TdfJ) bind to unknown ligands; the means by which these ligands are subsequently internalized are likewise unknown. After iron removal from transferrin and lactoferrin, the iron is subsequently transported through the periplasm and cytoplasm by FbpABC (blue ovals). An analogous heme-binding, periplasmic protein dependent uptake system has not yet been identified, but is expected to be distinct from FbpABC and is thus depicted in the figure as green ovals. The periplasmic binding protein and ABC transport system putatively employed for enterobactin internalization is encoded downstream of FetA (lavender ovals). No data are available on the fate of internalized ferric-enterobactin.
Another impediment to vaccine development efforts has been the capability of the gonococcus to block deposition of functional antibody by decoration of LOS with sialic acid. Specific epitopes of LOS can be modified by exogenously-supplied CMP-NANA, resulting in an LOS structure with unique physiochemical and size characteristics. This modification prevents the deposition of bactericidal antibodies directed at gonococcal porin [18]. In addition, antibodies generated against a conserved antigen, RmpM, similarly prevent deposition of functional anti-porin antibodies [19]. Thus, even antigens that are not susceptible to high-frequency variation can be rendered unavailable to immune factors by virtue of blocking antibody or sialylation of the cell surface.
Development of a protective anti-gonococcal vaccine also requires elicitation of mucosal immunity in the genital tract, where the infection first establishes a foothold. This is complicated by the absence of specialized inductive sites, which are present in other mucosal tissues (for example, Peyer's patches in the gut) and by the fact that this niche must be relatively tolerant of foreign antigens [20–22]. Studies in which antigens are delivered locally to the genital tract have, in large part, been less successful than those in which antigens are delivered to other mucosal sites, such as the nasal mucosa [23, 24]. Intranasal immunization has shown promise in the development of genital mucosal and systemic immune responses potentially effective against a variety of STI agents [25–27].
Iron transport proteins as promising vaccine antigens
In search of a cross-protective gonococcal vaccine, we have focused on receptors that are necessary for nutrient acquisition. One such nutrient is iron, which is consistently identified as an essential factor for bacterial survival in vivo and in tissue culture systems [28–30]. Figure 1 shows the receptor systems with which N. gonorrhoeae is known to acquire iron, along with a group of incompletely-characterized, potential transporters (Tdfs, Fig. 1). The TbpAB protein system is employed to bind transferrin, and relieve it of iron, which is then transported through the outer membrane. Subsequently, iron is bound by FbpA in the periplasm, which relays the nutrient to a membrane permease (FbpBC) for entry into the cytoplasm. The FbpABC protein system is also required to shuttle iron passed into the periplasm from lactoferrin, which is received and stripped of iron by the surface exposed LbpAB proteins. As indicated above, the LbpAB system is phase variable due to a repeat element within the structural genes. Moreover, the LbpAB locus is largely deleted in approximately 50% of gonococcal strains tested [31]. Thus, we have focused on the TbpAB system as a potential vaccine target due to its ubiquitous expression among isolates. In addition, this system is not subject to phase or antigenic variation like so many other outer membrane antigens. However, this system is repressed under high iron conditions, a situation likely experienced in vivo, as is true of other iron acquisition systems. We determined that expression of the TbpAB system was required to initiate signs and symptoms of urethritis in a male model of experimental infection [32]. The strain employed in these studies was a naturally occurring isolate that lacked a functional LbpAB locus. When a functional LbpAB locus was reconstituted into a TbpAB mutant, the resulting strain was capable of eliciting a urethral infection, suggesting that the Lbp system can functionally compensate for the absence of the Tbp system [33]. However, the fact remains that half of gonococcal isolates cannot express LbpAB, making these antigens poor vaccine targets.
Other possible iron transport systems to target for vaccine development include HpuAB and FetA (Fig. 1). The HpuAB system is necessary for gonococcal growth on hemoglobin as a sole iron source [34]. Presumably there is a dedicated periplasmic binding protein dependent system for transporting heme into the cell; however, to date such a system has not been identified. The HpuAB system, like the LbpAB, is subject to phase variation and was selectively expressed by gonococcal variants isolated from women during the first half of the menstrual cycle [35]. The final iron transport system characterized in N. gonorrhoeae consists of FetA and an associated ABC transport system (Fig. 1). FetA has been shown to bind to ferric enterobactin with weak affinity and to provide the ability to grow on ferric-enterobactin as a sole iron source [36]. There are predicted members of a periplasmic binding protein dependent systems encoded downstream of FetA; however, these gene products have not been well characterized. Likewise, the benefits that FetA or the downstream genes provide in vivo are unknown.
While the Tbp proteins are expressed in vivo [37] and are necessary to initiate infection in males [32], native infections result in limited amounts of Tbp-specific antibody [38]. This result concurs with those reported by others, cumulatively suggesting that gonococcal infections elicit only modest humoral and mucosal antibody responses [39–41]. Perhaps this is related to the Opa-mediated T-cell suppression mentioned above, and perhaps other gonococcal factors contribute as well [41]. Nonetheless, native infections generate modest immune responses, a concept that seems to correlate well with the lack of protective immunity elicited by infection. Thus, our approach to vaccine development has been to identify Tbp-specific epitopes that are well conserved among strains and which, when combined with an appropriate adjuvant and immunization route, will generate a protective immune response where native infection does not.
We developed a topology model of TbpA (Fig. 2; [42, 43]) based upon its homology with other outer membrane TonB-dependent transporters such as FepA and FhuA, expressed by E. coli. This class of transporters requires TonB-derived energy to accumulate ligands in the periplasm of Gram-negative bacteria. Several TonB-dependent transporters have been crystallized [44–50], and their conserved structures include a β-barrel, which forms a pore through the outer membrane, and an amino-terminal plug or hatch region, which occludes the pore until ligand and TonB-derived energy are provided. We have focused our vaccine development efforts on surface-exposed regions of TbpA, which are likely to be involved in ligand binding and accessible to immune factors. These regions (Fig. 2) have been identified by a combination of computer analysis, epitope insertion, loop deletion, and antibody binding experiments [42, 43, 51]. We found that portions of loops 2, 3 5, 7 and 10 are indeed surface exposed in N. gonorrhoeae. Of these, loop 5 appears to be critical for transferrin binding as this region retains transferrin binding characteristics when expressed in recombinant form in E. coli. In addition, deletion of this region of the protein abrogated transferrin binding and transferrin-iron internalization capabilities by N. gonorrhoeae.
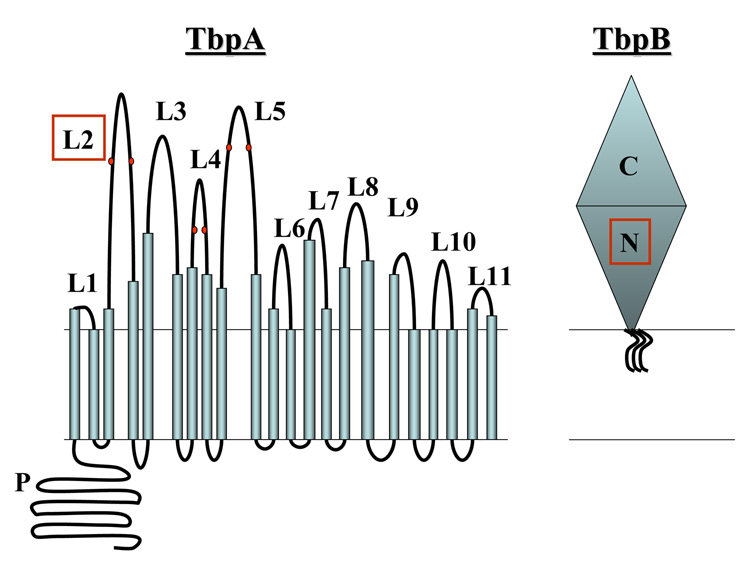
Figure 2. Models of TbpA and TbpB in the outer membrane of N. gonorrhoeae.
TbpA (left) is shown as a two domain protein consisting of a plug domain (P) and a β-barrel domain. The β-barrel is hypothesized to be made up of 22 transmembrane β-strands (blue cylinders), connected outside of the outer membrane by 11 putatively surface-exposed loops (L1–L11). Six cysteine residues (highlighted in red) are located in 3 putative loops (L2, L4, and L5). L2 (boxed in red) was included in a chimeric vaccine preparation (see text for details). TbpB (right) is shown as a lipid-modified, surface exposed protein with two domains: N and C. No details on the structure of TbpB are available since this protein is not similar to any proteins for which crystal structures have been solved. The N domain (highlighted in red) was included in a chimeric vaccine preparation (see text for details).
The second member of the transferrin-iron uptake system is TbpB, which is lipid modified and entirely surface exposed (Fig. 1 and Fig. 2). The presence of TbpB is not required for growth on transferrin, but TbpB makes iron internalization more efficient in short-term assays [52, 53]. No crystallized homologs of TbpB are available for homology modeling, thus hypothetical models lack specificity and detail (Fig. 2). TbpB is comprised of two similar lobes, each of which contains transferrin binding motifs [53]. The amino-terminal lobe is responsible for the unique ability of TbpB to retain transferrin binding capabilities following SDS-PAGE [53]. TbpB, unlike TbpA, preferentially binds holo-transferrin [54]. The ability to selectively bind the iron-laden form of transferrin likely contributes to the role that TbpB plays in increasing the association rate with and dissociation rate from the gonococcal cell surface. In addition to its discriminatory function, TbpB could play a more active role in iron removal from transferrin.
We took advantage of the adjuvanticity of detoxified forms of cholera toxin in generating TbpAB antigens for vaccine studies. The B subunit of cholera toxin has shown promise as a mucosal adjuvant in a number of studies, particularly when delivered intranasally [23, 55]. We chemically conjugated recombinant, full-length TbpA or TbpB with cholera toxin B subunits. These antigens were administered to female BALB/c mice intranasally, after which we analyzed the resulting mucosal and systemic immune responses. Both conjugated proteins elicited vaginal and serum antibodies, although TbpB was much more immunogenic than TbpA. The serum antibodies elicited in animals immunized with either protein conjugate were bactericidal in the presence of human complement. However, sera from the TbpA-immunized animals were more cross-reactive with heterologous strains than sera from TbpB-immunized animals [56]. This result is in agreement with those of Thomas et al. [57], who also found that recombinant TbpB alone generated a vigorous immune response, but the resulting antibodies only recognized the surface of the homologous strain. Thus, we conclude that the most effective and cross-reactive antibodies are generated by immunization with a combination of TbpA and TbpB conjugates and that the resulting antibodies generated against both proteins could have synergistic effects. A similar phenomenon has been described for outer membrane protein antigens from N. meningitidis [58]
In a second study [59], we produced hybrid proteins between the A2 subunit of cholera toxin and Tbp-specific peptides. The A2 subunit non-covalently interacts with the cholera toxin B subunits to generate GM1-ganglioside binding-competent pentamers. We generated one chimera between the A2 domain of cholera toxin and the amino terminal half of TbpB (Fig. 2). With the idea that TbpB would be more immunogenic and TbpA would generate cross-reactive antibodies, we created a second chimera which fused the amino terminus of TbpB with loop 2 of TbpA (Fig. 2) and the A2 cholera subunit. These chimeras were used as immunogens to vaccinate BALB/c mice. While intranasal immunization alone generated low levels of Tbp-specific antibodies, following an intraperitoneal boost, both mucosal and systemic antibodies were elicited against TbpB. Likewise detectable TbpA-specific antibodies were detectable in the serum after immunization with the L2-containing chimera. Interestingly, sera from the mice immunized with the double chimera were the most cross-reactive and were bactericidal against heterologous gonococcal strains. Furthermore, vaginal secretions from mice immunized with the double chimera were capable of interfering with transferrin-dependent growth of both the homologous strain, and a heterologous strain. Therefore, as with the previous study, the double chimera between L2 of TbpA and the amino terminus of TbpB generated a more biologically-functional antibody response, in which the TbpA- and TbpB-specific antibodies appeared to act in synergy. We are currently testing these and other Tbp-specific antigens to identify those that are protective in a mouse model of female genital tract infection [60].
While the transferrin receptor system is required to initiate infection in human males, expression of the Tbps is not necessary for gonococcal survival in the female mouse model [61]. Other iron sources appear to be available in the female genital tract besides transferrin, lactoferrin, and hemoglobin. Thus, we sought to identify other potential iron transport systems encoded within the gonococcal genome. Three putative iron transporters were identified by Turner et al. [62] and we identified a fourth when searching the genome sequence of gonococcal strain FA1090. These four TonB-dependent transporters were named TdfF, TdfG, TdfH and TdfJ [62, 63]. While the ligands for these putative transporters (cumulatively labeled Tdfs in Fig. 1) have not yet been identified, they all share homology with outer membrane transporters for ferric-siderophores or heme. We insertionally inactivated each of these transporters and found that one, TdfF, was expressed and required for growth within human cervical epithelial cells [63]. The inability of the tdfF mutant to grow within epithelial cells was overcome by addition of excess iron to the cell growth medium, suggesting that TdfF is responsible for iron acquisition within epithelial cells during iron restriction. Consistent with this hypothesis, we determined that TdfF expression is repressed under high iron conditions and induced under low iron conditions, as is the case with the other neisserial iron transport systems. Cumulatively, these results suggest that TdfF is important for in vivo survival of the gonococcus and therefore might prove to be a valuable vaccine antigen for future study. The role that the other Tdfs play in gonococcal growth and/or nutrient acquisition has not yet been defined; however, these outer membrane proteins could also serve as useful vaccine targets, even if their in vivo functions have not yet been elucidated.
Other potential vaccine antigens
Several antigens have been evaluated as possible vaccine antigens to protect against gonococcal disease, with varying degrees of success. Pili were early targets for vaccine development as they function in multiple aspects of gonococcal pathogenesis including attachment, host cell signaling and natural transformation. However, the high-frequency phase and antigenic variation deployed by this antigen limits the utility of such a vaccine. Recently, Hansen et al. [64] demonstrated that a surface exposed and variable epitope of pilin was critical for immunogenicity in mice. Without this epitope, the conserved regions of pilin alone were immunosilent but were not capable of actively suppressing immunity to other antigens [64]. Thus, given the diversity of gonococcal pili and the fact that the level of diversity correlates with immunogenicity of this antigen, the goal of a cross-protective pilin-based vaccine seems unrealistic.
Another potentially tempting outer membrane protein for vaccine development is Opa. While variable expression of Opa proteins perhaps limits their utility in this regard, one could envision employing a cocktail of Opa proteins as vaccine immunogens. In vivo growth of the gonococcus selects for Opa-positivity; however, no specific Opa protein has been identified as crucial for survival in a human male challenge model of gonococcal disease [14]. Moreover, recent evidence indicates that Opa protein binding to host cell receptors results in suppressed activation and proliferation of T-cells, which would have the undesirable effect of diminishing any acquired immune response [65]. Lee et al. [66] have recently extended this observation to membrane vesicles containing Opa proteins; thus a vaccine approach based upon vesicles derived from any of the Neisseriae should be devoid of Opa proteins in order to generate the most effective immune response.
Porin proteins are abundant in the outer membrane and necessary to allow diffusion of small, hydrophilic molecules into the periplasm. N. gonorrhoeae expresses one of two porin alleles: PorB1A (previously known as P.1A) or PorB1B (previously known as P.1B). The former allele is associated with gonococcal isolates capable of causing disseminated infections; the latter allele is more frequently identified among localized isolates. Expression of either allele can result in serum resistance, although the mechanisms employed differ between porin alleles [67]. The PorB protein is not subject to high frequency phase or antigenic variation and is essential for growth. Thus, this antigen would seem to be an ideal vaccine target. As indicated above, functional anti-PorB antibody deposition is blocked by LOS sialylation and by RmpM-specific antibodies. However, vaccination with preparations lacking RmpM could alleviate the latter problem. Sparling and co-workers tested a variety of immunization strategies to discern which presentation of PorB would result in functional PorB-specific antibodies [68]. A DNA-based delivery system generated the lowest level of antibody, but a booster with recombinant protein increased this level modestly. Immunization with recombinant PorB generated significantly more antibody, but the response was strain specific, generating antibodies that reacted only with those strains expressing the same allele as was present in the vaccine. Intranasal administration of PorB-containing outer membrane vesicles generated the most functional and potentially protective responses. Specific and cross-reactive antibodies were detected in the serum and mucosal secretions; serum antibodies were bactericidal in the presence of human complement [68]. These results suggest that vesicles, which retain outer membrane proteins in a native conformation, could be the preferred means of antigen delivery, particularly via the intranasal route. However, this enthusiasm is tempered to some degree by the knowledge that these vesicles also contain LOS, which can be immunostimulatory but also quite toxic. As indicated above, vesicles are also likely to contain one or more Opa proteins which could suppress the adaptive immune response. However, this effect would be masked in mouse models and mouse immunization studies as the Opa-receptor interaction is host restricted [66].
Predicted vaccine antigens
The term "reverse vaccinology", used by Rappuoli and co-workers [69, 70] refers to the process of identifying promising vaccine antigens by application of bioinformatic approaches to genomic sequence data. Employing this approach, several vaccine candidates have been identified in Neisseria meningitidis, which cumulatively have the potential to serve as a universal vaccine to prevent meningococcal disease caused by serogroup B [71]. Similarly, Comanducci et al. have identified a novel surface adhesin of N. meningitidis, which also has vaccine potential [72]. In the gonococcus, similar genomic searches have identified a peptidyl-prolyl isomerase that is involved in persistence in macrophages [73], and more recently a potential vaccine antigen with similarity to the E. coli OmpA protein [74]. This N. gonorrhoeae OmpA-like protein was found to facilitate interaction with human cervical epithelial cells and to be important for survival in the mouse female genital tract [74]. Presumably this sampling of antigens represents the tip of the iceberg, and as more genome sequences become available from other gonococcal strains, the opportunities for vaccine development will expand dramatically.
Conclusion
N. gonorrhoeae causes the very common sexually transmitted infection, gonorrhea, which has become increasingly difficult to treat. A single class of antimicrobial agent, the third generation cephalosporins, is now the sole recommended course of treatment for this infection due to increased resistance to other agents. The impact of the disease is disproportionately experienced by women, given the incidence of asymptomatic and ascending infections among this group. Women also serve as an important reservoir for the disease. In addition, HIV transmission is facilitated by concomitant N. gonorrhoeae infection in both men and women. Thus, identification of preventative measures to combat gonococcal infections is important and the subject of ongoing research. Protective vaccines to prevent gonorrhea have been sought for decades; however, antigenic variation by and poor immunogenicity of many potential targets has hampered these efforts. While there is a protective, capsule-based vaccine against the very similar Neisseria meningitidis, this option is not available for vaccine development against N. gonorrhoeae as the latter lacks a polysaccharide capsule. Moreover, the meningococcal vaccine is not protective against all serogroups. Most notably, the serogroup B capsule is not immunogenic but is possessed by N. meningitidis strains endemic in many industrialized nations. Thus, ongoing efforts are aimed at improving the meningococcal vaccine and developing a gonococcal vaccine.
Iron acquisition is critical for the survival of most bacterial pathogens; as such the surface exposed components that contribute to this process could represent conserved, vulnerable targets for vaccine development. We have focused our efforts on the transferrin-iron acquisition system since it is ubiquitously expressed by the pathogenic Neisseriae, required for infection, and not subject to high-frequency variation. Gonococcal and meningococcal transferrin binding proteins are similar in sequence [52, 75], suggesting that a vaccine comprised of these antigens could potentially protect against both gonococcal and meningococcal infections. Antibodies generated against meningococcal transferrin binding proteins have been shown to be protective in animal models of infection [76, 77]. Subunit vaccines comprised of the gonococcal transferrin binding proteins conjugated to or fused with the B subunit of cholera toxin elicited specific antibodies, both systemically and mucosally. These antibodies had biologically-important properties, including complement-dependent bactericidal activities and growth inhibitory functions. Utilizing both TbpA and TbpB in antigen preparations resulted in increased immunogenicity and cross-reactivity. Genomic sequencing and bioinformatic approaches have identified other, as yet uncharacterized, potential vaccine antigens. Some of these new gene products are important for various aspects of gonococcal infection or pathogenesis, interference with which could result in amelioration or prevention of disease.
Future perspective
Gonorrhea rates in the United States have declined from historic highs, but incidence has risen again in recent years. Estimates of unreported or unrecognized disease rival the reported incidence numbers. Added to the dramatic rise in antibiotic resistance among gonococcal isolates, this represents a public health crisis. Efforts to develop a vaccine to prevent this common infection have so far been unsuccessful. However, several candidate antigens have shown promise with regard to immunogenicity and elicitation of cross-reactive, biologically-relevant responses. A successful gonococcal vaccine is expected to be composed of one or several surface-exposed antigens. Immunization by the mucosal route may be the preferred method as this would elicit local genital tract antibodies, in addition to systemic responses that could prevent dissemination. Given that the gonococcus is capable of colonizing both males and females in addition to multiple niches within a single individual, it seems likely that distinct surface antigens are employed in these various environments. A successful vaccine would therefore be expected to consist of factors that are expressed and vulnerable to attack in each of these niches, which could necessitate the incorporation of antigenically and functionally diverse antigens into a cocktail, subunit-based vaccine. Appropriate adjuvants will have to be identified or developed, which increase immunogenicity of conserved epitopes and preserve protein conformation in the context of a mucosally-delivered antigen.
EXECUTIVE SUMMARY
Gonorrhea infections and complications
Gonorrhea is currently the second most commonly reported infectious disease and rates in the United States have increased over the last few years.
Gonococcal infections are under-reported and often asymptomatic, especially in women.
Asymptomatic infection leads to increased morbidity, as the disease ascends into the upper genital tract. Complications include epididymitis, salpingitis, ectopic pregnancy, pelvic inflammatory disease and infertility.
Increasing urgency for development of preventative approaches
Antibiotic resistance in N. gonorrhoeae has increased dramatically. A single class of antimicrobial agent is currently available for treatment of this disease.
HIV transmission is facilitated by a concomitant gonococcal infection. Treatment of the bacterial infection decreases virus titers in mucosal secretions.
There is optimism that prevention of gonococcal infections by vaccination would decrease HIV transmission rates as a secondary consequence.
Challenges in the development of a gonococcal vaccine
Gonococcal populations are antigenically heterogeneous since many surface structures are subject to high frequency phase and antigenic variation.
Promising vaccine antigens such as porin are blocked by sialylation of lipooligosaccharide.
Prevention of a sexually transmitted disease requires development of mucosal immunity, which is complicated by a paucity of inductive sites in the genital tract.
Iron transport proteins as promising vaccine antigens
The transferrin receptor system represents a promising vaccine target as it is expressed by all gonococci and not subject to high frequency phase or antigenic variation.
All or parts of the transferrin binding proteins are immunogenic in mice when conjugated to or genetically fused with a detoxified form of cholera toxin.
Antibodies generated against the gonococcal transferrin binding proteins exhibited potentially protective characteristics, including bactericidal and growth inhibitory activities.
Other outer membrane transporters potentially fulfill important functions during gonococcal growth and could serve as protective vaccine antigens.
Other potential vaccine antigens
Gonococcal pilin is composed of variable and conserved sequence domains. However, the variable regions are immunostimulatory and the conserved regions are immunosilent.
Opacity proteins are unlikely to represent good vaccine targets as they seem to diminish acquire immune responses.
Porin proteins are abundant in the outer membrane and may represent good vaccine targets if delivered in a native conformation.
Predicted vaccine antigens
Promising vaccine antigens can be identified from genomic sequence data employing bioinformatic approaches.
With the availability of additional gonococcal genome sequences, opportunities for vaccine development are expected to expand dramatically.
Future perspective
A successful gonococcal vaccine would be expected to contain one or several surface-exposed antigens with important functions in vivo.
A cocktail vaccine comprised of multiple antigenic types, and potentially antigens differentially expressed during various phases of in vivo colonization, would likely be the most effective.
Adjuvants can potentially affect the presentation and immunogenicity of promising vaccine antigens. The selection of adjuvant and delivery system is crucial to the success of any gonococcal vaccine development effort.
Acknowledgements
C.N. Cornelissen's research is supported by Public Health Service grants AI47141 and AI065555 from the National Institute of Allergy and Infectious Diseases at NIH. The author also acknowledges the current and past members of her laboratory and Ann Jerse for critical reading of this manuscript.
Bibliography
- 1.CDC. Trends in reportable sexually transmitted diseases in the United States, 2006: National surveillance data for Chlamydia, Gonorrhea, and Syphillis. Atlanta: Georgia; 2007. pp. 1–7. [Google Scholar]
- 2.WHO. Global prevalence and incidence of selected curable sexually transmitted infections: Overview and estimates. Geneva: WHO; 2001. [PubMed] [Google Scholar]
- 3.Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 2007;44:84–101. doi: 10.1086/511422. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein KT, Mehta SD, Rompalo AM, Erbelding EJ. Cost-effectiveness of screening strategies for gonorrhea among females in private sector care. Obstet. Gynecol. 2006;107:813–821. doi: 10.1097/01.AOG.0000204187.86600.0a. [DOI] [PubMed] [Google Scholar]
- 5.Farley TA, Cohen D, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prevent. Med. 2003;36:502–509. doi: 10.1016/s0091-7435(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 6.Turner CF, Rogers SM, Miller HG, et al. Untreated gonococcal and chlamydial infection in a probability sample of adults. J. Am. Med. Assoc. 2002;287:726–733. doi: 10.1001/jama.287.6.726. [DOI] [PubMed] [Google Scholar]
- 7. CDC. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. Morb. Mortal. Wkly. Rep. 2007;56:332–336. • The increased incidence of resistance to fluoroquinolones among Neisseria gonorrhoeae isolates led the CDC in 2006 to stop recommending this class of antibiotics to treat gonococcal infections.
- 8.McCarthy M. Drug-resistant gonorrhoeae spread in the USA. Lancet. 2007;369:1592. doi: 10.1016/S0140-6736(07)60729-6. [DOI] [PubMed] [Google Scholar]
- 9.Spencer SE, Valentin-Bon IE, Whaley K, Jerse AE. Inhibition of Neisseria gonorrhoeae genital tract infection by leading-candidate topical microbicides in a mouse model. J. Infect. Dis. 2004;189:410–419. doi: 10.1086/381125. [DOI] [PubMed] [Google Scholar]
- 10. Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nature Rev. Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. • Review of the role that sexually transmitted infections play in facilitating HIV transmission.
- 11.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 12.Criss AK, Kline KA, Seifert HS. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 2005;58:510–519. doi: 10.1111/j.1365-2958.2005.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayliss CD, Field D, Moxon ER. The simple contingency loci of Haemophilus influenzae and Neisseria meningitidis. J. Clin. Invest. 2001;107:657–662. doi: 10.1172/JCI12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerse AE, Cohen MS, Drown PM, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas GD, Anderson JE, Chen C-j, Cornelissen CN, Sparling PF. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect. Immun. 1999;67:455–459. doi: 10.1128/iai.67.1.455-459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CJ, Elkins C, Sparling PF. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect. Immun. 1998;66:987–993. doi: 10.1128/iai.66.3.987-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson SD, Stone B, Beucher M, Fu J, Sparling PF. Phase variation of the gonococcal siderophore receptor FetA. Mol. Microbiol. 2000;36:585–593. doi: 10.1046/j.1365-2958.2000.01873.x. [DOI] [PubMed] [Google Scholar]
- 18.Elkins C, Carbonetti NH, Varela VA, Stirewalt D, Klapper DG, Sparling PF. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 19.Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestecky J, Russell MW. Induction of mucosal immune responses in the human genital tract. FEMS Immunol. Med. Microbiol. 2000;27:351–355. doi: 10.1111/j.1574-695X.2000.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 21.Russell MW, Hedges SR, Wu H-Y, Hook EW, Mestecky J. Mucosal immunity in the genital tract: prospects for vaccines against sexually transmitted diseases - a review. Am. J. Reprod. Immun. 1999;42:58–63. doi: 10.1111/j.1600-0897.1999.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 22.Russell MW, Martin MH, Wu H-Y, Hollingshead SK, Moldoveanu Z, Mestecky J. Strategies of immunization against mucosal infections. Vaccine. 2001;19:s122–s127. doi: 10.1016/s0264-410x(00)00290-5. [DOI] [PubMed] [Google Scholar]
- 23. Johansson E-L, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 2001;69:7481–7486. doi: 10.1128/IAI.69.12.7481-7486.2001. • Demonstrated that the B subunit of cholera toxin could be employed as an antigen to stimulate localized and systemic antibody development following intranasal and intravaginal vaccination.
- 24.Wu H-Y, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect. Immun. 2000;68:5539–5545. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez HM, Figueredo M, Garrido N, Sanchez L, Sarracent J. Intranasal immunisation with a 62 kDa proteinase combined with cholera toxin or CpG adjuvant protects against Trichomonas vaginalis genital tract infections in mice. Int. J. Parisitol. 2005;35:1333–1337. doi: 10.1016/j.ijpara.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 2007;75:666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plante M, Jerse A, Hamel J, et al. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 2000;182:848–855. doi: 10.1086/315801. [DOI] [PubMed] [Google Scholar]
- 28.Burall LS, Harro JM, Li X, et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: Identification of 25 signiture-tagged mutants attenuated at least 100-fold. Infect. Immun. 2004;72:2922–2938. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldmann F, Sorsa LJ, Hildinger K, Schubert S. The salmochelin siderophore receptor IroN contributes to invasion of urothelial cells by extraintestinal pathogenic Escherichia coli in vitro. Infect. Immun. 2007;75:3183–3187. doi: 10.1128/IAI.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawlor MS, O'Connor C, Miller VL. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 2007;75:1463–1472. doi: 10.1128/IAI.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas GD, Sparling PF. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect. Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelissen CN, Kelley M, Hobbs MM, et al. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JE, Hobbs MM, Biswas GD, Sparling PF. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 2003;48:1325–1337. doi: 10.1046/j.1365-2958.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- 34.Rohde KH, Gillaspy AF, Hatfield MD, Lewis LA, Dyer DW. Interactions of haemoglobin with Neisseria meningitidis receptor HpuAB: the role of TonB and intact proton motive force. Mol. Microbiol. 2002;43:335–354. doi: 10.1046/j.1365-2958.2002.02745.x. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JE, Leonie PA, Miller WC, Chen CJ, Hobbs MM, Sparling PF. Selection for expression of the gonococcal hemoglobin receptor during menses. J. Inf. Dis. 2001;184:1621–1623. doi: 10.1086/324564. [DOI] [PubMed] [Google Scholar]
- 36.Carson SDB, Klebba PE, Newton SMC, Sparling PF. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 1999;181:2895–2901. doi: 10.1128/jb.181.9.2895-2901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S, King CA, Klein EK, et al. The gonococcal fur-regulated tbpA and tbpB genes are expressed during natural mucosal gonococcal infection. Infect. Immun. 2005;73:4281–4287. doi: 10.1128/IAI.73.7.4281-4287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price GA, Hobbs MM, Cornelissen CN. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect. Immun. 2004;72:277–283. doi: 10.1128/IAI.72.1.277-283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges SR, Mayo MS, Mestecky J, Hook EW, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrheae during uncomplicated genital infections. Infect. Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hedges SR, Sibley DA, Mayo MS, Hook EW, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 41.Pantelic M, Kim Y-J, Bolland S, Chen I, Shively J, Chen T. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect. Immun. 2005;73:4171–4179. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masri HP, Cornelissen CN. Specific ligand binding attributable to individual epitopes of gonococcal transferrin binding protein A. Infect. Immun. 2002;70:732–740. doi: 10.1128/iai.70.2.732-740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yost-Daljev MK, Cornelissen CN. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect. Immun. 2004;72:1775–1785. doi: 10.1128/IAI.72.3.1775-1785.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchanan SK, Smith BS, Venkatramani L, et al. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nature Struct. Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 45.Chimento DP, Mohanty AK, Kadner RJ, Wiener MC. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 2003;10:394–401. doi: 10.1038/nsb914. [DOI] [PubMed] [Google Scholar]
- 46.Cobessi D, Celia H, Folschweiller N, Schalk IJ, Abdallah MA, Pattus F. The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6A resolution. J. Mol. Biol. 2005;347:121–134. doi: 10.1016/j.jmb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Cobessi D, Celia H, Pattus F. Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J. Mol. Biol. 2005;352:893–904. doi: 10.1016/j.jmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J. Structural basis of gating by the outer membrane transporter FecA. Science. 2002;295:1715–1719. doi: 10.1126/science.1067313. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 50. Locher KP, Rees B, Koebnik R, et al. Transmembrane signaling across the ligand-gated FhuA receptor: Crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. • One of the first studies to present the crystal structure of a bacterial TonB-dependent transporter.
- 51.Boulton IC, Yost MK, Anderson JE, Cornelissen CN. Identification of discrete domains within gonococcal transferrin-binding protein A that are necessary for ligand binding and iron uptake functions. Infect. Immun. 2000;68:6988–6996. doi: 10.1128/iai.68.12.6988-6996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeRocco AJ, Cornelissen CN. Identification of transferrin-binding domains in TbpB expressed by Neisseria gonorrhoeae. Infect. Immun. 2007;75:3220–3232. doi: 10.1128/IAI.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornelissen CN, Sparling PF. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin M, Hajishengallis G, Metzger DJ, Michalek SM, Connell TD, Russell MW. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 2001;69:252–261. doi: 10.1128/IAI.69.1.252-261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect. Immun. 2005;73:3945–3953. doi: 10.1128/IAI.73.7.3945-3953.2005. • Demonstrated that gonococcal transferrin binding proteins, when chemically conjugated to cholera toxin B subunits, could generate potentially protective antibodies after intranasal vaccination of mice.
- 57.Thomas CE, Zhu W, Dam CNV, Davis NL, Johnson RE, Sparling PF. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan Equine Encephalitis viral replicon particles. Infect. Immun. 2006;74:1612–1620. doi: 10.1128/IAI.74.3.1612-1620.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weynants VE, Feron CM, Goraj DK, et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 2007;75:5434–5442. doi: 10.1128/IAI.00411-07. • Demonstrated synergism of antibodies generated against a combination of minor outer membrane proteins from Neisseria meningitides.
- 59. Price GA, Masri HP, Hollander AM, Russell MW, Cornelissen CN. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine. 2007;25:7247–7260. doi: 10.1016/j.vaccine.2007.07.038. • Demonstrated that genetic chimeras between epitopes of TbpA and TbpB fused with cholera toxin B subunits were immunogenic and capable of inducing potentially protective antibody responses in mice.
- 60.Jerse A. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jerse AE, Crow ET, Bordner AN, et al. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect. Immun. 2002;70:2549–2558. doi: 10.1128/IAI.70.5.2549-2558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner PC, Thomas CE, Stojiljkovic I, et al. Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiol. 2001;147:1277–1290. doi: 10.1099/00221287-147-5-1277. [DOI] [PubMed] [Google Scholar]
- 63.Hagen TA, Cornelissen CN. Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol. Microbiol. 2006;62:1144–1157. doi: 10.1111/j.1365-2958.2006.05429.x. [DOI] [PubMed] [Google Scholar]
- 64.Hansen JK, Demick KP, Mansfield JM, Forest KT. Conserved regions from Neisseria gonorrhoeae pilin are immunosilent and not immunosuppressive. Infect. Immun. 2007;75:4138–4147. doi: 10.1128/IAI.02015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 2002;3:229–236. doi: 10.1038/ni769. • Demonstrated that binding of neisserial Opa proteins to CEACAM1 expressing T cells resulted in suppression of lymphocyte function.
- 66.Lee HSW, Boulton IC, Reddin K, et al. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and supress CD4+ T lymphocyte function. Infect. Immun. 2007;75:4449–4455. doi: 10.1128/IAI.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ngampasutadol J, Ram S, Blom AM, et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Nat. Acad. Sci. USA. 2005;102:17142–17147. doi: 10.1073/pnas.0506471102. • Demonstrated that gonococcal resistance to complement-mediated killing is host restricted, providing one mechanism to explain the species specificity of gonococcal infections.
- 68.Zhu W, Thomas CE, Chen C-j, et al. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan Equine Encephalitis virus replicon particles. Infect. Immun. 2005;73:7558–7568. doi: 10.1128/IAI.73.11.7558-7568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mora M, Veggi D, Santini L, Pizza M, Rappuoli R. Reverse vaccinology. Drug Discov. Today. 2003;8:459–464. doi: 10.1016/s1359-6446(03)02689-8. [DOI] [PubMed] [Google Scholar]
- 70. Scarselli M, Giuliani MM, Adu-Bobie J, Pizza M, Rappuoli R. The impact of genomics on vaccine design. Trends Biotechnol. 2005;23:84–91. doi: 10.1016/j.tibtech.2004.12.008. • Recent review of the potential uses of genomic sequence date in vaccine development efforts.
- 71. Guiliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc. Nat. Acad. Sci. USA. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. • Demonstrated production of cross-reactive and potentially protective antibodies against five meningococcal vaccine antigens predicted by reverse vaccinology.
- 72.Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leuzzi R, Serino L, Scarselli M, et al. Ng-MIP, a surface-exposed lipoprotein on Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- 74.Serino L, Nesta B, Leuzzi R, et al. Identification of a new OmpA-like protein in Neisseria gonorrhoeae involved in the binding to human epithelial cells and in vivo colonization. Mol. Microbiol. 2007;64:1391–1403. doi: 10.1111/j.1365-2958.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- 75.Cornelissen CN, Anderson JE, Boulton IC, Sparling PF. Antigenic and sequence diversity in gonococcal transferrin-binding protein A (TbpA) Infect. Immun. 2000;68:4725–4735. doi: 10.1128/iai.68.8.4725-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danve B, Lissolo L, Mignon M, et al. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 77.West D, Reddin K, Matheson M, et al. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 2001;69:1561–1567. doi: 10.1128/IAI.69.3.1561-1567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]